Abstract
Arsenic is widely spread in our living environment and imposes a big challenge on human health worldwide. Arsenic damages biological systems through multiple mechanisms including the generation of reactive oxygen species. The transcription factor Nrf2 regulates the cellular antioxidant response that protects cells from various insults. In this study, the protective role of Nrf2 in arsenic toxicity was investigated in a human bladder urothelial cell line, UROtsa. Using an UROtsa cell line stably infected with Nrf2-siRNA, we clearly demonstrate that compromised Nrf2 expression sensitized the cells to As(III)- and MMA(III)-induced toxicity. On the other hand, the activation of the Nrf2 pathway by tert-butylhydroquinone (tBHQ) and sulforaphane (SF), the known Nrf2-inducers, rendered UROtsa cells more resistant to As(III)- and MMA(III). Furthermore, the wild type mouse embryo fibroblast (WT-MEF) cells were protected from As(III)- and MMA(III)-induced toxicity following Nrf2 activation by tBHQ or SF whereas neither tBHQ nor SF conferred protection in the Nrf2−/−-MEF cells, demonstrating that tBHQ- or SF-mediated protection against As(III)- and MMA(III)-induced toxicity depends on Nrf2 activation. These results, obtained by both loss of function and gain of function analyses, clearly demonstrate the protective role of Nrf2 in arsenic-induced toxicity. The current work lays the groundwork for using Nrf2 activators for therapeutic and dietary interventions against adverse effects of arsenic.
Keywords: Nrf2, Keap1, arsenic, arsenite, MMA(III), UROtsa
Arsenic is an environmental toxicant. Ground water contaminated with arsenic is the main source of human exposure worldwide and generates an important global health issue (Smith et al., 2000; Rossman, 2003; Tchounwou et al., 2003). Epidemiological studies have correlated arsenic exposure to various human diseases such as cancer, hyperkeratosis, atherosclerosis, diabetes and chronic obstructive pulmonary diseases (Cohen et al., 2000; Steinmaus et al., 2000; Tseng, 2002; Smith et al., 2006). Arsenic has been classified as a human carcinogen that induces tumors in the skin, lung, and bladder. However, it has been problematic to induce tumors in rodents using inorganic arsenic. Nevertheless, maternal exposure to inorganic arsenic induced several types of tumors in the offspring, demonstrating that arsenic is a complete carcinogen in animal models (Waalkes et al., 2003). Inorganic arsenic has also been demonstrated as a cocarcinogen to enhance ultraviolet-induced skin tumors in a hairless mouse model (Burns et al., 2004).
Many factors, such as chemical species and dose, determine the action of arsenic. High doses of arsenic mainly cause acute toxicity, while moderate doses of repetitive exposure are associated with various cancers. Different species of arsenic, such as As(III) vs. As(V) and inorganic vs. methylated organic As, display varying potency of toxicities (Carter et al., 2003). Following uptake, inorganic arsenic is converted primarily in the liver to methylated metabolites including monomethylarsonous acid [MMA(III)], monomethylarsonic acid [MMA(V)], dimethylarsinous acid [DMA(III)], and dimethylarsinic acid [DMA(V)] (Aposhian, 1997; Thomas et al., 2004; Kenyon et al., 2005). Trivalent arsenic species are more toxic than pentavalent species, and the methylated arsenic compounds are more toxic than the inorganic ones (Thomas et al., 2001; Aposhian et al., 2003).
Arsenic damages biological systems through multiple mechanisms, one of them being reactive oxygen species (ROS). ROS is generated by arsenic indirectly through many sources. For example, arsenic was shown to increase NAD(P)H oxidase activity, resulting in superoxide accumulation (Smith et al., 2001). Through direct binding with the cysteinyl sulfhydryl group in glutathione, arsenic is able to conjugate with glutathione and leads to subsequent removal of the arsenic-glutathion complex by multidrug associated transporters such as MRP1 and MRP2. As a consequence, glutathione is depleted, resulting in the formation of ROS (Scott et al., 1993; Delnomdedieu et al., 1994; Kala et al., 2000; Vernhet et al., 2001; Leslie et al., 2004). In addition to the non-protein sulfhydryl glutathione, endogenous sulfhydryl groups in proteins have been reported to interact with arsenic and play an important role in the detoxification of arsenic (Scott et al., 1993; Thompson, 1993; Sakurai et al., 2005). The exogenous antioxidant N-acetylcysteine is able to prevent arsenic-induced toxicity, implicating a role of ROS in arsenic-induced toxicity/carcinogenicity (Liu et al., 2003).
Vertebrates have evolved several defense mechanisms to cope with environmental insults. The antioxidant response is the major one used to neutralize ROS elicited by toxic exposure and thus to maintain cellular redox homeostasis (Venugopal and Jaiswal, 1996; Itoh et al., 1999). This antioxidant system is mediated by the transcription factor NF-E2-related factor 2 (Nrf2) through the antioxidant response element (ARE). The ARE is present in the promoters of genes encoding antioxidant enzymes and Phase II detoxification enzymes, such as glutathione S-transferase (GST), NAD(P)H quinone oxidoreductase (NQO1), γ-glutamylcysteine synthetase (γ-GCS), and heme oxygenase-1 (HO-1) (Wild et al., 1999; McMahon et al., 2001; Jaiswal, 2004; Kobayashi et al., 2004). These enzymes work in concert to elicit cytoprotective responses that defend cells from the toxic effects of environmental insults. Activation of the Nrf2 pathway has been clearly demonstrated to confer protection against toxic and carcinogenic effects of many environmental insults. This is illustrated by several findings indicating that Nrf2 knockout mice display increased sensitivity to chemical toxicants and carcinogens and are refractory to the protective actions of chemopreventive compounds (Chan and Kan, 1999; Aoki et al., 2001; Enomoto et al., 2001; Ramos-Gomez et al., 2001; Cho et al., 2002). Thus, it is believed that the activation of the Nrf2-mediated antioxidant response offers beneficial effects against many oxidative stress-related human diseases including cancer, neurodegenerative disease, aging, cardiovascular diseases, pulmonary fibrosis, and acute pulmonary injury (Motohashi and Yamamoto, 2004; Cho et al., 2006; Zhang, 2006). Fortunately, activity of Nrf2 can be modulated by dietary choice. Many health foods, for examples, curcumin, red grapes, green tea, cruciferous and leguminous vegetables, contain compounds that are able to activate the Nrf2-dependent cellular protective response (Surh, 2003; Lee and Surh, 2005).
