Conspectus
Nitrogen heterocycles are present in many compounds of enormous practical importance, ranging from pharmaceutical agents and biological probes to electroactive materials. Direct functionalization of nitrogen heterocycles through C-H bond activation constitutes a powerful means of regioselectively introducing a variety of substituents with diverse functional groups onto the heterocycle scaffold. Working together, our two groups have developed a family of Rh-catalyzed heterocycle alkylation and arylation reactions that are notable for their high level of functional-group compatibility. This Account describes our work in this area, emphasizing the relevant mechanistic insights that enabled synthetic advances and distinguished the resulting transformations from other methods.
We initially discovered an intramolecular Rh-catalyzed C-2-alkylation of azoles by alkenyl groups. That reaction provided access to a number of di-, tri-, and tetracyclic azole derivatives. We then developed conditions that exploited microwave heating to expedite these reactions. While investigating the mechanism of this transformation, we discovered that a novel substrate-derived Rh-N-heterocyclic carbene (NHC) complex was involved as an intermediate. We then synthesized analogous Rh–NHC complexes directly by treating precursors to the intermediate [RhCl(PCy3)2] with N-methylbenzimidazole, 3-methyl-3,4-dihydroquinazoline, and 1-methyl-1,4-benzodiazepine-2-one.
Extensive kinetic analysis and DFT calculations supported a mechanism for carbene formation in which the catalytically active RhCl(PCy3)2 fragment coordinates to the heterocycle before intramolecular activation of the C-H bond occurs. The resulting Rh-H intermediate ultimately tautomerizes to the observed carbene complex. With this mechanistic information and the discovery that acid co-catalysts accelerate the alkylation, we developed conditions that efficiently and intermolecularly alkylate a variety of heterocycles, including azoles, azolines, dihydroquinazolines, pyridines, and quinolines, with a wide range of functionalized olefins. We demonstrated the utility of this methodology in the synthesis of natural products, drug candidates, and other biologically active molecules.
In addition, we developed conditions to directly arylate these heterocycles with aryl halides. Our initial conditions that used PCy3 as a ligand were successful only for aryl iodides. However, efforts designed to avoid catalyst decomposition led to the development of ligands based on 9-phosphabicyclo[4.2.1]nonane (Phoban) that also facilitated the coupling of aryl bromides. We then replicated the unique coordination environment, stability, and catalytic activity of this complex using the much simpler tetrahydrophosphepine ligands and developed conditions that coupled aryl bromides bearing diverse functional groups without the use of a glovebox or purified reagents. With further mechanistic inquiry, we anticipate that researchers will better understand the details of the aforementioned Rh-catalyzed C-H bond functionalization reactions, resulting in the design of more efficient and robust catalysts, expanded substrate scope, and new transformations.

Introduction
Nitrogen heterocycles are present in many compounds of enormous practical importance, ranging from pharmaceutical agents and biological probes to electroactive materials. Tailoring the properties of these compounds to satisfy their specific functions necessitates the development of synthetic methods capable of regioselectively introducing a variety of substituents bearing diverse functional groups to the desired heterocycle scaffold. Direct functionalization of nitrogen heterocycles through C-H bond activation constitutes a powerful means to accomplish this important goal.1
This approach provides an atom-economical alternative to conventional procedures using halogenated or metallated starting materials.2 However, a potential catalyst must activate a relatively inert C-H bond in the presence of other C-H bonds and then efficiently functionalize the metallated carbon in order to provide the desired product.3 Despite these demanding requirements, a number of reactions that exploit directing groups,4 repulsive steric interactions,5 electron-rich substrates,6 C-H bond acidity,7 radical stability8, and even enzymes9 to selectively activate and functionalize a specific C-H bond with a transition metal catalyst have been developed. When applicable to a particular substrate class, these methods reduce reaction byproducts, increase the number of available substrates, and decrease the synthetic effort required for formation of the desired C-C bond.
Selecting the appropriate catalyst for a desired substrate can seem daunting. However, understanding the mechanisms of the individual transformations provides a rational approach to addressing this problem as well as a framework for the application and continued discovery of new transformations. Working together, principally via jointly supervised coworkers, our two groups have extensively applied this strategy toward the development of a family of Rh-catalyzed heterocycle alkylation and arylation reactions that are notable for the high level of functional group compatibility that is achieved. This account describes our work in this area, emphasizing the relevant mechanistic insights that enabled synthetic advances and distinguished the resulting transformations from other methods.
Intramolecular Alkylation of Azoles
The prevalence of 2-alkyl azoles in drugs led to our interest in developing a catalytic method for the alkylation of azoles at the 2-position.10 At the time we began our investigations in this area, a number of researchers had demonstrated the feasibility of directly arylating the 2-position of azoles with aryl halides using Pd catalysis11 and acylating this same site using Ru catalysis.12 We envisioned that the analogous alkylation reaction might be accomplished by formal hydroheteroarylation of an olefin by an azole in the presence of a transition metal catalyst.13
A number of late transition metal complexes were screened for their ability to catalyze the intramolecular alkylation of a benzimidazole bearing a pendant olefin, which led to the finding that Wilkinson’s catalyst (RhCl(PPh3)3) provided a single cyclization product 1 in 60% isolated yield (Scheme 1). Optimization of the reaction parameters led to the identification of [RhCl(coe)2]2 (coe = cyclooctene) as a highly effective Rh pre-catalyst and PCy3 as the optimal phosphine. This catalyst system was then applied toward the synthesis of a range of bi-, tri-, and tetracyclic 2-alkylimidazoles (Scheme 2, conditions a). In general, the cyclization provides products containing a five-membered ring as the major isomer unless an overriding steric bias, such as geminal alkene substitution or allylic α,α-dibranding, prevails. This preference is observed for both allyl- and homoallyl-substituted imidazoles due to rapid, competitive olefin isomerization that generates an allyl-substituted cyclization precursor regardless of the initial olefin position.
Scheme 1.
Scheme 2.
Extensive efforts to improve the efficiency of this reaction led to the discovery that Lewis and Brønsted acid additives, including 2,6-dimethylpyridinium chloride and magnesium bromide, provided marked increases in reaction rate and conversion.14 It was subsequently found that [PCy3H]Cl could be conveniently utilized to provide both the additive and the phosphine. This modification simplifies the reaction setup and renders the phosphine air stable for long-term storage.
In a collaborative effort with researchers at Abbott Laboratories, the cyclization reactions described above were reinvestigated using [PCy3H]Cl and a simplified protocol employing microwave heating (Scheme 2, conditions b).15 The use of a microwave reactor allows convenient access to the high temperatures needed to reduce reaction times to ≤ 20 min, and the initial reaction mixtures were assembled using only a N2 line to degas the solvent and reaction vessel prior to heating.16 A microwave procedure was also developed using commercially available Wilkinson’s catalyst in place of [RhCl(coe)2]2/PCy3. Even with this suboptimal catalyst, the desired cyclized products were obtained in moderate yields (data not shown).
