Abstract
Objective
To examine the frequency of allergic sensitization to staphylococcal superantigens in young children with mild to moderate atopic dermatitis (AD).
Study design
AD severity was assessed by objective Scoring AD (SCORAD). Serum IgE to staphylococcal enterotoxin (SE) A, SEB, SEC, SED and toxic shock syndrome toxin-1 (TSST-1) were measured by ImmunoCAP. Comparisons between mild and moderate AD were performed using logistic regressions.
Results
The prevalence of allergic sensitization to staphylococcal superantigens in patients with mild and moderate AD was 38% and 63%, respectively. Allergic sensitization to staphylococcal superantigens, particularly SEA and SED, was found to be associated with moderate AD, compared with mild AD.
Conclusions
Our results suggest that allergic sensitization to staphylococcal superantigens is common even among young children with mild to moderate AD, and such sensitization may contribute to the disease severity of these patients.
Keywords: allergic sensitization, enterotoxin, Staphylococcus aureus, staphylococcal superantigens, young children
Staphylococcus aureus (S. aureus) is believed to play a significant role in the pathogenesis of atopic dermatitis (AD).1 Up to 57% of S. aureus isolated from children with AD were found to secrete exotoxins with superantigen activity.2 These exotoxins are known as staphylococcal superantigens, which include staphylococcal enterotoxin (SE) A, SEB, SEC, SED and toxic shock syndrome toxin-1 (TSST- 1).2 In addition to their superantigen activity, staphylococcal superantigens also have been shown to induce inflammation via the production of superantigen-specific IgE in patients with AD.3 Although it is known that nearly 80% of patients with severe AD produce specific IgE against staphylococcal superantigens,2 the association of these specific IgE molecules among young children with mild and moderate AD has not been studied in detail. In this study, we analyzed the frequency of allergic sensitization to staphylococcal superantigens in patients with mild and moderate AD.
METHODS
Patients and IgE Measurement
The study was approved by the hospital’s local Institutional Review Board. Written and informed consent was obtained from all parents/guardians when appropriate. Fifty children from age 1 to 6 years who fulfilled the U.K. Working Party’s diagnostic criteria for AD were recruited.4 AD severity was measured by objective Scoring AD (SCORAD).5 Subjects were divided into 2 groups: mild AD, in which subjects had objective SCORAD of 15 or less; and moderate AD, in which subjects had objective SCORAD of more than 15, but less than 40. 6 Total serum IgE and specific IgE to staphylococcal enterotoxin (SEA), SEB, SEC, SED and toxic shock syndrome toxin (TSST)-1 were assayed in a commercial laboratory (Specialty Lab., Valencia, California). Specific IgE to a panel of common food and inhalant allergens also was assessed. The panel consisted of cow’s milk, egg white, soybean, wheat, peanut, house dust mites (Dermatophagoides farinae and Dermatophagoides pteronyssinus), Aspergillus fumigatus, Cladosporium herbarum, cockroach, cat, dog, western ragweed and Bermuda grass. The specific IgE assays were based on the ImmunoCAP system (Phadia, USA). Specific IgE concentrations of more than 0.35 kU/L were considered to be positive.
Statistical Analysis
The association between allergic sensitization to staphylococcal superantigens and total serum IgE or allergic sensitization to common food/inhalant allergens was analyzed by the Fisher exact test. Logistic regressions were performed for comparing the moderate AD group with the mild AD group. Subjects with positive IgE to staphylococcal superantigen(s) were assigned a value of 1 and those with negative IgE to staphylococcal superantigen(s) a value of 0. The analyses were performed by controlling for patients with AD with “high total IgE” (defined as subjects with total serum IgE > 150 IU/mL), as these patients have been shown to have more severe disease than patients with “low total IgE” (i.e. total serum IgE < 150 IU/mL).7 Odds ratios with 95% confidence were expressed. A p value of 0.05 or less was considered to be statistically significant.
RESULTS/DISCUSSION
The mean objective SCORADs for the mild and moderate AD groups were 9.9 ± 3.7 and 19.9 ± 2.5, respectively. Our results showed that the prevalence of allergic sensitization to staphylococcal superantigens in mild and moderate AD was 38% and 63%, respectively (Table I). These observations confirm previous beliefs that the prevalence of allergic sensitization to staphylococcal superantigens increases with the severity of AD.8
Table 1.
