Abstract
Endothelin receptor B (Ednrb) plays a critical role in the development of melanocytes and neurons and glia of the enteric nervous system. These distinct neural crest-derived cell types express Ednrb and share the property of intercalating into tissues, such as the intestine whose muscle precursor cells also express Ednrb. Such widespread Ednrb expression has been a significant obstacle in establishing precise roles for Ednrb in development. We describe here the production of an Ednrb allele floxed at exon 3 and its use in excising the receptor from mouse neural crest cells by use of Cre-recombinase driven by the Wnt1 promoter. Mice born with neural crest-specific excision of Ednrb possess aganglionic colon, lack trunk pigmentation, and die within five weeks due to megacolon. Ednrb receptor expression in these animals is absent only in the neural crest but present in surrounding smooth muscle cells. The absence of Ednrb from crest cells also results in a compensatory upregulation of Ednrb expression in other cells within the gut. We conclude that Ednrb loss only in neural crest cells is sufficient to produce the Hirschsprungs disease phenotype observed with genomic Ednrb mutations.
Endothelins make up a family of 21 amino acid peptides, ET-1, ET-2 and ET-3. . These peptides bind to two distinct G-protein-coupled receptors, ET-A and ET-B. The affinity of ET-A is greater for ET-1 and ET-2 than for ET-3 whereas the affinity of ET-B is the same for the three peptides. ET-1, the most ubiquitous, is produced by endothelial cells in the vasculature, epithelial cells in the kidney, heart cells, and some neurons, while ET-2 and ET-3 are both found in the gastrointestinal tract. Both ET-A and ET-B receptors are found in a variety of tissues and activate a number of different effectors. Both receptors are prominent in the vasculature where ET-A mediates vasoconstriction by activating smooth muscle contraction and ET-B receptors produce vasodilation by release of nitric oxide from the endothelial cells under certain conditions (Schneider et al., 2007). Both receptors can also act in mitogen signaling and are known to play a role in cancer (Grant et al., 2006; Lahav et al., 1996).
The endothelin receptors have discrete roles during embryonic development by influencing the fate of different neural crest cell populations. The cephalic neural crest invades the pharyngeal arches and gives rise to the bones and cartilage of the facial skeleton, dermis, and smooth muscle of the great arteries. The ET-A receptor appears in the cephalic neural crest cells as they emigrate from the neural tube while the ligand ET-1 is produced by the non-crest cells. Loss of the ET-A receptors results in cranial-facial defects such as a reduced or absent mandible, middle ear defects and alterations in the great arteries (Pla and Larue, 2003).
The ET-B receptor is required for normal development of the vagal and truncal neural crest (Heanue and Pachnis, 2007). The vagal neural crest cells give rise to enteric neurons and truncal neural crest are precursors for sympathetic and sensory neurons, and melanocytes. Global knockouts or naturally-occurring mutations of the ET-B receptor result in the absence of neural crest-derived neurons in the terminal colon and the lack of pigment in the skin of the trunk (Hosoda et al., 1994). Aganglionic colon is the hallmark of human Hirschprung’s disease (HSCR) and ET-B deficient mice are an animal model of this congenital disease. In the gut, the ET-B message has been found in both neural crest-derived cells and mesenchymal-derived smooth muscle precursors (Wu et al., 1999). Because the null mutants lack the ET-B receptor on both the crest cells and the mesenchymal cells, it has been difficult to determine which cells require ET-B signaling for normal ENS development. To overcome this difficulty we have generated a floxed Ednrb allele (Flex3) to permit the targeting of a null ET-B allele to particular tissues in both the embryo and adult. In this study we have used Wnt1 to drive Cre-recombinase expression within neural crest cells.