The intramolecular alkylation reaction has subsequently been applied to the synthesis of complex bioactive compounds. For example, the potent c-Jun N-terminal kinase inhibitor 3, originally prepared in 14 linear steps and 6% overall yield,17 was prepared in 11 linear steps and 13% overall yield by relying on our C-H functionalization reaction as the key step in the sequence (Scheme 3).18 More highly substituted derivatives 4, ent-4, and 5, which would be very difficult to prepare by alternative methods, could be readily synthesized just as rapidly in 15 and 17% overall yields, respectively, and resulted in the identification of even more potent inhibitors (Figure 1).
Scheme 3.
Figure 1.
Methylated Derivatives of Kinase Inhibitor 3.
Investigation of the Mechanism of C-H Activation
Having established the utility of this new method for the intramolecular alkylation of imidazoles, we undertook an investigation of the mechanism of this novel transformation.19 Initial deuterium tracer and crossover experiments revealed that rapid H/D exchange occurred between the heterocycle 2-position and pendant and external olefins. In order to shed further light on this exchange and to minimize the number of concurrent processes, the non-isomerizable substrate 6 was heated in the presence of stoichiometric quantities of [RhCl(coe)2]2 and PCy3 at a temperature substantially below that required for cyclization in the absence of acid co-catalysts (Scheme 4).
Scheme 4.
A single compound, assigned as the square planar Rh(I)-N-heterocyclic carbene (NHC) complex 7 by X-ray crystallography (Figure 2), formed under these conditions. This result was surprising, given that literature precedent generally indicates the formation of Rh(III)- or Ir(III)-hydride complexes following C-H bond activation by neutral Rh(I) or Ir(I) species.3 Nonetheless, 7 catalyzed the cyclization of benzimidazole derivative 8 at the same rate as [RhCl(coe)2]2/PCy3 (Scheme 5). Furthermore, a kinetic investigation of the cyclization of 8 catalyzed by complex 7 indicated that the reaction was zero order in [8] and first order in [7]. These results are consistent with a mechanism in which the resting state of the catalyst contains a single molecule of bound substrate. Carbene complex 7 forms at a lower temperature than that at which cyclization occurs and is observed throughout the reaction, leading to the conclusion that 7 is the resting state of the catalyst.
Figure 2.

ORTEP Diagram of 7.
Scheme 5.
Similar Rh-NHC complexes have been obtained from the reaction of [RhCl(coe)2]2 and PCy3 with a number of additional heterocycles, including benzimidazole,20 3,4-dihydroquinazoline,21 and a 1,4-benzodiazepine-2-one22 (Scheme 6, Figure 3). Prior to these examples, the direct formation of metal-NHC complexes from the corresponding heterocycles had not been reported. The novelty of this transformation, in addition to its centrality to our alkylation chemistry, led us to explore its mechanism in greater detail.
Scheme 6.
Figure 3.

ORTEP Diagrams of 10–12.
Closer examination of the reaction of 3-methyl-3,4-dihydroquinazoline 13, [RhCl(coe)2]2, and PCy3 at 45 °C in THF revealed the initial formation of a N-bound Rh-heterocycle complex, 14 (Scheme 7).21 The structure of this complex was established unambiguously by extensive multi-dimensional NMR experiments on a variety of 2H, 13C, and 15N labeled 3-methyl-3,4-dihydroquinazolines. Upon further heating at 75 °C, this material was converted to the Rh-NHC complex 11. Careful kinetic analysis established the N-bound complex 14 as an intermediate in the formation of 11.
Scheme 7.
Deuterium labeling tracer experiments using C2-D-14 led to formation of the carbene complex possessing 88% deuterium at the N1 position. In addition, a double-labeling crossover experiment carried out using equal amounts of C2-D-13 and 15N1-13 revealed only a minor amount of crossover product (14N1-H-11/15N1-D-11). Together, these data are consistent with an intramolecular H-transfer in the conversion of 14 to 11. The deuterium kinetic isotope effect on rate of conversion of 14 to 11 was measured, and the observed kH/kD (1.8 ± 0.1) is consistent with cleavage of the C2-H bond during or prior to the rate limiting step. Activation parameters (ΔH‡ = 26.0 ± 0.3 kcal/mol and ΔS‡ = −10.3 ± 0.8 cal/mol·K) were also obtained and reveal the dramatic extent to which metal-mediation facilitates heterocycle-to-NHC tautomerization.
In an effort to gain further insight into the microscopic steps of C-H activation, these detailed mechanistic data were augmented with DFT calculations. A model system using 3-methyl-3,4-dihydropyrimidine in place of 13 and PMe3 in place of PCy3 was constructed based upon the structures of 14 and 11. Conversion of starting complex A, the structure of which is in agreement with all spectroscopic data for 11, to I was most consistent with a mechanism shown in Figure 4. Importantly, this mechanism indicated that the aforementioned Rh(III)-hydride complex (G in Figure 4) does indeed lie on the reaction coordinate, but that the NHC tautomer is thermodynamically more stable and readily accessible via an intermolecular H migration.
Figure 4.
Calculated Reaction Coordinate for Carbene Formation.
While N-heterocyclic carbenes are commonly utilized as ancillary ligands on transition metal complexes,23 the results highlighted above marked the first time that such complexes had been implicated as intermediates in a catalytic reaction.24 More significantly, the known formation of stable metal-NHC complexes derived from myriad metals and carbene precursors, most notably azoles,23 non-aromatic heterocycles,25 heterocycles with only a single heteroatom,26 and even certain acyclic molecules,27 suggested that a number of additional substrates might also be compatible with Rh-catalyzed alkylation reaction conditions. Taken together with our synthesis of metal-NHC complexes directly from a variety of heterocycles, these precedents provided strong evidence that a greatly expanded substrate scope of Rh-catalyzed alkylation could be realized.
Intermolecular Alkylation of Heterocycles
Azoles
Initial efforts to achieve this goal focused on the intermolecular alkyation of various azoles with alkenes. Reaction conditions were initially evaluated for the intermolecular alkylation of benzimidazole using 3,3-dimethyl-1-butene which proceeded efficiently with catalytic amounts of [RhCl(coe)2]2 and either [PCy3H]Cl or PCy3 and 2,6-dimethylpyridinium chloride (LutCl) (Scheme 8).14 Heterocycles capable of stabilizing M-NHC complexes served as effective substrates, including 1-methylbenzimidazole, benzthiazole, benzoxazole, 4,5-dimethylthiazole, and purine. Moreover, a broad functional group tolerance on the heterocycle was observed, and a variety of terminal olefins were compatible with the reaction conditions. In particular, both electron rich and electron poor olefins underwent coupling efficiently, and numerous functional groups, including silyl ethers, esters, acetals, phthalimides, and nitriles were tolerated.
Scheme 8.