The prevalence of allergic sensitization to staphylococcal superantigens among children with mild to moderate atopic dermatitis
| SEA IgE+ | SEB IgE+ | SEC IgE+ | SED IgE+ | TSST-1 IgE+ | IgE+ to one or more staphylococcal superantigens | |
|---|---|---|---|---|---|---|
| Mild AD (n = 34) | 6 (18%) | 4 (12%) | 6 (18%) | 1 (3%) | 8 (24%) | 13 (38%) |
| Moderate AD (n = 16) | 9 (56%) | 5 (31%) | 5 (31%) | 4 (25%) | 6 (38%) | 10 (63%) |
Our results are also in keeping with a previous study that showed nearly 80% allergic sensitization to staphylococcal superantigens among children with severe AD.2 Allergic sensitization to SEA and TSST-1 was the most common sensitization to staphylococcal superantigens among our population of mild and moderate AD (Table I).
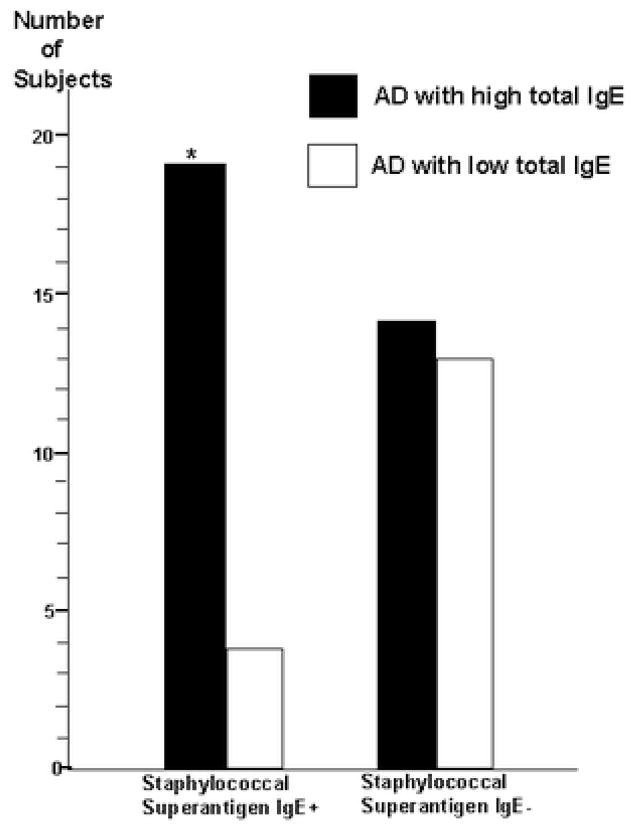
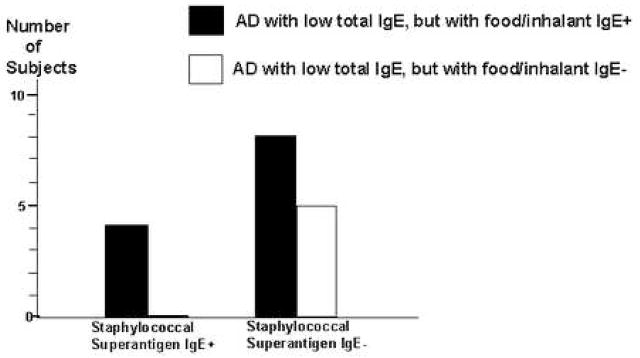
The prevalence of subjects with AD with high total IgE in our study was 66% (33/50). Among patients with AD with high total IgE, 58% (19/33) had allergic sensitization to at least one staphylococcal superantigen, compared with only 23% (4/17) of patients with AD with low total IgE (p<0.04)(Figure 1). These results are consistent with recent data showing an association of allergic sensitization to microbial antigens with patients with AD who have high total serum IgE,7,8 rather than low total serum IgE.9 We further analyzed whether allergic sensitization to staphylococcal superantigens may be associated with a subgroup of patients with AD with low total IgE, but with allergic sensitization to common food/inhalant allergen(s). Our data shows that 71% (12/17) of subjects with AD with low total IgE have allergic sensitization to at least one or more common food/inhalant allergens, compared with all the subjects with high total IgE. Our results confirmed that allergic sensitization to cow’s milk, egg white, house dust mites (D. farinae or D. pteronyssinus) and house pets (dog or cat) are among the most common food/inhalant allergic sensitizations in children with AD.10,11,12 As shown by Reefer et al in adults,7 we found no association between allergic sensitization to staphylococcal superantigens and common food/inhalant allergens among children with AD with low total IgE (p=0.3)(Figure 2).