Being the last target organ for arsenic exposure before excretion, the urinary bladder is exposed not only to inorganic arsenic but also to the organic metabolites. As reported in human studies, urinary arsenic species, including MMA(III), MMA(V), DMA(III), and DMA(V), were detected in populations exposed to inorganic arsenic in their drinking water (Hsueh et al., 1998; Aposhian et al., 2000; Mandal et al., 2001). Both MMA(III) and DMA(III) are significantly more toxic than inorganic arsenic. However, retention time for DMA(III) is very transient, which leads to the hypothesis that MMA(III) is the form of arsenic species responsible for the majority of toxicity and carcinogenicity in humans following the uptake of inorganic arsenic. MMA(III) comprises 7–11% of urinary arsenic and has been reported to be 20 fold more toxic than inorganic arsenic in various cell types (Aposhian et al., 2000; Petrick et al., 2000). In the current work, the functional role of Nrf2 in arsenic-induced toxicity was studied using both inorganic arsenic [As(III) in the form of sodium arsenite] and an organic arsenic metabolite [MMA(III)] in a human bladder urothelium cell line.
Materials and methods
Construction of retrovirus vectors containing Nrf2-shRNA
The vector pRS used for cloning Nrf2 siRNA was purchased from OriGene. Four different shRNA constructers were made. The 29-mer short hairpin sequence was inserted between the BamH1 and HindIII cloning sites. The 29 mer sequences used were:
TGGCATCACCAGAACACTCAGTGGAATCT,
CCTGGAAGTGTCAAACAGAATGGTCCTAA,
TGACTTCAGTCAGCGACGGAAAGAGTATG, and
CCAGTCTTCATTGCTACTAATCAGGCTCA.
Chemicals, cell culture, transfection, and induction
Sodium arsenite, tBHQ, sulforaphane, polybrene and puromycin were purchased from Sigma. MMA(III) was synthesized in the Synthetic Chemistry Core at the University of Arizona. UROtsa cells were generously provided by Drs. Mary Ann and Donald Sens (University of North Dakota). The passage number of UROtsa cells used for this study was from 59 to 69. UROtsa cells were grown in DMEM medium enriched with 5% FBS according to the original condition described by Petzoldt et al. (Petzoldt et al., 1995). All mammalian cells were incubated at 37 °C in a humidified incubator containing 5% CO2. For establishing stable cell lines harboring control siRNA and Nrf2-siRNA, five plasmids containing five different shRNA (four Nrf2 siRNAs and one control) were first transfected into SD-3443, an amphotropic packaging cell line from ATCC. Viruses produced in the supernatant were collected and used to infect UROtsa cells in the presence of 2 μg/ml polybrene. 48 h after infection, cells were grown in the medium containing 3 μg/ml of puromycin for selection. Stable cell lines were established once all the cells in the negative control plate were killed. Stable cell lines were continuously grown in the medium containing 3 μg/ml of puromycin.
Cell Viability
As(III)- and MMA(III)-induced toxicity was measured by functional change of mitochondria using 3-(4, 5 dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) (Sigma). For the MTT assay of UROtsa cells, approximately 8×103 cells per well were seeded in a 96-well plate and incubated overnight. Cells were treated with several concentrations of As(III) or MMA(III) for 24 h followed by the addition of 20 μl of 2 mg/ml MTT directly into the medium. After incubation (37°C for 0.5–3 hrs), the plate was centrifuged and the medium removed. 100 μl of isopropanol/HCl was added into each well and crystals were dissolved by shaking the plate at room temperature. Absorbance was measured by a plate reader at 570 nm. Triplicate wells were used for each sample and the experiments were repeated at least three times to get means and standard deviations.
ROS detection
Cells were either left untreated or treated with 20 μM As(III) for 16 hr. Following washing with PBS, fresh medium containing dichlorofluorescein (DCF) (Sigma, 10 μg/ml final concentration) was added and the plates were incubated for 20–60 minutes. Cells were then washed twice with PBS and trypsinized. After further washing with PBS, cells were resuspended in PBS to approximate 106 cells per ml. Fluorescence was measured using flow cytometry with excitation at 488 nm and emission at 515–545 nm. All steps were handled in the dark.
NQO1 enzymatic activity and glutathione concentration
NQO1 activity was measured as the dicoumarol-inhibitable fraction of DCPIP reduction (De Haan et al., 2002). Cells were trypsinized, washed and resuspended in 2xTE buffer (20 mM Tris-HCl, 2 mM EDTA, pH7.4). Cells were disrupted by three cycles of freeze/thaw and centrifuged for 5 min at 12,000 g. An aliquot of supernatant was mixed with the reaction buffer (25 mM Tris-HCl [pH7.4], 0.18 mM NADPH, 0.2 mg/ml BSA, and 0.01% Tween-20 in a total volume of 1 ml), in the absence or presence of 20 μM dicoumarol. The reaction was started by the addition of 40 μM DCPIP. Reduction of DCPIP was measured as the difference of two readings at 600 nm at room temperature between a one-minute interval. NQO1 activity was calculated as the dicoumarol-inhibitable part of DCPIP reduction (nmol DCPIP reduced/min/μg protein). Protein amounts were measured using Quant-iT™ Protein Assay Kit from Invitrogen. All the chemicals used for this assay were purchased from Sigma. Intracellular glutathione concentrations were measured using QuantiChrom glutathione assay kit from BioAssay Systems. The procedure was performed according to the manufacturer’s instruction. Three independent experiments were performed for the calculation of means and standard deviations.
Real time RT-PCR
Total mRNAs were extracted using the TRIZOL reagent (Invitrogen) and equal amounts of RNA were reverse-transcribed to cDNA with Transcriptor First Strand cDNA Synthesis Kit (Roche). Quantification of cDNA amount for Nrf2, NQO1, HO-1, and β-actin in each sample was performed with LightCycler 480 probes master kit (Roche). All primer sets were tested prior to use in this work to ensure that only a single product of the correct size was amplified. Taqman probes were from the universal probe library (Roche): hNrf2 (#70), hNQO1 (#87), hHO1 (#25), hGAPDH (#25), and hkeap1 (#10). Oligos used for primers were synthesized by IDT.
| hNrf2: | forward (acacggtccacagctcatc) and reverse (tgtcaatcaaatccatgtcctg); |
| hNQO1: | forward (atgtatgacaaaggacccttcc) and reverse (tcccttgcagagagtacatgg); |
| hHO1: | forward (aactttcagaagggccaggt) and reverse (ctgggctctccttgttgc); |
| hGAPDH: | forward (ctgacttcaacagcgacacc) and reverse (tgctgtagccaaattcgttgt). |
Triplicate reactions were performed for each sample. Cycling was performed as follows: one cycle of initial denaturation (95°C for 10 min), 40 cycles of amplification (95°C for 10 sec and 60°C for 20 sec), and a cooling program (50°C for 5 sec). Mean crossing point (Cp) values and standard deviations were determined. Crossing point values were normalized to the respective crossing point values of the hGAPDH reference gene. Data is presented as a fold change in gene expression in Nrf2-siRNA cells compared to control-siRNA cells.
Antibodies, and immunoblot analysis
The Nrf2 antibody was purchased from Santa Cruz. Cells were lysed in sample buffer (50 mM Tris-HCl [pH 6.8], 2% SDS, 10% Glycerol, 100 mM DTT, 0.1% bromophenol blue). Following sonication, cell lysates were electrophoresed through SDS-polyacrylamide gels, and subjected to immunoblot analysis.