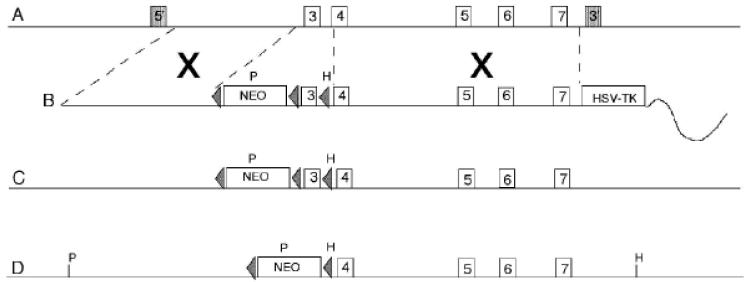
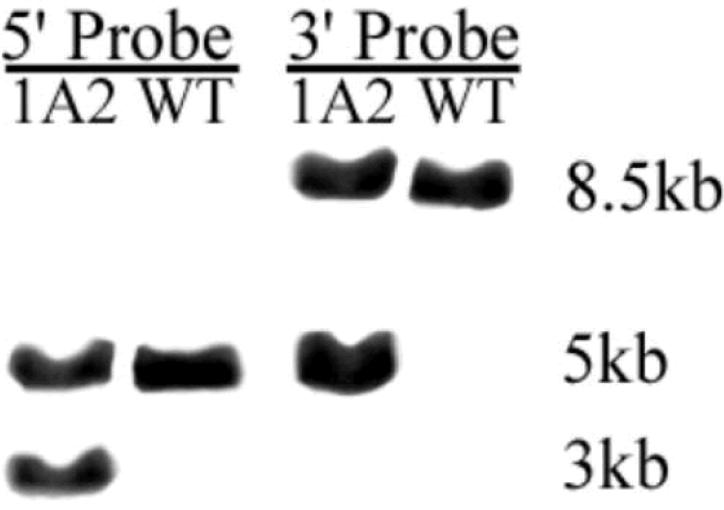
To generate a conditional Ednrb knock-out mouse loxP sites were inserted to flank exon 3 of Ednrb. Upon deletion of exon 3, exon 2 is spliced to exon 4 resulting in a out of frame transcript which generates a null mutation (Figure 1). The targeting vector was constructed as described in the Methods. The targeting vector was linearized by digestion with PacI and introduced by electroporation into murine SV/129 R1 embryonic stem cells (Nagy et al., 1993). ES cells that integrated the targeting vector either by homologous or random integration were selected by growth on G418. Gancyclovir (GANC) selected against clones that contained the HSV-TK cassette, thus enriching for clones that integrated the Neo cassette by homologous recombination. The resulting Neor, GANCr colonies were replicated and expanded. DNA was isolated from replica ES clones, digested with Hindlll, electrophoresed on agarose gels, transferred to charged nylon membranes and hybridized to a unique radiolabeled 3′ probe. Seventeen clones were identified as correctly targeted by the appearance of a 5 kb band representing a gene modified by homologous recombination as well as the 8.5 kb band representing the unmodified gene. DNA from 9 replicas of clones that appear to be correctly targeted on the 3′ side were digested with PstI, electrophoresed on agarose gels, transferred to charged nylon membranes and hybridized to a unique radiolabeled 5′ probe (Figure 2). All 9 clones were identified as correctly targeted by the appearance of a 3 kb targeted band in addition to the 5 kb native band. Portions of these clones were sequenced to confirm the integrity of the loxP sites and the presence of the floxed exon 3.
Figure 1.
A) murine chromosome 14. B) linearized Ednrb-cKO targeting vector. C) Ednrb-cKO targeted chromosome 14. D) Ednrb-cKO targeted chromosome after Cre-excision of exon 3. Numbered boxes indicate the location of exons in the 8.8 kb targeting vector. Triangles represent loxP sites flanking the Neomycin cassette (neo). Dashed lines indicate regions of homology, X represents location of homologous recombination. P represents an introduced PstI site used in genotyping the 5′ probe, H represents an introduced HindIII restriction site used in genotyping using the 3′ probe. The shaded boxes on A indicate the location of the 5′ probe and 3′ probes, respectively.
Figure 2.