3,4-Dihydroquinazolines
Quinazoline-derived metal-carbene complexes are less well represented in the literature than their azole congeners, but the results of our study of complex 11 provided ample motivation to explore the Rh-catalyzed alkylation of this heterocycle class.28 Furthermore, the quinazoline skeleton, in its various oxidation states, is a common structural motif in natural products and pharmaceuticals with a range of biological activities.29 We initially investigated the alkylation of 3,4-dihydroquinazoline because it can be readily converted to the analogous quinazolines, quinazolinones, or 1,2,3,4-tertahyrdroquinazolines by choice of an appropriate oxidant or reductant.30
Analysis by 1H NMR spectroscopy established that quantitative C2 alkylation of 3,4-dihydroquinazoline occurred with n-hexene in the presence of our Rh catalyst. To simplify product analysis, oxidation of the crude alkylation product to the corresponding alkylated quinazoline was accomplished using MnO2 (Scheme 9). In contrast to the azole alkylation discussed above, both styrene and methylenecyclohexane, a 1,1-disubstituted alkene, coupled efficiently, and N-methyldihydroquinazoline also proved to be a viable substrate.
Scheme 9.
Intramolecular dihydroquinazoline alkylation is also of interest because the process can be used to form ring-fused pyrrolo[2,1-b]quinazolines, a common scaffold for medically relevant natural products.29 To demonstrate the applicability of this method, we undertook a total synthesis of vasicoline (Scheme 10). Cyclization of 15 proved to be particularly challenging, but a conformationally rigid cyclohexyl-phoban ligand, which had previously provided ruthenium alkylidene catalysts with enhanced stability,31 proved to be an effective ligand to facilitate the desired reaction.
Scheme 10.
Azolines
Non-aromatic azolines were next targeted as potential substrates since the wealth of literature on azoline-derived carbene ligands suggested that the corresponding azolines should be compatible with our NHC mechanism.25 We were particularly interested in the alkylation of oxazolines as such a method would constitute a formal C1 olefin homologation without the use of toxic CO gas. The alkylated oxazolines could subsequently be converted to carboxylic acids or esters using well-known chemistry.32
Alkylation of 4,4-dimethyl-2-oxazoline was achieved with a large number of alkenes displaying a range of functionality in the presence of our Rh-phosphine catalyst (Scheme 11).33 Under optimal conditions the alkylation could often be performed at 45 °C, a temperature significantly lower than that used in the coupling of aromatic azoles. Both 1,1- and 1,2-disubstituted alkenes were effective coupling partners, and cyclohexene and methylenecylcohexane underwent coupling with good to moderate yield. Even α-methylstyrene, which results in a new stereocenter, coupled with modest efficiency.
Scheme 11.
Pyridines
In all of the substrates compatible with the Rh-catalyzed alkylation chemistry discussed thus far, the reactive carbon atom is flanked by two heteroatoms. This motif presumably stabilizes the proposed Rh-NHC intermediates common to this family of reactions.23 However, a number of groups have investigated the formation of NHC complexes in which two donating heteroatoms are not required.26,27 While these complexes were not necessarily formed via the activation of a C-H bond, they did indicate the possibility that such complexes could function as catalytic intermediates. The Carmona and Esteruelas groups recently reported the synthesis of 2-substituted-pyridine- and quinoline-based Os, Ru, and Ir-NHC complexes directly from the corresponding heterocycles and a late transition metal complex.26 The authors propose mechanisms similar to those shown in Figure 4, but emphasize the necessity of substitution ortho to the heterocycle ring nitrogen in order to drive the equilibrium from an N-bound to the desired NHC complexes (Scheme 7, Figure 4). We therefore sought to determine whether our Rh/PCy3 catalyst system could be used to not only activate but also alkylate these heterocycles.
Slight modifications to the conditions optimized for azole alkylation enabled the alkylation of a number of 2-substituted pyridines (Scheme 12).34 Increasing the bulk of the 2-substituent from methyl to isopropyl led to an increase in both alkylation rate and isolated yield of alkylated product, and 2-triisopropylsilylpyridine also proved to be an effective substrate. Consistent with the findings of Carmona and Esteruelas for carbene formation,26 pyridine was alkylated in less than 5% yield when heated in the presence of excess olefin and catalyst.
Scheme 12.
A variety of quinolines were also alkylated under the reaction conditions. Parent quinoline was nearly quantitatively converted to the corresponding alkylated quinoline, and both ether and ester substitution were tolerated in the quinoline 6-position. A wide range of olefin substitution patterns were also compatible with the reaction conditions.
While substitution ortho to the pyridine nitrogen was required to obtain high yields of alkylated products, an ortho-silyl group serves as a suitable blocking group that can readily be removed to provide mono-alkylated pyridines. For example, treatment of 18 with aqueous HF in refluxing THF provided the mono-alkylated pyridine product 19 in good yield (Scheme 13).
Scheme 13.
In a preliminary evaluation of catalyst loading, quinoline was alkylated with neohexene in 91% yield using only 0.5 mol% of the Rh catalyst (Scheme 14).
Scheme 14.
Rh-Catalyzed Direct Arylation of Heterocycles
Our successes in the area of Rh-catalyzed heterocycle alkylation led us to postulate the feasibility of the corresponding arylation, as such a transformation would provide a highly efficient route to pharmaceutically relevant compounds.35 At the time we began our research in this area, direct heterocycle arylation had seen only limited literature precedent with Miaura’s pioneering work using Pd catalysis as the most notable example.11 Furthermore, we hoped that the novel mode of activation available using Rh-catalysis might offer regioselectivity and substrate scope different from those observed with existing Pd and Cu-based catalysts.36
Discovery and Optimization of Rh-Catalyzed Heterocycle Arylation
Aryl iodides were identified as suitable coupling partners for the arylation of benzimidazole using catalytic amounts of [RhCl(coe)2]2 and PCy3 in the presence of triethylamine (Scheme 15).20 In promising initial studies, a range of heterocycles, including benzimidazoles, benzoxazoles, 3,4-dihydroquinazoline and 3,3-dimethyl oxazoline, and both electron-rich and poor aryl halides coupled in moderate to good yields.
Scheme 15.
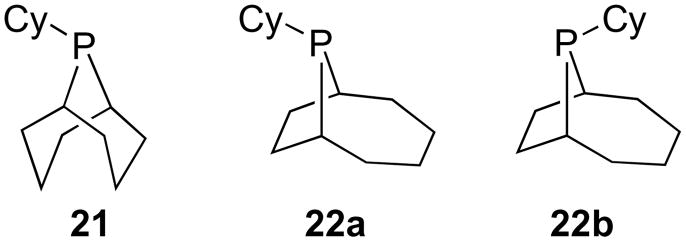
In addition, hydrodehalogenation of the aryl iodide coupling partner was identified as a key side reaction under the reaction conditions.37 This process resulted from the dehydrogenation of the cyclohexyl groups of PCy3, which led to the formation of reactive Rh-hydride complexes and ligand decomposition.38 Our efforts to find phosphines that would maintain the unique steric and electronic qualities of PCy3, while reducing the ability of the phosphine to undergo dehydrogenation, led to the exploration of P-substituted phobanes as used for the previously discussed dihydroquinazoline alkylation (Figure 5).28 Superior results were obtained using the [4.2.1] phoban isomers (22a and 22b) as opposed to the [3.3.1] isomer (21) used in the alkylation reaction.