Results
An UROtsa-derived cell line, UROtsa-725, has reduced endogenous Nrf2 and impaired Nrf2-mediated antioxidant response
The UROtsa cell line was used to examine the functional importance of Nrf2 activation in arsenic-induced toxicity. To overcome the problem of having low transfection efficiency, a retrovirus-mediated infection method was chosen for establishment of stable cell lines harboring Nrf2 shRNA. Five stable cell lines were established: four shRNAs targeting different regions of Nrf2 and one scrambled siRNA as a negative control. Once the selection was completed, endogenous levels of the Nrf2 protein were measured by immunoblot analysis with anti-Nrf2 antibodies. The UROtsa-725 (U725, UROtsa cells stably transfected with a vector containing Nrf2 siRNA: TGGCATCACCAGAACACTCAGTGGAATCT) had maximum reduction of the Nrf2 protein with approximately 50% knockdown, compared to UROtsa-724 (U724, UROtsa cells stably infected with a vector containing a scrambled siRNA) (Fig. 1A). Thus, UROtsa-725 and UROtsa-724 were used for measuring mRNA of Nrf2 and the downstream effects of Nrf2 down-regulation: (i) mRNA expression of Nrf2, Keap1, NQO1, and HO-1 (Fig. 1B), (ii) enzymatic activity of NQO1 (Fig. 1C), (iii) intracellular glutathione concentration (Fig. 1D), and (iv) constitutive and induced levels of ROS following As(III) treatment (Fig. 1E). Stable transfection of Nrf2 siRNA reduced mRNA expression of Nrf2, as well as its downstream genes NQO1 and HO-1, while it had no effect on Keap1 mRNA (Fig. 1B). The 30–50% knockdown of the mRNAs of Nrf2 or its downstream target genes in URotsa-725 was not as pronounced as expected, which may due to the factor that only a single copy of shRNA was incorporated into each cell, in addition to the weak U6 polymerase III promoter used. However, regardless the moderate reduction of the Nrf2 mRNA expression, the functional difference of the Nrf2 downstream effects between UROtsa-724 and UROtsa-725 cell lines was significant by all the measurements tested. For example, when the cell lysates of UROtsa-724 and UROtsa-725 were tested for the enzymatic activity of NQO1 by DCPIP reduction, the NQO1 activity was decreased in UROtsa-725 and it was significant (Fig. 1C). Nrf2 is known to regulate cellular redox conditions by transcriptional activation of many enzymes involving glutathione biosynthesis such as gamma-glutamylcysteine synthetase, glutathione synthase, and xCT cysteine antiporter (Shih et al., 2003). Therefore, the intracellular glutathione concentrations of these two cell lines were compared. Impaired Nrf2 expression in UROtsa-725 resulted in a decrease of the reduced form of glutathione even in unchallenged cells, demonstrating the impact of Nrf2 on cellular redox homeostasis (Fig. 1D). To further verify that UROtsa-725 has reduced redox buffering capacity, intracellular ROS levels were measured by DCF. Fluorescence data from flow cytometry measurement were converted into bar graphic and presented in Figure 1E. Both the basal and As(III)-induced levels of ROS in UROtsa-725 were much higher, compared to UROtsa-724 (Fig. 1E). H2O2 was included as a positive control for the assay (data not shown). Taken together, these results demonstrate that a stable cell line with the impaired Nrf2-dependent antioxidant response was established.
Fig. 1. UROtsa-725 had reduced endogenous Nrf2.
(A) UROtsa-725 and its negative control URotsa-724 were derived from URotsa cells stably infected with Nrf2-siRNA or scrambled-siRNA using retrovirus-based vectors. Equal amounts of total lysates were subjected to immunoblot analysis with anti-Nrf2 and anti-tubulin antibodies. (B) The mRNA levels of Nrf2, Keap1, NQO1, and HO-1 in UROtsa-724 and UROtsa-725 were compared by real-time RT-PCR. (C) NQO1 activities in the lysates of UROtsa-724 and UROtsa-725 were measured using DCPIP as a substrate. (D) Intracellular glutathione concentrations in UROtsa-724 and UROtsa-725 were measured using the QuantiChrom glutathione assay kit. (E) UROtsa-724 and UROtsa-725 cell lines were either left untreated or treated with 20 μM As(III) for 16 h. Following incubation of cells with DCF, ROS was detected using flow cytometry. The bar graph represents the means of fluorescence values.
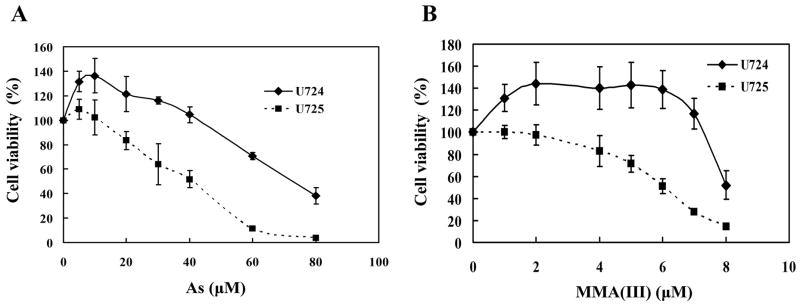
Down-regulation of Nrf2 sensitizes the human bladder urothelium cells to As(III)- and MMA(III)-induced toxicity
To demonstrate the protective role of Nrf2 against arsenic-induced toxicity, cellular toxicity assays were performed in UROtsa-724 and UROtsa-725 following As(III) or MMA(III) treatment. Remarkably, the UROtsa-725 cell line had increased sensitivity to both As(III) and MMA(III) compared to the control cell line, UROtsa-724 (Fig. 2A and 2B). Consistence with the previous findings, MMA(III) is much more potent in inducing cell death than As(III) (Fig. 2A and 2B). These results indicate that a 30–50% reduction of the Nrf2-mediated response makes UROtsa cells more susceptible to As(III) and MMA(III), demonstrating the critical role of Nrf2 in cytoprotection against arsenic toxicity.
Fig. 2. Down-regulation of Nrf2 sensitizes cells to As(III)- and MMA(III)-induced toxicity.
UROtsa-724 and UROtsa-725 were treated with the indicated concentrations of As(III) (A) or MMA(III) (B) for 24h. Cell viability was measured by the MTT assay.