Southern Blots showing PstI digested DNA from ES cell clone 1A2 after the specific binding of a 3′ radiolabeled probe to the targeting vector (lane 1), DNA from untransfected ES cells (lane 2). Southern blots showing HindIII digested DNA from ES cell clone 1A2 after specific binding of a 5′ radiolabeled probe to the targeting vector (lane 3) , and DNA from untransfected ES cells (lane 4).
Eight correctly targeted clones were expanded and two of these clones were karyotyped. The karyotypically normal ES clones, 1A2 and 1D8, were microinjected into C57BL/6 blastocysts to produce chimeric founders. Highly chimeric founders were mated to C57BL/6 females. F1 pups derived from the gene-targeted ES cells have agouti coat color while F1 pups derived from the host C57BL/6 blastocyst have black coat color. Only the 1A2 chimeric males produced agouti F1 pups. These pups were genotyped by PCR to identify those carrying the gene-targeted allele. Note that the neo cassette is intact and has not been deleted.
To determine whether ET-B function could be ablated in a particular tissue, we targeted the excision of Ednrb to neural crest cells of developing fetuses and neonatal animals. To do this we used mice in which Cre-recombinase, driven by the neural crest specific Wnt1 promoter (Danielian et al., 1998), is expressed at about embryonic day (E) 8. These Wnt1 Cre mice were mated to the floxed Ednrb mice to generate mice heterozygous for both Wnt1 Cre and floxed Ednrb (Wnt1 Cre+/− Ednrb+/flex3). These mice were then mated with a strain homozygous for floxed stop YFP (Srinivas et al., 2001) and floxed Ednrb (R26RYFP/YFP Ednrbflex3/flex3) to generate offspring with neural crest cells that express YFP and either lack the Ednrb receptor (Wnt1 Cre+/− R26RYFP/+ Ednrbflex3/flex3 henceforth called Ednrb-null) or contain one functional receptor copy (Wnt1 Cre+/− R26RYFP/+ Ednrbflex3/+; called heterozygous). All the neural crest cells in the fetuses and neonatal mice with the genotypes of interest express YFP. These YFP+ animals are easily recognized by the fluorescence of the head, a feature resulting from the vast contribution it receives from neural crest cells (Noden and Trainor, 2005). The YFP expression allows the segregation of neural crest cells from surrounding cells by fluorescence activated cell sorting (FACS) for molecular analysis. Here we exploited this marker to separate enteric neural crest-derived cells (ENCCs) from smooth muscle progenitor cells and enterocytes from null and heterozygous Ednrb animals. In order to verify that Ednrb was deleted from ENCCs but not from smooth muscle progenitors, we examined the colons from YFP+ fetuses and neonatal mice with a fluorescent microscope. We are able to distinguish unambiguously a phenotype by the position of the most caudal ENCC. Those animals with colons in which the ENCC are at the anus are considered heterozygous while those in which the ENCC had clearly not reached the anus are null. Genotype was confirmed by PCR analysis of tissue from each preparation. The intestines of heterozygous and null mice were placed in separate pools and dissociated into cells that were separated by FACS into YFP positive and negative populations.
Lysates of these separate cell populations obtained from E14 to postnatal day(P) 7 small and large intestine were combined and analyzed for ET-B expression by Western blot (Figure 3). The blots showed that both the YFP+ ENCC and YFP negative cells (enterocytes and mesenchymal smooth muscle precursors) from Ednrb+/− mice expressed ET-B. However, in tissue from the null mice the receptor was not expressed in YFP+ENCCs but was produced by YFP negative smooth muscle cells and enterocytes. These results indicate that ET-B is absent only from the ENCCs and supports the idea that Ednrb is selectively deleted from neural crest cells. It is of interest that the YFP negative cells from the null produced increased amounts of ET-B compared to the heterozygote, a result suggesting a compensatory response in ET-B expression in smooth muscle cells (i.e. YFP negative cells). Although the reason for this response is unclear, we speculate that increase in receptor may arise in order to enhance endothelin peptide clearance by smooth muscle cells in the absence of ET-B in the ENCCs.