Figure 5.
Structures of [3.3.1] (21), exo-[4.2.1] (22a), and endo-[4.2.1] (22b) Isomers of 9-Cyclohexyl-9-phosphabicyclononane.
Microwave heating was employed to facilitate reaction set-up and to conveniently reach the higher temperatures needed to minimize reaction time. Following optimization of the reaction conditions, 2-phenylbenzimidazole was produced from the coupling of benzimidazole and iodobenzene in a 95% yield. More importantly, bromobenzene was also coupled to benzimidazole in 80% isolated yield under the same reaction conditions (Scheme 16).
Scheme 16.
The direct arylation of heterocycles with aryl bromides using a Rh/22a/b catalyst exhibited considerable functional group tolerance39 and provided access to 2-arylbenzimidazoles incorporating a wide variety of functional groups, including nitrile, chloride, alkoxy, ketone, and amide substituents (data not shown). The reaction conditions were compatible with a number of different heterocycles, including N-methylbenzimidazole, benzoxazole, 3,4-dihydroquinazoline and bis-arylimidazoles.
Development of New Ligands
Efforts to understand the enhanced arylation activity afforded through the use of 22a/b revealed the formation of P-olefin complex 23 under the arylation reaction conditions (Scheme 17).40 This complex was prepared in good yield, and its structure was confirmed by single crystal X-ray analysis (Figure 6). The structure clearly showed that one of the ligands had been selectively dehydrogenated to generate a P-olefin binding motif, while the second was left intact. The stability of this complex even under extended heating at 125 °C indicated the existence of tighter chelation of the rhodium center relative to the analogous complex formed in situ from Rh/PCy3, which underwent multiple rounds of cyclometallation/β-hydride elimination ultimately leading to complete decomposition.
Scheme 17.
Figure 6.

ORTEP diagram of 23.
Complex 23 catalyzed the arylation of benzimidazole with a rate and final yield similar to those obtained with the use of [RhCl(coe)2]2/22a/b. Thus, the capacity of 22a/b to form a stable bidentate P-olefin Rh complex, and not solely the sterics and electronics of the phosphines themselves, is largely responsible for the superior activity of the arylation catalysts derived from phobane ligands. Based on this hypothesis, we sought to simplify the phosphine ligand while maintaining the P-olefin binding motif that conferred the unique activity of 23 through the use of (Z)-2,3,6,7-tetrahydrophosphepines (Figure 7).41
Figure 7.

(Z)-2,3,6,7-Tetrahydrophosphepine skeleton.
A family of these ligands was synthesized, and the Rh complex (27) of (Z)-1-cyclohexyl-2,3,6,7-tetrahydro-1H-phosphepine (25) was prepared (Scheme 18). The structure of 27 was confirmed by single crystal X-ray analysis (Figure 8). Notably, the Rh-binding motifs found in 23 and 27 exhibited a great deal of similarity, indicating that removing the 2-carbon bridge from 22a did not significantly alter the desired coordination geometry.
Scheme 18.
Figure 8.

ORTEP Diagram of 27.
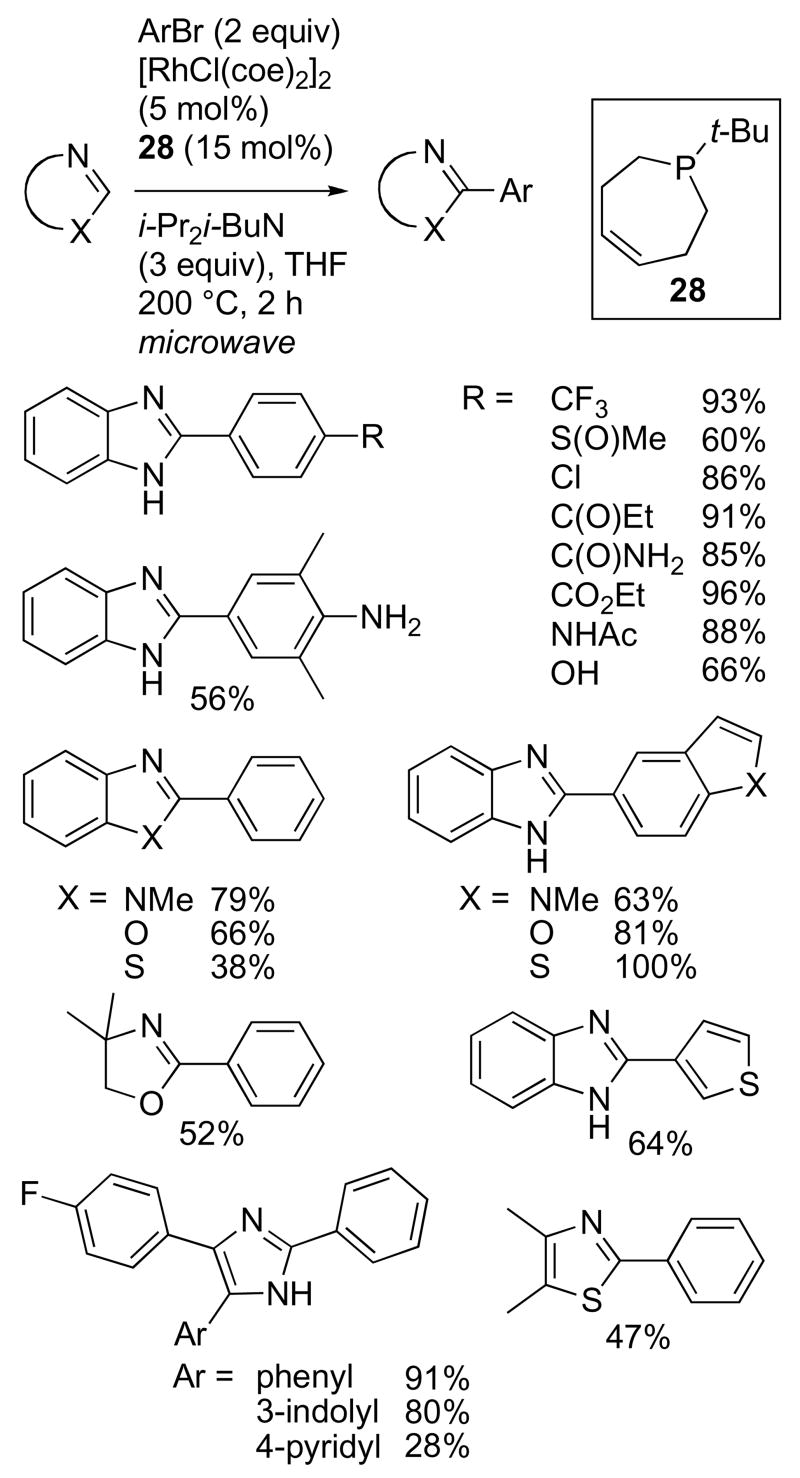
The tert-butyl-substituted phosphepine 28 (Figure 7, R = t-Bu) provided the highest conversion and and essentially no hydrodehalogenation of PhBr during the course of the arylation of benzimidazole, with optimal conditions being microwave heating at 200 °C for 2 h with THF as the solvent.42 The unique reactivity of these ligands compared with those previously investigated suggests that the hemi-labile P-olefin coordination modulates the reactivity of the Rh center to allow the desired heterocycle arylation while reducing off-cycle hydrodehalogenation. At this point we do not have very much information about the mechanism of the arylation reaction, compared to the progress that has been made in the alkylation reactions discussed earlier in this article. However, if N-heterocycle carbene intermediates are also involved in the arylation, a reasonable hypothesis for the mechanism of the overall process is illustrated in Scheme 19. In this context, the Rh(I)-NHC intermediate is particularly attractive, since the low oxidation state at Rh prepares the metal center for oxidative addition of an aryl halide. However, further mechanistic investigation will be required to determine whether the mechanisms of the alkylation and arylation reactions do proceed by analogous pathways.