Activation of Nrf2 by tBHQ and SF protects human bladder urothelium cells from As(III)- and MMA(III)-induced toxicity
The results in Figure 2 were obtained by reducing Nrf2 expression to make cells more sensitive to As(III)- and MMA(III)-induced toxicity. It is conceivable that activation of Nrf2 by its inducers should elicit the cytoprotective response, resulting in increased resistance of cells to the arsenic challenge. For this purpose, two well-studied Nrf2 activators tBHQ and SF were chosen. Since no data exists in terms of the duration of Nrf2 activation by tBHQ and SF in UROtsa cells, a sets of pilot experiments were performed to define the optimal conditions for tBHQ and SF treatment in terms of concentration and duration of pretreatment and treatment. Optimal Nrf2 induction and duration were monitored by Nrf2 expression using immunoblot analysis (data not shown). 40 μM (24 h) pretreatment plus 5 μM (24h) cotreatment with tBHQ significantly increased resistance of UROtsa cells to both As(III) and MMA(III) (Fig. 3A and 3B). In accordance with tBHQ, 8 μM (24 h) pretreatment plus 0.5 μM (24h) cotreatment with SF also increased survival of UROtsa cells following As(III)- or MMA(III)-treatment (Fig. 3A and 3B).
Fig. 3. tBHQ or SF pretreatment protects cells from As(III)- and MMA(III)-induced toxicity.

(A and B) UROtsa cells were pretreated and co-treated with DMSO (diamond), tBHQ (triangle) or SF (square). Treatment conditions are: 40 μM (24 h) pretreatment and 5 μM (24h) cotreatment for tBHQ, 8 μM (24 h) pretreatment and 0.5 μM (24h) cotreatment for SF. During the cotreatment time period, 5 μM tBHQ or 0.5 μM SF was added along with the indicated concentrations of As(III) or MMA(III). (C–F) Cell viability assay was carried out in WT-MEF (C and D) and Nrf2−/− MEF cells (E and F). The pretreatment and cotreatment conditions for tBHQ or SF were same as described in Figure 3A or 3B.
To further confirm that the protective effect of tBHQ or SF on As(III)- and MMA(III)-induced toxicity is attributed to the specific activation of the Nrf2 pathway, the same experiments were carried out in the WT-MEF and Nrf2−/−-MEF cells with the same pretreatment and cotreatment conditions for tBHQ or SF as that used in UROtsa cells. tBHQ or SF rendered the WT-MEF cells more resistant to toxic effects of both As(III) and MMA(III) (Fig 3C and 3D). In sharp contrast, tBHQ or SF aggravates, rather than ameliorates, As(III)- and MMA(III)-induced toxicity in the Nrf2−/−-MEF cells (Fig 3E and 3F), demonstrating that both tBHQ and SF are able to protect UROtsa cells from arsenic-induced toxicity through the Nrf2 pathway. Taken together, these data provide strong evidence that activation of the Nrf2 pathway protects cells from arsenic-induced toxicity.
Discussion
In the current study, the impact of Nrf2 on As(III)- and MMA(III)-induced toxicity has been elucidated by the finding that reduced expression of Nrf2 sensitized UROtsa cells to As(III)- and MMA(III)-induced toxicity while activation of Nrf2 by pretreatment and cotreatment with tBHQ or SF increased cellular resistance to As(III) and MMA(III). In accordance with our finding, SF was demonstrated to reduce intracellular arsenic concentration and increase resistance to arsenic-toxicity in primary mouse hepatocytes (Shinkai et al., 2006). Thus, activation of Nrf2 represents a beneficial effect of cells to confer protection against arsenic toxicity. Paradoxically, As(III) was reported to activate the Nrf2 pathway (He et al., 2006; Kimura et al., 2006; Massrieh et al., 2006). The puzzling question is why does the highly toxic arsenic induce the Nrf2-mediated beneficial response? These seemingly conflicting results can be explained by the phenomena called adaptive response or hormesis, i.e. preconditioning cells with sublethal doses of toxic compounds increases cellular resistance to similar types of toxic compounds (Mattson and Cheng, 2006). Hormesis has been described since 1956 although it is still not very clear how it works. It is speculated that the stress-inducible transcription factor Nrf2 contributes significantly to the hormesis phenomena. Activation of the ARE-Nrf2-Keap1 pathway by As(III) at either low or high concentration is beneficial and is likely to be an attempt of cells to counteract the damaging effects, although the protective mechanism of the Nrf2 pathway may be masked by cell death effects at high concentrations of arsenic. In the MTT assays performed with UROtsa cells, a peak at low concentrations of As(III) or MMA(III) was observed, which represents an increased rate of cell proliferation in response to low doses of As(III) or MMA(III) exposure (Fig 2A and 2B). This beneficial low dose of As(III) exposure was also reported by others. For example, 0.1 to 1 μM As(III) exposure provides a protective effect against oxidative stress and DNA damage in human keratinocyte and fibroblast cells (Snow et al., 2005); Chronic low dose As(III) exposure enhances self tolerance in liver epithelial cells (Romach et al., 2000). Obviously, given the complexity of gene expression profile in response to a toxicant, it is likely that the net outcome in a given exposure portfolio is dependent on batteries of genes working in concert. Nevertheless, activation of an array of Nrf2 downstream target genes preconditions cells to a defensive mode for toxic/carcinogenic insults. Following arsenic exposure, a number of pathways that lead to different responses are activated. The net outcome is dictated by species, dose of arsenic, and duration of exposure. With high concentrations or repetitive doses of arsenic exposure, the Nrf2-dependent defense response is outweighed by the deteriorative effects induced by arsenic, resulting in acute toxicity or cell transformation. Therefore, it is believed that specific and potent activation of Nrf2 to elicit cellular hormesis by a reagent with low toxicity such as sulforaphane should be a great strategy to combat arsenic-induced damage.
The current study clearly demonstrates that Nrf2 protects cells from acute toxicity of arsenic, as measured by the MTT assay. Previously, it has been reported that arsenic induced both forms of cell death in human osteogenic sarcoma cell line U-2OS cells: low doses of arsenic resulted in apoptosis whereas high doses elicited necrosis. In addition, low dose arsenic-induced apoptosis was a slow process, required at least 48 hr for detection while high dose arsenic induced necrosis rapidly (Komissarova et al., 2005). Although whether Nrf2 protects cells from apoptotic cell death or necrotic cell death is not addressed in this study, it is envisioned that Nrf2 is able to protect both forms of cell death by acting on the early events of arsenic toxic effects, such as blockage of ROS formation through increased redox buffering capacity, detoxification/removal of arsenic and its reactive metabolites. Interestingly, a recent report indicates that arsenic was able to induce an apoptotic response through the NF-κB (P50)-dependent stabilization of GADD45α and subsequent induction of JNK(Song et al., 2006). JNK has been shown to be required for activation of Nrf2 by several-Nrf2 inducers, which leads to the suggestion that JNK activates Nrf2 through direct phosphorylation of Nrf2 (Kong et al., 2001; Keum et al., 2003). However, the phosphorylation site in Nrf2 that is absolutely required for its activity in vivo has not been detected so far. It is not clear, at this point, whether JNK activation plays any role in the Nrf2-mediated protection against arsenic toxicity.