Figure 3.
Western Blot. ET-B protein is expressed in 1. YFP negative cells (i.e. enterocytes and smooth muscle precursors) and 2. YFP-positive ENCCs isolated from the intestines of Ednrb+/− preparations. In contrast the expression of ET-B protein is increased in 3. YFP negative cells but is absent from 4. YFP+ ENCCs from null animals. A 50 Kd marker protein is shown in the rightmost column. Equal amounts of lysate were loaded as measured by staining of ß-actin (not shown). The protein came from cells obtained from preparations ranging in age between E14 to P 7.
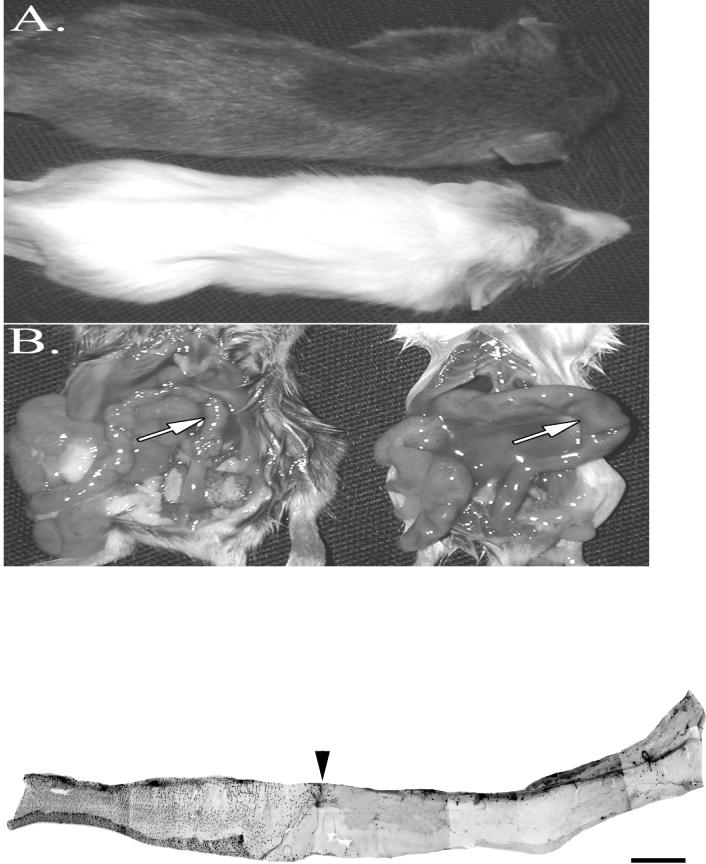
The phenotypes of the neonatal mice generated here resembled those observed with naturally occurring or global deletions of Ednrb (Hosoda et al.,1994). The coat of the heterozygous mice was entirely pigmented while the nulls lacked pigment in the trunk (Figure 4A). The null mice also showed an enlarged abdomen, failure to gain weight after P25 (data not shown) and the presence of an enlarged colon (Figure 4B). All of the null mice died around five weeks after birth. Examination of the colon in postnatal null mice showed the absence of ganglion cells in the distal colon, a defining characteristic of HSCR (Figure 4C). Together these results indicate that the absence of ET-B from only ENCCs is sufficient to produce all the characteristics of a null Ednrb-mediated Hirschsprung’s disease, and that compensatory up-regulation of Ednrb expression in neighboring tissues cannot prevent disease onset. By introducing the DßH-EdnrB transgene into (sl/sl) null Ednrb rats Gariepy et al (1998) found that most null transgenic animals did not develop aganglionic colons. This result implies that introduction of a functional gene into neural crest cells is sufficient to rescue the aganglionic phenotype, a result consistent with ours.
Figure 4.