Scheme 19.
Substrate Scope of Heterocycle Arylation Using Rh-Phosphepine Catalyst 28
The optimized reaction conditions exhibited very high functional group tolerance, and allowed the use of aryl halides and heterocycles containing acidic NH and OH groups that have yet to be demonstrated as viable azole direct arylation substrates using Pd or Cu catalysis (Scheme 20).36 In particular, sulfinyl, chloro, acetamide, free hydroxy, and free amine groups were all tolerated; however, ortho substitution was not. Electron rich heteroaryl bromides, including 5-bromo-1-methylindole, 5-bromobenzoxazole, 5-bromobenzothiazole, and 3-bromothiophene, also underwent coupling in excellent yields. These results are particularly notable given that these heterocycles undergo electrophilic metallation by Pd catalysts, which could cause regioselectivity problems in Pd-catalyzed direct arylations using these substrates.43
Scheme 20.
A variety of additional heterocycles were also compatible with the Rh-catalyzed arylation conditions. Unprotected N-H benzimidazoles were optimal, N-methylbenzimidazole and benzoxazole coupled in good yield, and benzthiazole was a viable substrate. Pharmaceutically important bisarylimidazoles were also excellent arylation substrates, and both indolyl- and pyridyl-subsitution, common to a number of known drug candidates, can be present on the imidazole ring.44 Finally, arylation of 4,5-dimethylthiazole and the non-aromatic 4,4-dimethyloxazoline provided moderate yields of the corresponding arylated products.
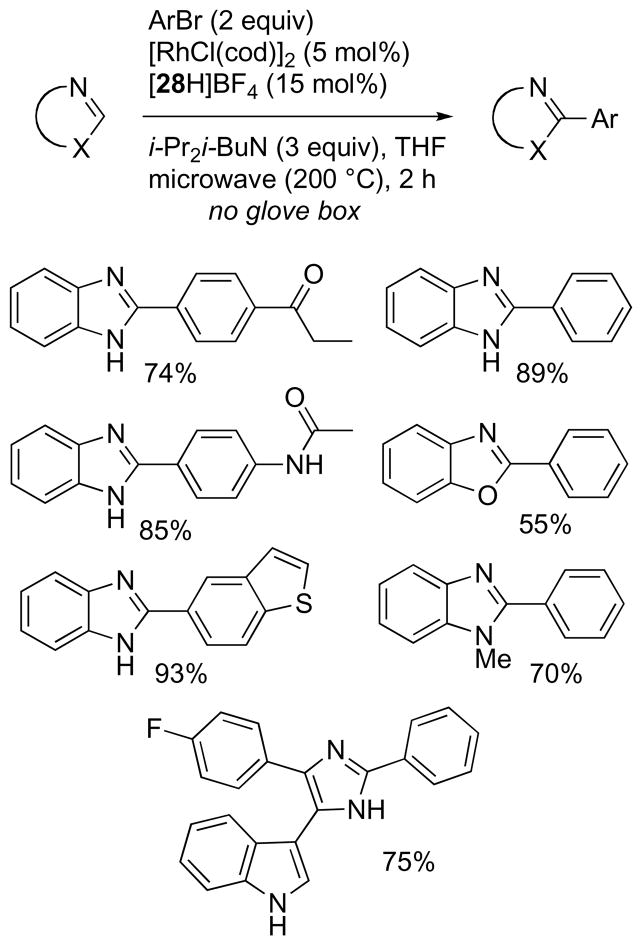
The arylation protocol was greatly simplified through the use of [28H]BF4, an air-stable surrogate of 2845 that is now commercially available from Sigma-Aldrich, and [RhCl(cod)]2 (cod = cyclooctadiene), a much less expensive, air-stable Rh source (Scheme 21). Using these catalyst precursors, the reaction mixtures can be assembled without the use of a glovebox and purified reagents and with only a N2 line to provide an inert atmosphere in the microwave vessel. In general, high yields of the desired products were obtained using standard lab equipment, so it is anticipated that these conditions would be most suitable for practical applications. Comparable results were obtained with dioxane as solvent (data not shown).
Scheme 21.
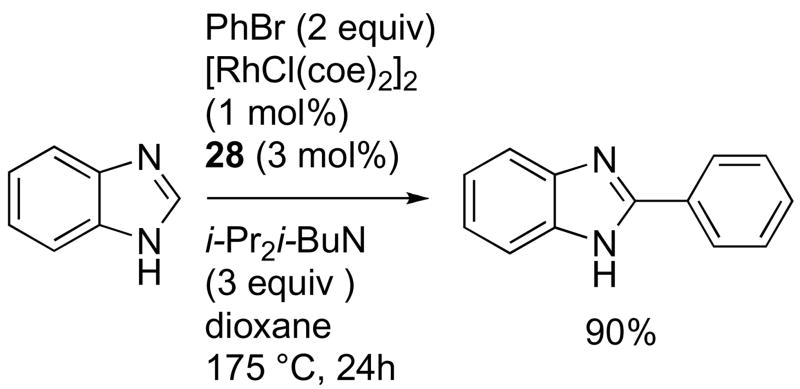
Preliminary investigation of catalyst loading was also performed under conventional heating, which is most applicable to large-scale reactions. At 1% loading of the [RhCl(coe)2]2 precatalyst in dioxane at 175 °C, a high yield of arylation product was obtained within 24 h (Scheme 22).
Scheme 22.
Summary and Outlook
In summary, we have used mechanistic insight to guide the development of efficient, functional group tolerant Rh-catalyzed heterocycle alkylation and arylation reactions. This work commenced with the identification of an intramolecular azole alkylation catalyzed by a Rh(I)-phosphine complex. The discovery that this reaction proceeds via a Rh-NHC intermediate inspired the extension of this reaction to the intermolecular alkylation of a variety of substrates compatible with this intermediate, including azoles, azolines, 3,4-dihydroquinazolines, pyridines, and quinolines.