Many of the Nrf2 downstream-target genes seem to have apparent functions in inhibiting arsenic toxicity directly or indirectly. For example, (1) both subunits of γ-GCS, the rate-limiting enzyme in glutathione biosynthesis, were upregulated by Nrf2, which is further supported by the finding that Nrf2 knockout cells had reduced intracellular glutathione levels (Wild et al., 1999; Chan and Kwong, 2000; Shih et al., 2003). Thus, Nrf2 likely increases the rate of glutathione synthesis in response to arsenic exposure to compensate for glutathione depletion during removal of the arsenic-glutathione complexes. By doing so, Nrf2 maintains cellular redox homeostasis and inhibits arsenic-induced ROS. Similarly, another intracellular redox-regulating protein thioredoxin is activated by tBHQ in an Nrf2-ARE-dependent manner. Working in concert, glutathione and thioredoxin redox buffering systems then counterbalance the production of ROS during arsenic exposure, resulting in enhanced resistance (Kim et al., 2003). (2) In addition to induce γ-GCS, Nrf2 was shown to maintain intracellular glutathione levels through ARE-dependent induction of the gene encoding the xCT cysteine/glutamate exchange transport that is important in the uptake of cysteine (Sasaki et al., 2002; Shih et al., 2003). (3) Overexpression of glutathione S-transferase Pi, an Nrf2 downstream gene, increased cellular resistance to inorganic arsenic (Liu et al., 2001; Nishinaka et al., 2007). (4) Transcription of the multidrug resistance proteins (including Mrp1-6) and P-glycoprotein was reported to be activated by Nrf2 (Vernhet et al., 2001; Hayashi et al., 2003; Maher et al., 2005; Xu et al., 2005). Functioning as transporters, these proteins were shown to play crucial roles in arsenic transport (Kala et al., 2000; Leslie et al., 2004; Kimura et al., 2006). Evidently, detailed mechanistic investigations of Nrf2-dependnet protection in response to arsenic is worth further investigation.
The finding that both tBHQ and SF are able to protect UROtsa cells from As(III)- and MMA(III)-induced toxicity provides a molecular basis for therapeutic or dietary intervention. Using Nrf2 inducers as a strategy for chemoprevention appears to be promising. Kensler and coworkers have pioneered clinical trials in residents of Qidong in China who are at high risk of aflatoxin-induced liver cancer. They have provided evidence that oral administration of Nrf2 inducers, such as oltipraz (a synthetic anti-cancer drug) and SF-rich broccoli extract, offer beneficial effects (Wang et al., 1999; Kensler et al., 2000; Kensler et al., 2005). Targeting the activation of Nrf2 may prove to be a superior approach for designing and developing more effective chemopreventive drugs. More significantly, many of the naturally occurring compounds isolated from plants have recently been identified as Nrf2 activators that include isothiocyanates (e.g. SF found in cruciferous vegetables) and polyphenols (epigallocatechin-3-gallate in green tea and caffeic acid phenethyl ester in honeybee propollis) (Zhang et al., 1994; Suganuma et al., 1999; Orsolic et al., 2003; Shen et al., 2005). Diet-based Nrf2 inducers offer greater advantages over therapeutic drugs due to their general acceptance, low toxicity, and cost-effectiveness. Thus, using natural products to boost the Nrf2-dependent antioxidant response in populations at high risk for arsenic exposure is an excellent choice to combat arsenic-induced damages.
Acknowledgments
This study was supported by the NIH grants ES015010-01 and ES04940. We thank Jefferson Y. Chan for his gift of the wild type and Nrf2−/− MEF cells.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aoki Y, Sato H, Nishimura N, Takahashi S, Itoh K, Yamamoto M. Accelerated DNA adduct formation in the lung of the Nrf2 knockout mouse exposed to diesel exhaust. Toxicol Appl Pharmacol. 2001;173:154–160. doi: 10.1006/taap.2001.9176. [DOI] [PubMed] [Google Scholar]
- Aposhian HV. Enzymatic methylation of arsenic species and other new approaches to arsenic toxicity. Annu Rev Pharmacol Toxicol. 1997;37:397–419. doi: 10.1146/annurev.pharmtox.37.1.397. [DOI] [PubMed] [Google Scholar]
- Aposhian HV, Gurzau ES, Le XC, Gurzau A, Healy SM, Lu X, Ma M, Yip L, Zakharyan RA, Maiorino RM, Dart RC, Tircus MG, Gonzalez-Ramirez D, Morgan DL, Avram D, Aposhian MM. Occurrence of monomethylarsonous acid in urine of humans exposed to inorganic arsenic. Chem Res Toxicol. 2000;13:693–697. doi: 10.1021/tx000114o. [DOI] [PubMed] [Google Scholar]
- Aposhian HV, Zakharyan RA, Avram MD, Kopplin MJ, Wollenberg ML. Oxidation and detoxification of trivalent arsenic species. Toxicol Appl Pharmacol. 2003;193:1–8. doi: 10.1016/s0041-008x(03)00324-7. [DOI] [PubMed] [Google Scholar]
- Burns FJ, Uddin AN, Wu F, Nadas A, Rossman TG. Arsenic-induced enhancement of ultraviolet radiation carcinogenesis in mouse skin: a dose-response study. Environ Health Perspect. 2004;112:599–603. doi: 10.1289/ehp.6655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter DE, Aposhian HV, Gandolfi AJ. The metabolism of inorganic arsenic oxides, gallium arsenide, and arsine: a toxicochemical review. Toxicol Appl Pharmacol. 2003;193:309–334. doi: 10.1016/j.taap.2003.07.009. [DOI] [PubMed] [Google Scholar]
- Chan JY, Kwong M. Impaired expression of glutathione synthetic enzyme genes in mice with targeted deletion of the Nrf2 basic-leucine zipper protein. Biochim Biophys Acta. 2000;1517:19–26. doi: 10.1016/s0167-4781(00)00238-4. [DOI] [PubMed] [Google Scholar]