Figure 1.
Allergic sensitization of staphylococcal superantigens is significantly associated with patients with AD who have high total IgE (> 150 IU/mL)(*p<0.04)(Fisher exact test)
Figure 2.
No association between allergic sensitization to staphylococcal superantigens and that to common food/inhalant allergens in patients with AD with low total IgE (< 150 IU/mL)(p=0.3)(Fisher’s exact test)
Allergic sensitization to each of 5 staphylococcal superantigens was at least 2 to 3 times more likely to be associated with moderate AD than mild AD, although we found only IgE sensitization to SEA and SED was significantly associated with moderate AD, compared with mild AD (p=0.01 and 0.05, respectively)(Table II). It is known that SEA and SED are structurally similar and both may be co-expressed by certain strains of S. aureus.13,14 One previous study indicated that SEA- and SED-producing S. aureus may be the predominant superantigen-producing strains in certain populations with AD.15 These studies and ours support that SEA, SED and their specific IgE may play an important role in the pathogenesis of AD.
Table 2.
Odds ratios for mild vs moderate atopic dermatitis in relation to allergic sensitization to staphylococcal superantigens
| SEA IgE+ | SEB IgE+ | SEC IgE+ | SED IgE+ | TSST-1 IgE+ | IgE+ to one or more staphylococcal superantigens | |
|---|---|---|---|---|---|---|
| Mild AD (n = 34) | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| Moderate AD (n = 16) | 6.3 * (1.4–27.4; p=0.01) | 3.0 * (0.6–14.8; p=0.17) | 2.0 * (0.4–7.9; p=0.50) | 10.0 * (1.0–103.8; p=0.05) | 2.0 * (0.5–6.6; p=0.40) | 2.5 * (0.7–8.9; p=0.20) |
95% confidence intervals with p values.
Skin barrier defects have been proposed as the primary defects in the pathogenesis of AD.16 This concept is supported by the association of null mutations in filaggrin (FLG), a protein with important barrier functions, in moderate to severe AD.17 More recently, association of null mutations in FLG also has been confirmed in patients with mild to moderate AD.18 However, the external triggers of inflammation in these patients remain unclear. More than 90% of children with AD are colonized with S. aureus.19 It has been shown that children with severe AD exhibited cutaneous T-lymphocyte inflammation consisting of T-lymphocyte receptor variable β repertoire that correspond to the staphylococcal superantigens found on their skin.20 Staphylococcal superantigens also have been shown to induce a T helper 2 (Th2)(“allergic”) inflammation, which is characteristic of the immune response found in acute AD lesions.21,22 A consequence of a Th2 response is the production of IgE molecules, which may include superantigen-specific IgE in genetically-susceptible individuals. On re-encounter of staphylococcal superantigens, basophils bearing the relevant superantigen-specific IgE molecules release histamine or other inflammatory mediators, further aggravating the cutaneous inflammation of AD.3 There may be other mechanisms by which S. aureus trigger AD inflammation that have yet to be defined.
Young children with mild to moderate AD constitute the majority of patients with AD.23 Addressing any potential trigger in this population is an important clinical problem. An emphasis should be placed on the restoration of skin barrier functions.24 This approach could minimize potential pathogen and allergen triggers including S. aureus and its superantigens. Routine use of antibiotics to reduce S. aureus colonization, in the absence of clinical signs of infection, is not recommended due to the concern of inducing bacterial resistance. In addition to their anti-inflammatory effects, both topical corticosteroids and topical calcineurin inhibitors have been shown to decrease S. aureus colonization and improve barrier functions in AD.25,26 Timely use of these medications should be encouraged for symptomatic control.
Acknowledgments
This work was supported by a CReFF award to PYO from the General Clinical Research Center, National Center for Research Resources at the University of Southern California (MO1 RR 00043). The authors disclose no potential conflicts of interest.