We then hypothesized that this electron-rich low-valent Rh(I) complex might also be ideally suited to affect the oxidative addition step required in the corresponding heterocycle arylation. Indeed, methods for coupling aryl iodides and ultimately aryl bromides to the previously mentioned heterocycles were developed. These methods were notable in the extremely high functional group tolerance and unique selectivity compared to Cu- and Pd-catalyzed methods.
Further mechanistic inquiry will lead to an increased understanding of the intimate details of the aforementioned Rh-catalyzed C-H functionalization reactions enabling the design of more efficient and robust catalysts. Moreover, many additional classes of nitrogen heterocycles should be capable of undergoing C-H bond activation to provide NHC-metal intermediates, and it is likely that this important new pathway for C-H bond activation will make possible new catalytic C-H bond functionalization methods.
Acknowledgments
This work was supported by the NIH GM069559 to J.A.E. and by the Director and Office of Energy Research, Office of Basic Energy Sciences, Chemical Sciences Division, U.S. Department of Energy, under Contract DE-AC03-76SF00098 to R.G.B.
Biographies
Robert Bergman received his chemistry undergraduate degree at Carleton College and his Ph. D. from the University of Wisconsin under the direction of Jerome Berson, followed by postdoctoral work with Ronald Breslow at Columbia University. He joined the faculty of the California Institute of Technology in 1967 and ten years later accepted a professorship at the University of California, Berkeley. At Caltech his research focused on reaction mechanisms, first on organic systems and gradually moving into the area of organometallic reactions and catalysis. This emphasis continued after his move to Berkeley and has supported his collaboration with Prof. Ellman on catalytic applications of C-H activation reactions.
Jonathan Ellman received his chemistry undergraduate degree from Massachusetts Institute of Technology and his Ph.D. from Harvard University, working with David A. Evans. After carrying out postdoctoral research with Peter G. Schultz at the University of California at Berkeley, he joined the faculty in 1992 and is currently a Professor of Chemistry. His laboratory is primarily engaged in the design of chemical tools for biological studies and in the development of new synthesis methods. He has collaborated with Robert Bergman since 2000 on the study and application of new C-H bond functionalization reactions.
Jared Lewis received his B.S. in chemistry from the University of Illinois working with Eric Oldfield and his Ph. D. from the University of California, Berkeley under the direction of Jonathan Ellman and Robert Bergman. He is currently an NIH postdoctoral fellow with Frances Arnold at the California Institute of Technology.
References
- 1.(a) Dyker G. Transition Metal Catalyzed Coupling Reactions Under C-H Activation. Angew Chem Int Ed. 1999;38:1698. doi: 10.1002/(SICI)1521-3773(19990614)38:12<1698::AID-ANIE1698>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]; (b) Kakiuchi F, Murai S. Activation of CH Bonds: Catalytic Reactions. Top Curr Chem. 1999;3:47. [Google Scholar]; (c) Miura M, Nomura M. Direct Arylation via Cleavage of Activated and Unactivated C-H Bonds. Top Curr Chem. 2002;219:212. [Google Scholar]; (d) Campeau L-C, Fagnou K. Palladium-Catalyzed Direct Arylation of Simple Arenes in Synthesis of Biaryl Molecules. Chem Commun. 2006:1253. doi: 10.1039/b515481m. [DOI] [PubMed] [Google Scholar]; (e) Daugulis O, Zaitsev VG, Shabashov D, Pham Q-N, Lazareva A. Regioselective Functionalization of Unreactive Carbon-Hydrogen Bonds. Synlett. 2006;20:3382. [Google Scholar]; (f) Alberico D, Scott ME, Lautens M. Aryl-aryl bond formation by transition-metal-catalyzed direct arylation. Chem Rev. 2007;107:174–238. doi: 10.1021/cr0509760. [DOI] [PubMed] [Google Scholar]
- 2.Diederich F, Stang PJ, editors. Metal-catalyzed Cross-coupling Reactions. Wiley-VCH; New York: 1998. [Google Scholar]
- 3.(a) Crabtree RH. Alkane C-H Activation and Functionalization with Homogeneous Transition Metal Catalysts: a Century of Progress-a New Millennium in Prospect. Dalton. 2001:2437–2450. [Google Scholar]; (b) Bercaw JE, Labinger JA. Understanding and Exploiting C-H Bond Activation. Nature. 2002;417:507–514. doi: 10.1038/417507a. [DOI] [PubMed] [Google Scholar]; (c) Bergman RG. Organometallic Chemistry: C-H Activation. Nature. 2007;446:391–393. doi: 10.1038/446391a. [DOI] [PubMed] [Google Scholar]
- 4.(a) Daugulis O, Zaitsev VG. Anilide Ortho-Arylation by Using C-H Activation Methodology. Angew Chem Int Ed. 2005;4:4046. doi: 10.1002/anie.200500589. [DOI] [PubMed] [Google Scholar]; (b) Kalyani D, Deprez NR, Desai LV, Sanford MS. Oxidative C-H Activation/C-C Bond Forming Reactions: Synthetic Scope and Mechanistic Insights. J Am Chem Soc. 2005;127:7330. doi: 10.1021/ja051402f. [DOI] [PubMed] [Google Scholar]; (c) Kakiuchi F, Murai S. Catalytic C-H/Olefin Coupling. Acc Chem Res. 2002;35:826–834. doi: 10.1021/ar960318p. [DOI] [PubMed] [Google Scholar]
- 5.(a) Cho J-Y, Iverson CN, Smith MR. Steric and Chelate Directing Effects in Aromatic Borylation. J Am Chem Soc. 2000;122:12868. [Google Scholar]; (b) Ishiyama T, Takagi J, Kousaku I, Miyaura N, Anastasi NR, Hartwig JF. Mild Iridium-Catalyzed Borylation of Arenes. High Turnover Numbers, Room Temperature Reactions, and Isolation of a Potential Intermediate. J Am Chem Soc. 2002;124:390. doi: 10.1021/ja0173019. [DOI] [PubMed] [Google Scholar]