- Chan K, Kan YW. Nrf2 is essential for protection against acute pulmonary injury in mice. Proc Natl Acad Sci U S A. 1999;96:12731–12736. doi: 10.1073/pnas.96.22.12731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho HY, Jedlicka AE, Reddy SP, Kensler TW, Yamamoto M, Zhang LY, Kleeberger SR. Role of NRF2 in protection against hyperoxic lung injury in mice. Am J Respir Cell Mol Biol. 2002;26:175–182. doi: 10.1165/ajrcmb.26.2.4501. [DOI] [PubMed] [Google Scholar]
- Cho HY, Reddy SP, Kleeberger SR. Nrf2 defends the lung from oxidative stress. Antioxid Redox Signal. 2006;8:76–87. doi: 10.1089/ars.2006.8.76. [DOI] [PubMed] [Google Scholar]
- Cohen SM, Shirai T, Steineck G. Epidemiology and etiology of premalignant and malignant urothelial changes. Scand J Urol Nephrol Suppl. 2000:105–115. doi: 10.1080/00365590050509869. [DOI] [PubMed] [Google Scholar]
- De Haan LH, Boerboom AM, Rietjens IM, van Capelle D, De Ruijter AJ, Jaiswal AK, Aarts JM. A physiological threshold for protection against menadione toxicity by human NAD(P)H:quinone oxidoreductase (NQO1) in Chinese hamster ovary (CHO) cells. Biochem Pharmacol. 2002;64:1597–1603. doi: 10.1016/s0006-2952(02)01383-7. [DOI] [PubMed] [Google Scholar]
- Delnomdedieu M, Basti MM, Otvos JD, Thomas DJ. Reduction and binding of arsenate and dimethylarsinate by glutathione: a magnetic resonance study. Chem Biol Interact. 1994;90:139–155. doi: 10.1016/0009-2797(94)90099-x. [DOI] [PubMed] [Google Scholar]
- Enomoto A, Itoh K, Nagayoshi E, Haruta J, Kimura T, O'Connor T, Harada T, Yamamoto M. High sensitivity of Nrf2 knockout mice to acetaminophen hepatotoxicity associated with decreased expression of ARE-regulated drug metabolizing enzymes and antioxidant genes. Toxicol Sci. 2001;59:169–177. doi: 10.1093/toxsci/59.1.169. [DOI] [PubMed] [Google Scholar]
- Hayashi A, Suzuki H, Itoh K, Yamamoto M, Sugiyama Y. Transcription factor Nrf2 is required for the constitutive and inducible expression of multidrug resistance-associated protein 1 in mouse embryo fibroblasts. Biochem Biophys Res Commun. 2003;310:824–829. doi: 10.1016/j.bbrc.2003.09.086. [DOI] [PubMed] [Google Scholar]
- He X, Chen MG, Lin GX, Ma Q. Arsenic induces NAD(P)H-quinone oxidoreductase I by disrupting the Nrf2 x Keap1 x Cul3 complex and recruiting Nrf2 x Maf to the antioxidant response element enhancer. J Biol Chem. 2006;281:23620–23631. doi: 10.1074/jbc.M604120200. [DOI] [PubMed] [Google Scholar]
- Hsueh YM, Huang YL, Huang CC, Wu WL, Chen HM, Yang MH, Lue LC, Chen CJ. Urinary levels of inorganic and organic arsenic metabolites among residents in an arseniasis-hyperendemic area in Taiwan. J Toxicol Environ Health A. 1998;54:431–444. doi: 10.1080/009841098158728. [DOI] [PubMed] [Google Scholar]
- Itoh K, Ishii T, Wakabayashi N, Yamamoto M. Regulatory mechanisms of cellular response to oxidative stress. Free Radic Res. 1999;31:319–324. doi: 10.1080/10715769900300881. [DOI] [PubMed] [Google Scholar]
- Jaiswal AK. Nrf2 signaling in coordinated activation of antioxidant gene expression. Free Radic Biol Med. 2004;36:1199–1207. doi: 10.1016/j.freeradbiomed.2004.02.074. [DOI] [PubMed] [Google Scholar]
- Kala SV, Neely MW, Kala G, Prater CI, Atwood DW, Rice JS, Lieberman MW. The MRP2/cMOAT transporter and arsenic-glutathione complex formation are required for biliary excretion of arsenic. J Biol Chem. 2000;275:33404–33408. doi: 10.1074/jbc.M007030200. [DOI] [PubMed] [Google Scholar]
- Kensler TW, Chen JG, Egner PA, Fahey JW, Jacobson LP, Stephenson KK, Ye L, Coady JL, Wang JB, Wu Y, Sun Y, Zhang QN, Zhang BC, Zhu YR, Qian GS, Carmella SG, Hecht SS, Benning L, Gange SJ, Groopman JD, Talalay P. Effects of glucosinolate-rich broccoli sprouts on urinary levels of aflatoxin-DNA adducts and phenanthrene tetraols in a randomized clinical trial in He Zuo township, Qidong, People’s Republic of China. Cancer Epidemiol Biomarkers Prev. 2005;14:2605–2613. doi: 10.1158/1055-9965.EPI-05-0368. [DOI] [PubMed] [Google Scholar]
- Kensler TW, Curphey TJ, Maxiutenko Y, Roebuck BD. Chemoprotection by organosulfur inducers of phase 2 enzymes: dithiolethiones and dithiins. Drug Metabol Drug Interact. 2000;17:3–22. doi: 10.1515/dmdi.2000.17.1-4.3. [DOI] [PubMed] [Google Scholar]
- Kenyon EM, Del Razo LM, Hughes MF. Tissue distribution and urinary excretion of inorganic arsenic and its methylated metabolites in mice following acute oral administration of arsenate. Toxicol Sci. 2005;85:468–475. doi: 10.1093/toxsci/kfi107. [DOI] [PubMed] [Google Scholar]
- Keum YS, Owuor ED, Kim BR, Hu R, Kong AN. Involvement of Nrf2 and JNK1 in the activation of antioxidant responsive element (ARE) by chemopreventive agent phenethyl isothiocyanate (PEITC) Pharm Res. 2003;20:1351–1356. doi: 10.1023/a:1025737622815. [DOI] [PubMed] [Google Scholar]
- Kim YC, Yamaguchi Y, Kondo N, Masutani H, Yodoi J. Thioredoxin-dependent redox regulation of the antioxidant responsive element (ARE) in electrophile response. Oncogene. 2003;22:1860–1865. doi: 10.1038/sj.onc.1206369. [DOI] [PubMed] [Google Scholar]
- Kimura A, Ishida Y, Hayashi T, Wada T, Yokoyama H, Sugaya T, Mukaida N, Kondo T. Interferon-gamma plays protective roles in sodium arsenite-induced renal injury by up-regulating intrarenal multidrug resistance-associated protein 1 expression. Am J Pathol. 2006;169:1118–1128. doi: 10.2353/ajpath.2006.060024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi A, Ohta T, Yamamoto M. Unique function of the Nrf2-Keap1 pathway in the inducible expression of antioxidant and detoxifying enzymes. Methods Enzymol. 2004;378:273–286. doi: 10.1016/S0076-6879(04)78021-0. [DOI] [PubMed] [Google Scholar]
- Komissarova EV, Saha SK, Rossman TG. Dead or dying: the importance of time in cytotoxicity assays using arsenite as an example. Toxicol Appl Pharmacol. 2005;202:99–107. doi: 10.1016/j.taap.2004.06.010. [DOI] [PubMed] [Google Scholar]