Phenotype of null Ednrb mice. A. The null 24 day old Ednrb mouse has a white trunk coat and a pigmented coat on the head and hindquarters while the Ednrb+/− littermate has a pigmented trunk coat. B. Terminal portion of large intestine. The descending colon of the null Ednrb mouse (right side) is enlarged (arrow). The same region (arrow) of the Ednrb+/− colon is not distended. C. Micrograph of aganglionic colon from a P2 null Ednrb mouse. The entire colon was immunostained with Hu antibody which was visualized with fluorescent conjugated secondary antibody (CY2). The arrowhead shows the demarcation between the region contaning ganglia (shown as black spots) and the caudal aganglionic region. Bar=1mm.
In summary, we have focused here on the role of Ednrb in development of the enteric nervous system, and visualized targeted cells by YFP co-expression. This allowed us to identify ENCCs with a specific phenotype for the first time in living tissue. Furthermore, cell sorting permitted us to directly demonstrate the absence of the ET-B receptor protein in the targeted cell type and its presence in native intestinal smooth muscle. Previously, Ednrb mRNA (not protein) had been reported in cultures of intestinal smooth muscle precursors (Wu et al.,1999). We also showed for the first time the capacity for compensatory ET-B receptor up-regulation in native intestinal smooth muscle cells. It should be noted that a floxed Ednrb mouse has been described in which exons 3 and 4 together were flanked with loxP sites (Bagnall, et al., 2006; Ge et al., 2006). By crossing with Tie-cre transgenic mice (Bagnall et al., 2006) or aquaporin-cre trangenic mice (Ge et al., 2006), these investigators reported tissue specific Ednrb deletion in endothelial cells or renal collecting duct cells, respectively. Here we have shown that the combined use of tissue-specific Ednrb gene knock-out and cell-type specific fluorescent labeling of targeted cells offers unique opportunities to employ complementary in vivo and in vitro strategies to address the role(s) of this enigmatic G-protein coupled receptor in development.
MATERIALS AND METHODS
Construction of targeting vector
A 129/SvJ BAC clone containing the Endothelin B receptor gene and obtained by PCR screening the129/Sv ResGen BAC DNA Pools (Invitrogen Inc, Carlsbad, CA) was used as the source of genomic DNA in generating the Ednrb-cKO targeting vector. The targeting construct contains 6.8 kb of 129/SV genomic DNA containing Ednrb exons 3-7. The 5′ homology arm contains the 3′-most 2.2 kb of intron 2. A floxed Neo cassette derived from from pFlox-1 (Chui et al., 1997) was introduced at the 3′ end of intron 2. A third loxP site was introduced into the 5′-most end of intron 3, resulting in exon 3 being flanked by loxP sites. The 3′ homology arm is 4.6 kb in length and includes intron 4 through exon 7. The MCI-TK cassette (Thomas and Cappechi, 1987) was introduced at the junction between the 3′ homology arm and the vector sequence. A PacI site for linearizing the targeting vector was introduced at the junction between the vector sequence and the 5′ homology arm.
Genotyping of mice and mouse fetuses
DNA was extracted from tail biopsies. PCR reactions for the floxed allele were carried out for 35 cycles at 94 °C for 45 sec, 62° C for 45 sec, 72° C for 70 sec and were the same for the wild allele except the annealing temperature was 56° C. Primers (PNT 5, ETB 3) used to detect the presence of floxed exon 3 gave a PCR product of 912 bp which included exon 3 and the inserted lox P sites. The primers (ETBS2,ETBEX 3) to detect intact Ednrb yielded a product of 480 bp and included the sequence within exon 3. The following PCR primers were used to detect the presence of the different alleles (written 5′ to 3′): Floxed Ednrb Forward, PNT 5: 5′ TGG AAT GTG TGC GAG GCC 3′ Reverse, ETB 3 : 5′ CAG CCA GAA CCA CAG AGA CCA CCC 3′ ; Wild-type Ednrb: Forward, ETBS2 : 5′ CTG AGG AGA GCC TGA TTG TGC CAC 3′, Reverse, ETBEX 3: 5′ CGA CTC CAA GAA GCA ACA GCT CG 3′. The Primers used to identify the presence of Wnt1 cre (Druckenbrod and Epstein, 2005) and YFP R-26R (Soriano, 1999) have been described.