- 6.(a) Tunge JA, Foresee LN. Mechanistic Studies of Fujiwara Hydroarylation. C-H Activation versus Electrophilic Aromatic Substitution. Organometallics. 2005;24:6440–6444. [Google Scholar]; (b) Yanagisawa S, Sudo T, Noyori R, Itami K. Direct C-H Arylation of (Hetero)arenes with Aryl Iodides via Rhodium Catalysis. J Am Chem Soc. 2006;128:11748–11749. doi: 10.1021/ja064500p. [DOI] [PubMed] [Google Scholar]; (c) Ferreira EM, Stoltz BM. Catalytic C-H Bond Functionalization with Palladium(II): Aerobic Oxidative Annulations of Indoles. J Am Chem Soc. 2003;125:9578–9579. doi: 10.1021/ja035054y. [DOI] [PubMed] [Google Scholar]; (d) Jia CG, Piao DG, Oyamada JZ, Lu WJ, Kitamura T, Fujiwara Y. Efficient Activation of Aromatic C-H Bonds for Addition to C-C Multiple Bonds. Science. 2000;287:1992–1995. doi: 10.1126/science.287.5460.1992. [DOI] [PubMed] [Google Scholar]
- 7.(a) Garcia-Cuadrado D, Braga AAC, Maseras F, Echavarren AM. Proton Abstraction Mechanism for the Palladium-Catalyzed Intramolecular Arylation. J Am Chem Soc. 2006;128:1066–1067. doi: 10.1021/ja056165v. [DOI] [PubMed] [Google Scholar]; (b) Stuart DR, Fagnou K. The Catalytic Cross-Coupling of Unactivated Arenes. Science. 2007;316:1172–1175. doi: 10.1126/science.1141956. [DOI] [PubMed] [Google Scholar]
- 8.Chen MS, White MC. A Predictably Selective Aliphatic C-H Oxidation Reaction for Complex Molecule Synthesis. Science. 2007;318:783–787. doi: 10.1126/science.1148597. [DOI] [PubMed] [Google Scholar]
- 9.Peters MW, Meinhold P, Glieder A, Arnold FH. Regio- and Enantioselective Alkane Hydroxylation with Engineered Cytochromes P450 BM-3. J Am Chem Soc. 2003;125:13442–13450. doi: 10.1021/ja0303790. [DOI] [PubMed] [Google Scholar]
- 10.Tan KL, Bergman RG, Ellman JA. Annulation of Alkenyl-Substituted Heterocycles via Rhodium-Catalyzed Intramolecular C-H Activated Coupling Reactions. J Am Chem Soc. 2001;123:2685. doi: 10.1021/ja0058738. [DOI] [PubMed] [Google Scholar]
- 11.Pivsa-Art S, Satoh T, Kawamura Y, Miura M, Nomura M. Palladium-Catalyzed Arylation of Azole Compounds with Aryl Halides in the Presence of Alkali Metal Carbonates and the use of Copper Iodide in the Reaction. Bull Chem Soc Jpn. 1998;71:467–473. [Google Scholar]
- 12.(a) Moore EJ, Pretzer WR, O’Connel TJ, Harris J, LaBounty L, Cou L, Grimmer SS. Catalytic and Regioselective Acylation of Aromatic Heterocycles using Carbon Monoxide and Olefins. J Am Chem Soc. 1992;114:5888. [Google Scholar]; (b) Chatani N, Fukuyama T, Kakiuchi F, Murai SRu. 3(CO)12-Catalyzed Coupling of Heteroaromatic C-H/CO/Olefins. Regioselective Acylation of the Imidazole Ring. J Am Chem Soc. 1996;118:493. [Google Scholar]
- 13.Ritleng V, Sirlin C, Pfeffer M. Ru-, Rh-, and Pd-Catalyzed C-C Bond Formation Involving C-H Activation and Addition on Unsaturated Substrates: Reactions and Mechanistic Aspects. Chem Rev. 2002;102:1731–1769. doi: 10.1021/cr0104330. [DOI] [PubMed] [Google Scholar]
- 14.(a) Tan KL, Bergman RG, Ellman JA. Intermolecular Coupling of Isomerizable Alkenes to Heterocycles via Rhodium-Catalyzed C-H Bond Activation. J Am Chem Soc. 2002;124:13964. doi: 10.1021/ja0281129. [DOI] [PubMed] [Google Scholar]; (b) Tan KL, Park S, Ellman JA, Bergman RG. Intermolecular Coupling of Alkenes to Heterocycles via C-H Bond Activation. J Org Chem. 2004;69:7329. doi: 10.1021/jo048666p. [DOI] [PubMed] [Google Scholar]
- 15.Tan KL, Vasudevan A, Bergman RG, Ellman JA, Souers AJ. Microwave-Assisted C-H Bond Activation: A Rapid Entry into Functionalized Heterocycles. Org Lett. 2003;5:2131–2134. doi: 10.1021/ol030050j. [DOI] [PubMed] [Google Scholar]
- 16.Larhed M, Moberg C, Hallberg A. Microwave-Accelerated Homogeneous Catalysis in Organic Chemistry. Acc Chem Res. 2002;35:717–727. doi: 10.1021/ar010074v. [DOI] [PubMed] [Google Scholar]
- 17.Graczyk PP, Khan A, Bhatia GS, Palmer V, Medland D, et al. The neuroprotective action of JNK3 inhibitors based on the 6,7-dihydro-5H-pyrrolo[1,2-a]imidazole scaffold. Bioorg Med Chem Lett. 2005;15:4666. doi: 10.1016/j.bmcl.2005.07.076. [DOI] [PubMed] [Google Scholar]
- 18.Rech JC, Yato M, Duckett D, Ember B, LoGrasso PV, Bergman RG, Ellman JA. Synthesis of Potent Bicyclic Bisarylimidazole c-Jun N-Terminal Kinase Inhibitors by Catalytic C-H Bond Activation. J Am Chem Soc. 2007;129:490–491. doi: 10.1021/ja0676004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tan KL, Bergman RG, Ellman JA. Intermediacy of an N- Heterocyclic Carbene Complex in the Catalytic C-H Activation of a Substituted Benzimidazole. J Am Chem Soc. 2002;124:3202. doi: 10.1021/ja017351d. [DOI] [PubMed] [Google Scholar]
- 20.Lewis JC, Wiedemann SH, Bergman RG, Ellman JA. Arylation of Heterocycles via Rhodium-Catalyzed C-H Bond Functionalization. Org Lett. 2004;6:35. doi: 10.1021/ol035985e. [DOI] [PubMed] [Google Scholar]
- 21.Wiedemann SH, Lewis JC, Bergman RG, Ellman JA. Experimental and Computational Studies on the Mechanism of N-Heterocycle C-H Activation by Rh(I) J Am Chem Soc. 2006;128:2452. doi: 10.1021/ja0576684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gribble M, Ellman JA, Bergman RG. Synthesis of a Benzodiazepine-derived Rhodium NHC Complex by C-H Bond Activation. Organometallics. 2008;27:2152. doi: 10.1021/om8000839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Herrmann WA. N-Heterocyclic Carbenes. Part 31. N-Heterocyclic Carbenes: A New Concept in Organometallic Catalysis. Angew Chem, Int Ed. 2002;41:1290–1309. doi: 10.1002/1521-3773(20020415)41:8<1290::aid-anie1290>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 24.For a more recent example see Clement ND, Cavell KJ. Transition-Metal-Catalyzed Reactions Involving Imidazolium Salt/N-Heterocyclic Carbene Couples as Substrates. Angew Chem Int Ed. 2004;43:3845. doi: 10.1002/anie.200454166.