- Kong AN, Owuor E, Yu R, Hebbar V, Chen C, Hu R, Mandlekar S. Induction of xenobiotic enzymes by the MAP kinase pathway and the antioxidant or electrophile response element (ARE/EpRE) Drug Metab Rev. 2001;33:255–271. doi: 10.1081/dmr-120000652. [DOI] [PubMed] [Google Scholar]
- Lee JS, Surh YJ. Nrf2 as a novel molecular target for chemoprevention. Cancer Lett. 2005;224:171–184. doi: 10.1016/j.canlet.2004.09.042. [DOI] [PubMed] [Google Scholar]
- Leslie EM, Haimeur A, Waalkes MP. Arsenic transport by the human multidrug resistance protein 1 (MRP1/ABCC1). Evidence that a tri-glutathione conjugate is required. J Biol Chem. 2004;279:32700–32708. doi: 10.1074/jbc.M404912200. [DOI] [PubMed] [Google Scholar]
- Liu J, Chen H, Miller DS, Saavedra JE, Keefer LK, Johnson DR, Klaassen CD, Waalkes MP. Overexpression of glutathione S-transferase II and multidrug resistance transport proteins is associated with acquired tolerance to inorganic arsenic. Mol Pharmacol. 2001;60:302–309. doi: 10.1124/mol.60.2.302. [DOI] [PubMed] [Google Scholar]
- Liu L, Trimarchi JR, Navarro P, Blasco MA, Keefe DL. Oxidative stress contributes to arsenic-induced telomere attrition, chromosome instability, and apoptosis. J Biol Chem. 2003;278:31998–32004. doi: 10.1074/jbc.M303553200. [DOI] [PubMed] [Google Scholar]
- Maher JM, Cheng X, Slitt AL, Dieter MZ, Klaassen CD. Induction of the multidrug resistance-associated protein family of transporters by chemical activators of receptor-mediated pathways in mouse liver. Drug Metab Dispos. 2005;33:956–962. doi: 10.1124/dmd.105.003798. [DOI] [PubMed] [Google Scholar]
- Mandal BK, Ogra Y, Suzuki KT. Identification of dimethylarsinous and monomethylarsonous acids in human urine of the arsenic-affected areas in West Bengal, India. Chem Res Toxicol. 2001;14:371–378. doi: 10.1021/tx000246h. [DOI] [PubMed] [Google Scholar]
- Massrieh W, Derjuga A, Blank V. Induction of endogenous Nrf2/small maf heterodimers by arsenic-mediated stress in placental choriocarcinoma cells. Antioxid Redox Signal. 2006;8:53–59. doi: 10.1089/ars.2006.8.53. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Cheng A. Neurohormetic phytochemicals: Low-dose toxins that induce adaptive neuronal stress responses. Trends Neurosci. 2006;29:632–639. doi: 10.1016/j.tins.2006.09.001. [DOI] [PubMed] [Google Scholar]
- McMahon M, Itoh K, Yamamoto M, Chanas SA, Henderson CJ, McLellan LI, Wolf CR, Cavin C, Hayes JD. The Cap’n’Collar basic leucine zipper transcription factor Nrf2 (NF-E2 p45-related factor 2) controls both constitutive and inducible expression of intestinal detoxification and glutathione biosynthetic enzymes. Cancer Res. 2001;61:3299–3307. [PubMed] [Google Scholar]
- Motohashi H, Yamamoto M. Nrf2-Keap1 defines a physiologically important stress response mechanism. Trends Mol Med. 2004;10:549–557. doi: 10.1016/j.molmed.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Nishinaka T, Ichijo Y, Ito M, Kimura M, Katsuyama M, Iwata K, Miura T, Terada T, Yabe-Nishimura C. Curcumin activates human glutathione S-transferase P1 expression through antioxidant response element. Toxicol Lett. 2007;170:238–247. doi: 10.1016/j.toxlet.2007.03.011. [DOI] [PubMed] [Google Scholar]
- Orsolic N, Sver L, Terzic S, Tadic Z, Basic I. Inhibitory effect of water-soluble derivative of propolis and its polyphenolic compounds on tumor growth and metastasizing ability: a possible mode of antitumor action. Nutr Cancer. 2003;47:156–163. doi: 10.1207/s15327914nc4702_8. [DOI] [PubMed] [Google Scholar]
- Petrick JS, Ayala-Fierro F, Cullen WR, Carter DE, Vasken Aposhian H. Monomethylarsonous acid (MMA(III)) is more toxic than arsenite in Chang human hepatocytes. Toxicol Appl Pharmacol. 2000;163:203–207. doi: 10.1006/taap.1999.8872. [DOI] [PubMed] [Google Scholar]
- Petzoldt JL, Leigh IM, Duffy PG, Sexton C, Masters JR. Immortalisation of human urothelial cells. Urol Res. 1995;23:377–380. doi: 10.1007/BF00698738. [DOI] [PubMed] [Google Scholar]
- Ramos-Gomez M, Kwak MK, Dolan PM, Itoh K, Yamamoto M, Talalay P, Kensler TW. Sensitivity to carcinogenesis is increased and chemoprotective efficacy of enzyme inducers is lost in nrf2 transcription factor-deficient mice. Proc Natl Acad Sci U S A. 2001;98:3410–3415. doi: 10.1073/pnas.051618798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romach EH, Zhao CQ, Del Razo LM, Cebrian ME, Waalkes MP. Studies on the mechanisms of arsenic-induced self tolerance developed in liver epithelial cells through continuous low-level arsenite exposure. Toxicol Sci. 2000;54:500–508. doi: 10.1093/toxsci/54.2.500. [DOI] [PubMed] [Google Scholar]
- Rossman TG. Mechanism of arsenic carcinogenesis: an integrated approach. Mutat Res. 2003;533:37–65. doi: 10.1016/j.mrfmmm.2003.07.009. [DOI] [PubMed] [Google Scholar]
- Sakurai T, Ochiai M, Kojima C, Ohta T, Sakurai MH, Takada NO, Qu W, Waalkes MP, Himeno S, Fujiwara K. Preventive mechanism of cellular glutathione in monomethylarsonic acid-induced cytolethality. Toxicol Appl Pharmacol. 2005;206:54–65. doi: 10.1016/j.taap.2004.11.008. [DOI] [PubMed] [Google Scholar]
- Sasaki H, Sato H, Kuriyama-Matsumura K, Sato K, Maebara K, Wang H, Tamba M, Itoh K, Yamamoto M, Bannai S. Electrophile response element-mediated induction of the cystine/glutamate exchange transporter gene expression. J Biol Chem. 2002;277:44765–44771. doi: 10.1074/jbc.M208704200. [DOI] [PubMed] [Google Scholar]
- Scott N, Hatlelid KM, MacKenzie NE, Carter DE. Reactions of arsenic(III) and arsenic(V) species with glutathione. Chem Res Toxicol. 1993;6:102–106. doi: 10.1021/tx00031a016. [DOI] [PubMed] [Google Scholar]
- Shen G, Xu C, Hu R, Jain MR, Nair S, Lin W, Yang CS, Chan JY, Kong AN. Comparison of (−)-Epigallocatechin-3-Gallate Elicited Liver and Small Intestine Gene Expression Profiles Between C57BL/6J Mice and C57BL/6J/Nrf2 (−/−) Mice. Pharm Res. 2005;22:1805–1820. doi: 10.1007/s11095-005-7546-8. [DOI] [PubMed] [Google Scholar]