Animals
Pregnant mice from timed mating were killed by cervical dislocation. The University of Wisconsin Animal Care Committee approved these procedures. The day of the vaginal plug was considered embryonic day (E) 0.5. Fetuses were removed from the uterus between E10.5-18, identified for the presence of YFP in the head and staged. From these the gastrointestinal tract was removed and the location of the most caudal YFP+ cells was determined. Postnatal pups between 3-7 days old were sacrificed and the phenotype of the gut identified as above.
Selection of cells by FACS
The gastrointestinal tract was removed from YFP+ E14.5-18 fetuses and postnatal day 3-7 pups. The location of the ENCC wavefront identified the phenotype as either null or heterozygous. The stomach was separated from the small intestine and the latter was combined with the large intestine. The intestines from null and heterozygous fetuses were pooled separately and dissociated. Dissociation was achieved by incubation in a mixture of 5mg/ml collagenase and 2 mg/ml DNAase for 10-20 minutes at 37° C, washing in PBS, and trituration through a series of siliconized pipettes with openings of decreasing size. The dissociate was resuspended in DMEM/F12 buffered with Hepes. The dissociates was filtered through 20 um Nitex, and the YFP+ ENCCs were separated from YFP negative cells for both heterozygote and null mice by a Becton-Dickinson VantageSE fluorescent activated cell sorter. The cells were centrifuged and sonicated in protein lysis buffer consisting of 50mM Tris-HCl, pH7.4, 150mM NaCl, 2mM EDTA, 1 % NP40, 0.1% SDS, 5mM AMSF, 20 ug/ml aprotonin, 5 ug/ml leupeptin.
Western blot
Protein lysates were quantified with BCA (Pierce) and 20ug of protein was separated on a 8-16 % gradient Tris-Hepes SDS- polyacrylamide gel and transferred to a PVDF membrane, which was incubated with either rabbit anti-Ednrb (1/200, Santa Cruz, Ca) or monoclonal mouse anti-ß-actin (1/1000 Sigma). The membrane was washed, incubated with HRP-conjugated monoclonal mouse anti-Rabbit IgG, Light Chain Specific anti-rabbit (1/5000) or goat anti-mouse IgG (1/10,000), washed again, and incubated with ECL reagents (Pierce, Rockford, Il) to visualize the protein.
Acknowledgements
Generation of the mouse was supported by grant: HL47053. We are grateful to Andras Nagy for providing the RI ES cells and to Manorama C. John and Joe Warren of the University of Wisconsin Biotechnology Center Transgenic Animal Facility for their assistance.