- 25.Hillier AC, Sommer WJ, Yong BS, Petersen JL, Cavallo L, Nolan SP. A Combined Experimental and Theoretical Study Examining the Binding of N-Heterocyclic Carbenes (NHC) to the Cp*RuCl (Cp =η5-C5Me5) Moiety: Insight into Stereoelectronic Differences between Unsaturated and Saturated NHC Ligands. Organometallics. 2003;22:4322. [Google Scholar]
- 26.(a) Alvarez E, Conejero S, Paneque M, Petronilho A, Poveda ML, Serrano O, Carmona E. Iridium(III)-Induced Isomerization of 2-Substituted Pyridines to N-Heterocyclic Carbenes. J Am Chem Soc. 2006;128:13060. doi: 10.1021/ja0646592. [DOI] [PubMed] [Google Scholar]; (b) Esteruelas MA, Fernandez-Alvarez FJ, Onate E. Stabilization of NH Tautomers of Quinolines by Osmium and Ruthenium. J Am Chem Soc. 2006;128:13044. doi: 10.1021/ja064979l. [DOI] [PubMed] [Google Scholar]
- 27.Canac Y, Soleilhavoup M, Conejero S, Bertrand G. Stable Non-N-Heterocyclic Carbenes (Non-NHC): Recent Progress. J Organomet Chem. 2004;689:3857–3865. [Google Scholar]
- 28.Wiedemann SH, Ellman JA, Bergman RG. Rhodium-Catalyzed Direct C-H Addition of 3,4-Dihydroquinazolines to Alkenes and Their Use in the Total Synthesis of Vasicoline. J Org Chem. 2006;71:1969. doi: 10.1021/jo052345b. [DOI] [PubMed] [Google Scholar]
- 29.Studies of these alkaloids has been reviewed comprehensively and continue to be reviewed annually. Johne S. In: The 2nd supplements to the 2nd edition of Rodd's Chemistry of Carbon Compounds. Sainsbury M, editor. IV I/J. Elsevier; Amsterdam: 2000. pp. 203–231.Michael JP. Quinoline, quinazoline and acridone alkaloids. Nat Prod Rep. 2007;24:223. doi: 10.1039/b509528j.
- 30.(a) Kreher R, Bergmann U. N2-Eliminations Under the Influence of Electrophiles. Part 14. Synthesis of 3,4-Dihydroquinazolines via Cyclic N-Diazonium Ions. Heterocycles. 1981;16:1693. [Google Scholar]; (b) Zharekeev BK, Telezhenetskaya MV, Khashimov KN, Yunusov SY. Reduction of Some Alkaloids of Peganum harmala by Sodium Tetrahydroborate. Khim Prir Soedin. 1974:679. [Google Scholar]
- 31.(a) Klingler RJ, Chen MJ, Rathke JW, Kramarz KW. Phosphines on the Thermodynamics of the Cobalt-Catalyzed Hydroformylation System. Organometallics. 2007;26:352–357. [Google Scholar]; (b) Dwyer CL, Kirk MM, Meyer WH, Janse van Rensburg W, Forman GS. Rotational Isomerism of a Phoban-Derived First-Generation Grubbs Catalyst. Organometallics. 2006;25:3806–3812. [Google Scholar]
- 32.Gant TG, Meyers AI. The Chemistry of 2-Oxazolines (1985-Present) Tetrahedron. 1994;50:2297–2360. [Google Scholar]
- 33.Wiedemann SH, Bergman RG, Ellman JA. Rhodium-Catalyzed Direct C-H Addition of 4,4-Dimethyl-2-oxazoline to Alkenes. Org Lett. 2004;6:1685. doi: 10.1021/ol049417q. [DOI] [PubMed] [Google Scholar]
- 34.Lewis JC, Bergman RG, Ellman JA. Rh(I)-Catalyzed Alkylation of Quinolines and Pyridines via C-H Bond Activation. J Am Chem Soc. 2007;129:5332. doi: 10.1021/ja070388z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hlasta DJ, Zificsak CA. Current Methods for the Synthesis of 2-Substituted Azoles. Tetrahedron. 2004;60:8991–9016. [Google Scholar]
- 36.(a) see ref 1a, c, d and f. Do H-Q, Daugulis O. Copper-Catalyzed Arylation of Heterocycle C-H Bonds. J Am Chem Soc. 2007;129:12404–12405. doi: 10.1021/ja075802+.Chiong HA, Daugulis O. Palladium-Catalyzed Arylation of Electron-Rich Heterocycles with Aryl Chlorides. Org Lett. 2007;9:1449–1451. doi: 10.1021/ol0702324.Bellina F, Calandri C, Cauteruccio S, Rossi R. Efficient and Highly Regioselective Direct C-2 Arylation of Azoles, Including Free (NH)-Imidazole-Benzimidazole and -Indole with Aryl Halides. Tetrahedron. 2007;63:1970–1980.
- 37.Alonso F, Beletskaya IP, Yus M. Metal-Mediated Reductive Hydrodehalogenation of Organic Halides. Chem Rev. 2002;102:4009–4091. doi: 10.1021/cr0102967. [DOI] [PubMed] [Google Scholar]
- 38.Christ ML, Sabo-Etienne S, Chaudret B. Highly Selective Dehydrogenative Silylation of Ethylene Using the Bis(dihydrogen) Complex RuH2(H2)2(PCy3)2 as Catalyst Precursor. Organometallics. 1995;14:1082–1084. [Google Scholar]
- 39.Lewis JC, Wu JY, Bergman RG, Ellman JA. Microwave-Promoted Rhodium-Catalyzed Arylation of Heterocycles Through C-H Bond Activation. Angew Chem Int Ed. 2006;118:1619–1621. doi: 10.1002/anie.200504289. [DOI] [PubMed] [Google Scholar]
- 40.Johnson JB, Rovis T. More than Bystanders: The Effects of Olefins on Transition-Metal-Catalyzed Cross-Coupling Reactions. Angew Chem Int Ed. 2008;47:840–871. doi: 10.1002/anie.200700278. [DOI] [PubMed] [Google Scholar]
- 41.van Assema SGA, Ehlers AW, de Kanter FJJ, Schakel M, Spek AL, Lutz M, Lammertsma K. Bidentate Phosphorus Baskets by Intramolecular Phosphinidene Addition. Chem Eur J. 2006;12:4333–4340. doi: 10.1002/chem.200501531. [DOI] [PubMed] [Google Scholar]
- 42.Lewis JC, Berman A, Bergman RG, Ellman JA. Rh(I)-Catalyzed Arylation of Heterocycles via C-H Bond Activation: Expanded Scope Through Mechanistic Insight. J Am Chem Soc. 2008;130:2493–2500. doi: 10.1021/ja0748985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.See reference 36c.
- 44.Chang LL, Sidler KL, Cascieri MA, de Laszlo S, Koch G, Li B, MacCoss M, Mantlo N, O’Keefe S, Pang M, Rolando A, Hagmann WK. Substituted Imidazoles as Glucagon Receptor Antagonists. Bioorg Med Chem Lett. 2001;11:2549. doi: 10.1016/s0960-894x(01)00498-x. [DOI] [PubMed] [Google Scholar]
- 45.Netherton MR, Fu GC. Air-Stable Trialkylphosphonium Salts: Simple, Practical, and Versatile Replacements for Air-Sensitive Trialkylphosphines. Applications in Stoichiometric and Catalytic Processes. Org Lett. 2001;3:4295–4298. doi: 10.1021/ol016971g. [DOI] [PubMed] [Google Scholar]