We would like to thank the Nursing and Research Staff in the Division of Clinical Immunology and Allergy, especially Cindy Burrola, RN, Dalila Ortega and Neveen Abdelghani, Pharm.D., for their assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ong PY, Leung DYM. Immune dysregulation in atopic dermatitis. Curr Allergy Asthma Rep. 2006;6:384–9. doi: 10.1007/s11882-996-0008-5. [DOI] [PubMed] [Google Scholar]
- Nomura I, Tanaka K, Tomita H, Katsunuma T, Ohya Y, Ikeda N, et al. Evaluation of the staphylococcal exotoxins and their specific IgE in childhood atopic dermatitis. J Allergy Clin Immunol. 1999;104:441–6. doi: 10.1016/s0091-6749(99)70390-8. [DOI] [PubMed] [Google Scholar]
- Leung DYM, Harbeck R, Bina P, Reiser RF, Yang E, Norris DA, et al. Presence of IgE antibodies to staphylococcal exotoxins on the skin of patients with atopic dermatitis. Evidence for a new group of allergens. J Clin Invest. 1993;92:1374–80. doi: 10.1172/JCI116711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams HC, Burney PG, Pembroke AC, Hay RJ. The U.K. Working Party’s Diagnostic Criteria for Atopic Dermatitis. III. Independent hospital validation. Br J Dermatol. 1994;131:406–16. doi: 10.1111/j.1365-2133.1994.tb08532.x. [DOI] [PubMed] [Google Scholar]
- Kunz B, Oranje AP, Labreze L, Stalder JF, Ring J, Taieb A. Clinical validation and guidelines for the SCORAD index: consensus report of the European Task Force on Atopic Dermatitis. Dermatology. 1997;195:10–9. doi: 10.1159/000245677. [DOI] [PubMed] [Google Scholar]
- Oranje AP, Glazenburg EJ, Wolkerstorfer A, de Waard-van der Spek FB. Practical issues on interpretation of scoring atopic dermatitis: the SCORAD index, objective SCORAD and the three-item severity score. Br J Dermatol. 2007;157:645–8. doi: 10.1111/j.1365-2133.2007.08112.x. [DOI] [PubMed] [Google Scholar]
- Reefer AJ, Santinover SM, Wilson BB, Woodfolk JA. The relevance of microbial allergens to the IgE antibody repertoire in atopic and nonatopic eczema. J Allergy Clin Immunol. 2007;120:156–63. doi: 10.1016/j.jaci.2007.03.042. [DOI] [PubMed] [Google Scholar]
- Schnopp C, Grosch J, Ring J, Ollert M, Mempel M. Microbial allergen-specific IgE is not suitable to identify the intrinsic form of atopic eczema in children. J Allergy Clin Immunol. 2008;121:267–8. doi: 10.1016/j.jaci.2007.08.052. [DOI] [PubMed] [Google Scholar]
- Novak N, Allam JP, Bieber T. Allergic hyperreactivity to microbial components: a trigger factor of “intrinsic” atopic dermatitis? J Allergy Clin Immunol. 2003;112:215–6. doi: 10.1067/mai.2003.1590. [DOI] [PubMed] [Google Scholar]
- Hill DJ, Sporik R, Thorburn J, Hosking CS. The association of atopic dermatitis in infancy with immunoglobulin E food sensitization. J Pediatr. 2000;137:475–9. doi: 10.1067/mpd.2000.108207. [DOI] [PubMed] [Google Scholar]
- Hill DJ, Heine RG, Hosking CS, Brown J, Thiele L, Allen KJ, et al. IgE food sensitization in infants with eczema attending a dermatology department. J Pediatr. 2007;151:359–63. doi: 10.1016/j.jpeds.2007.04.070. [DOI] [PubMed] [Google Scholar]
- Schafer T, Heinrich J, Wjst M, Adam H, Ring J, Wichmann HE. Association between severity of atopic eczema and degree of sensitization to aeroallergens in schoolchildren. J Allergy Clin Immunol. 1999;104:1280–4. doi: 10.1016/s0091-6749(99)70025-4. [DOI] [PubMed] [Google Scholar]
- Breuer K, Wittmann M, Bosche B, Kapp A, Werfel T. Severe atopic dermatitis is associated with sensitization to staphylococcal enterotoxin B (SEB) Allergy. 2000;55:551–5. doi: 10.1034/j.1398-9995.2000.00432.x. [DOI] [PubMed] [Google Scholar]