Literature Cited
- Bagnall AJ, Kelland NF, Gulliver-Sloan F, Davenport AP, Gray GA, Yanagisawa M, Webb DJ, Kotelevtsev YV. Deletion of endothelial cell endothelin B receptors does not affect blood pressure or sensitivity to salt. Hypertension. 2006;48:286–293. doi: 10.1161/01.HYP.0000229907.58470.4c. [DOI] [PubMed] [Google Scholar]
- Chui D, Oh-Eda M, Liao YF, Panneerselvam K, Lal A, Marek KW, Freeze HH, Moremen KW, Fukuda MN, Marth JD. Alpha-mannosidase-II deficiency results in dyserythropoiesis and unveils an alternate pathway in oligosaccharide biosynthesis. Cell. 1997;90:157–167. doi: 10.1016/s0092-8674(00)80322-0. [DOI] [PubMed] [Google Scholar]
- Danielian PS, Muccino D, Rowitch DH, Michael SK, McMahon AP. Modification of gene activity in mouse embryos in utero by a tamoxifen-inducible form of Cre recombinase. Curr Biol. 1998;8:1323–1326. doi: 10.1016/s0960-9822(07)00562-3. [DOI] [PubMed] [Google Scholar]
- Druckenbrod NR, Epstein ML. The pattern of neural crest advance in the cecum and colon. Dev Biol. 2005;287:125–133. doi: 10.1016/j.ydbio.2005.08.040. [DOI] [PubMed] [Google Scholar]
- Gariepy CE, Williams SC, Richardson JA, Hammer RE, Yanagisawa M. Transgenic expression of the endothelin-B receptor prevents congenital intestinal aganglionosis in a rat model of Hirschsprung disease. J Clin Invest. 1998;102:1092–101. doi: 10.1172/JCI3702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge Y, Bagnall A, Stricklett PK, Strait K, Webb DJ, Kotelevtsev Y, Kohan DE. Collecting duct-specific knockout of the endothelin B receptor causes hypertension and sodium retention. Am J Physiol. Renal Physiol. 2006;291:F1274–80. doi: 10.1152/ajprenal.00190.2006. [DOI] [PubMed] [Google Scholar]
- Grant K, Loizidou M, Taylor I. Endothelin-1: a multifunctional molecule in cancer. Br J Cancer. 2003;88:163–166. doi: 10.1038/sj.bjc.6700750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heanue TA, Pachnis V. Enteric nervous system development and Hirschsprung’s disease: advances in genetic and stem cell studies. Nat Rev Neurosci. 2007;8:466–479. doi: 10.1038/nrn2137. [DOI] [PubMed] [Google Scholar]
- Hosoda K, Hammer RE, Richardson JA, Baynash AG, Cheung JC, Giaid A, Yanagisawa M. Targeted and natural (piebald-lethal) mutations of endothelin-B receptor gene produce megacolon associated with spotted coat color in mice. Cell. 1994;79:1267–1276. doi: 10.1016/0092-8674(94)90017-5. [DOI] [PubMed] [Google Scholar]
- Lahav R, Ziller C, Dupin E, Le Douarin NM. Endothelin 3 promotes neural crest cell proliferation and mediates a vast increase in melanocyte number in culture. Proc Natl Acad Sci U S A. 1996;93:3892–3897. doi: 10.1073/pnas.93.9.3892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy A, Rossant J, Nagy R, Abramow-Newerly W, Roder J. Derivation of completely cell culture-derived mice from early-passage embryonic stem cells. Proc. Natl. Acad. Sci. U S A. 1993;90:8424–8428. doi: 10.1073/pnas.90.18.8424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noden DM, Trainor PA. Relations and interactions between cranial mesoderm and neural crest populations. J Anat. 2005;207:575–601. doi: 10.1111/j.1469-7580.2005.00473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pla P, Larue L. Involvement of endothelin receptors in normal and pathological development of neural crest cells. Int J Dev Biol. 2003;47:315–325. [PubMed] [Google Scholar]
- Schneider MP, Boesen EI, Pollock DM. Contrasting actions of endothelin ET(A) and ET(B) receptors in cardiovascular disease. Annu Rev Pharmacol Toxicol. 2007;47:731–759. doi: 10.1146/annurev.pharmtox.47.120505.105134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- Srinivas S, Watanabe T, Lin CS, William CM, Tanabe Y, Jessell TM, Costantini F. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev Biol. 2001;1:4. doi: 10.1186/1471-213X-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas KR, Capecchi MR. Site-directed mutagenesis by gene targeting in mouse embryo-derived stem cells. Cell. 1987;51:503–512. doi: 10.1016/0092-8674(87)90646-5. [DOI] [PubMed] [Google Scholar]
- Wu JJ, Chen JX, Rothman TP, Gershon MD. Inhibition of in vitro enteric neuronal development by endothelin-3: mediation by endothelin B receptors. Development. 1999;126:1161–1173. doi: 10.1242/dev.126.6.1161. [DOI] [PubMed] [Google Scholar]