- Shih AY, Johnson DA, Wong G, Kraft AD, Jiang L, Erb H, Johnson JA, Murphy TH. Coordinate regulation of glutathione biosynthesis and release by Nrf2-expressing glia potently protects neurons from oxidative stress. J Neurosci. 2003;23:3394–3406. doi: 10.1523/JNEUROSCI.23-08-03394.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinkai Y, Sumi D, Fukami I, Ishii T, Kumagai Y. Sulforaphane, an activator of Nrf2, suppresses cellular accumulation of arsenic and its cytotoxicity in primary mouse hepatocytes. FEBS Lett. 2006;580:1771–1774. doi: 10.1016/j.febslet.2006.02.031. [DOI] [PubMed] [Google Scholar]
- Smith AH, Lingas EO, Rahman M. Contamination of drinking-water by arsenic in Bangladesh: a public health emergency. Bull World Health Organ. 2000;78:1093–1103. [PMC free article] [PubMed] [Google Scholar]
- Smith AH, Marshall G, Yuan Y, Ferreccio C, Liaw J, von Ehrenstein O, Steinmaus C, Bates MN, Selvin S. Increased mortality from lung cancer and bronchiectasis in young adults after exposure to arsenic in utero and in early childhood. Environ Health Perspect. 2006;114:1293–1296. doi: 10.1289/ehp.8832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KR, Klei LR, Barchowsky A. Arsenite stimulates plasma membrane NADPH oxidase in vascular endothelial cells. Am J Physiol Lung Cell Mol Physiol. 2001;280:L442–449. doi: 10.1152/ajplung.2001.280.3.L442. [DOI] [PubMed] [Google Scholar]
- Snow ET, Sykora P, Durham TR, Klein CB. Arsenic, mode of action at biologically plausible low doses: What are the implications for low dose cancer risk? Toxicol Appl Pharmacol. 2005;207:557–564. doi: 10.1016/j.taap.2005.01.048. [DOI] [PubMed] [Google Scholar]
- Song L, Li J, Zhang D, Liu ZG, Ye J, Zhan Q, Shen HM, Whiteman M, Huang C. IKKbeta programs to turn on the GADD45alpha-MKK4-JNK apoptotic cascade specifically via p50 NF-kappaB in arsenite response. J Cell Biol. 2006;175:607–617. doi: 10.1083/jcb.200602149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmaus C, Moore L, Hopenhayn-Rich C, Biggs ML, Smith AH. Arsenic in drinking water and bladder cancer. Cancer Invest. 2000;18:174–182. doi: 10.3109/07357900009038249. [DOI] [PubMed] [Google Scholar]
- Suganuma M, Okabe S, Sueoka N, Sueoka E, Matsuyama S, Imai K, Nakachi K, Fujiki H. Green tea and cancer chemoprevention. Mutat Res. 1999;428:339–344. doi: 10.1016/s1383-5742(99)00059-9. [DOI] [PubMed] [Google Scholar]
- Surh YJ. Cancer chemoprevention with dietary phytochemicals. Nat Rev Cancer. 2003;3:768–780. doi: 10.1038/nrc1189. [DOI] [PubMed] [Google Scholar]
- Tchounwou PB, Patlolla AK, Centeno JA. Carcinogenic and systemic health effects associated with arsenic exposure--a critical review. Toxicol Pathol. 2003;31:575–588. doi: 10.1080/01926230390242007. [DOI] [PubMed] [Google Scholar]
- Thomas DJ, Styblo M, Lin S. The cellular metabolism and systemic toxicity of arsenic. Toxicol Appl Pharmacol. 2001;176:127–144. doi: 10.1006/taap.2001.9258. [DOI] [PubMed] [Google Scholar]
- Thomas DJ, Waters SB, Styblo M. Elucidating the pathway for arsenic methylation. Toxicol Appl Pharmacol. 2004;198:319–326. doi: 10.1016/j.taap.2003.10.020. [DOI] [PubMed] [Google Scholar]
- Thompson DJ. A chemical hypothesis for arsenic methylation in mammals. Chem Biol Interact. 1993;88:89–14. doi: 10.1016/0009-2797(93)90086-e. [DOI] [PubMed] [Google Scholar]
- Tseng CH. An overview on peripheral vascular disease in blackfoot disease-hyperendemic villages in Taiwan. Angiology. 2002;53:529–537. doi: 10.1177/000331970205300505. [DOI] [PubMed] [Google Scholar]
- Venugopal R, Jaiswal AK. Nrf1 and Nrf2 positively and c-Fos and Fra1 negatively regulate the human antioxidant response element-mediated expression of NAD(P)H:quinone oxidoreductase1 gene. Proc Natl Acad Sci U S A. 1996;93:14960–14965. doi: 10.1073/pnas.93.25.14960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernhet L, Seite MP, Allain N, Guillouzo A, Fardel O. Arsenic induces expression of the multidrug resistance-associated protein 2 (MRP2) gene in primary rat and human hepatocytes. J Pharmacol Exp Ther. 2001;298:234–239. [PubMed] [Google Scholar]
- Waalkes MP, Ward JM, Liu J, Diwan BA. Transplacental carcinogenicity of inorganic arsenic in the drinking water: induction of hepatic, ovarian, pulmonary, and adrenal tumors in mice. Toxicol Appl Pharmacol. 2003;186:7–17. doi: 10.1016/s0041-008x(02)00022-4. [DOI] [PubMed] [Google Scholar]
- Wang JS, Shen X, He X, Zhu YR, Zhang BC, Wang JB, Qian GS, Kuang SY, Zarba A, Egner PA, Jacobson LP, Munoz A, Helzlsouer KJ, Groopman JD, Kensler TW. Protective alterations in phase 1 and 2 metabolism of aflatoxin B1 by oltipraz in residents of Qidong, People's Republic of China. J Natl Cancer Inst. 1999;91:347–354. doi: 10.1093/jnci/91.4.347. [DOI] [PubMed] [Google Scholar]
- Wild AC, Moinova HR, Mulcahy RT. Regulation of gamma-glutamylcysteine synthetase subunit gene expression by the transcription factor Nrf2. J Biol Chem. 1999;274:33627–33636. doi: 10.1074/jbc.274.47.33627. [DOI] [PubMed] [Google Scholar]
- Xu C, Li CY, Kong AN. Induction of phase I, II and III drug metabolism/transport by xenobiotics. Arch Pharm Res. 2005;28:249–268. doi: 10.1007/BF02977789. [DOI] [PubMed] [Google Scholar]
- Zhang DD. Mechanistic studies of the Nrf2-Keap1 signaling pathway. Drug Metab Rev. 2006;38:769–789. doi: 10.1080/03602530600971974. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Kensler TW, Cho CG, Posner GH, Talalay P. Anticarcinogenic activities of sulforaphane and structurally related synthetic norbornyl isothiocyanates. Proc Natl Acad Sci U S A. 1994;91:3147–3150. doi: 10.1073/pnas.91.8.3147. [DOI] [PMC free article] [PubMed] [Google Scholar]