- Tomi NS, Kranke B, Aberer E. Staphylococcal toxins in patients with psoriasis, atopic dermatitis, and erythroderma, and in healthy control subjects. J Am Acad Dermatol. 2005;53:67–72. doi: 10.1016/j.jaad.2005.02.034. [DOI] [PubMed] [Google Scholar]
- Breuer K, Haussler S, Kapp A, Werfel T. Staphylococcus aureus: colonizing features and influence of an antibacterial treatment in adults with atopic dermatitis. Br J Dermatol. 2002;147:55–61. doi: 10.1046/j.1365-2133.2002.04872.x. [DOI] [PubMed] [Google Scholar]
- Elias PM, Hatano Y, Williams ML. Basis for the barrier abnormality in atopic dermatitis: Outside-inside-outside pathogenic mechanisms. J Allergy Clin Immunol. 2008 Mar 6; doi: 10.1016/j.jaci.2008.01.022. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer CN, Irvine AD, Terron-Kwiatkowski A, Zhao Y, Liao H, Lee SP, et al. Common loss-of-function variants of the epidermal barrier protein filaggrin are a major predisposing factor for atopic dermatitis. Nat Genet. 2006;38:441–6. doi: 10.1038/ng1767. [DOI] [PubMed] [Google Scholar]
- Brown SJ, Relton CL, Liao H, Zhao Y, Sandilands A, Wilson IJ, et al. Filaggrin null mutations and childhood atopic eczema: a population-based case-control study. J Allergy Clin Immunol. 2008;121:940–46. doi: 10.1016/j.jaci.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeger PH, Lenz W, Boutonnier A, Fournier JM. Staphylococcal skin colonization in children with atopic dermatitis: prevalence, persistence, and transmission of toxigenic and nontoxigenic strains. J Infect Dis. 1992;165:1064–8. doi: 10.1093/infdis/165.6.1064. [DOI] [PubMed] [Google Scholar]
- Bunikowski R, Mielke ME, Skarabis H, Worm M, Anagnostopoulos I, Kolde G, et al. Evidence for a disease-promoting effect of Staphylococcus aureus-derived exotoxins in atopic dermatitis. J Allergy Clin Immunol. 2000;105:814–9. doi: 10.1067/mai.2000.105528. [DOI] [PubMed] [Google Scholar]
- Ardern-Jones MR, Black AP, Bateman EA, Ogg GS. Bacterial superantigen facilitates epithelial presentation of allergen to T helper 2 cells. Proc Natl Acad Sci U S A. 2007;104:5557–62. doi: 10.1073/pnas.0700733104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamid Q, Boguniewicz M, Leung DYM. Differential in situ cytokine gene expression in acute versus chronic atopic dermatitis. J Clin Invest. 1994;94:870–6. doi: 10.1172/JCI117408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerson RM, Williams HC, Allen BR. What is the cost of atopic dermatitis in preschool children? Br J Dermatol. 2001;144:514–22. doi: 10.1046/j.1365-2133.2001.04077.x. [DOI] [PubMed] [Google Scholar]
- Gutman AB, Kligman AM, Sciacca J, James WD. Soak and smear: a standard technique revisited. Arch Dermatol. 2005;141:1556–9. doi: 10.1001/archderm.141.12.1556. [DOI] [PubMed] [Google Scholar]
- Hung SH, Lin YT, Chu CY, Lee CC, Liang TC, Yang YH, et al. Staphylococcus colonization in atopic dermatitis treated with fluticasone or tacrolimus with or without antibiotics. Ann Allergy Asthma Immunol. 2007;98:51–6. doi: 10.1016/S1081-1206(10)60859-9. [DOI] [PubMed] [Google Scholar]
- Xhauflaire-Uhoda E, Thirion L, Pierard-Franchimont C, Pierard GE. Comparative effect of tacrolimus and betamethasone valerate on the passive sustainable hydration of the stratum corneum in atopic dermatitis. Dermatology. 2007;214:328–32. doi: 10.1159/000100884. [DOI] [PubMed] [Google Scholar]