Abstract
The presence of necrosis within a diffuse glioma is a powerful predictor of poor prognosis, yet little is known of its origins. Intravascular thrombosis is a frequent finding in glioblastoma [GBM; World Health Organization (WHO) grade IV] specimens and could potentially be involved in astrocytoma progression to GBM or represent a surrogate marker of GBM histology. We investigated whether intravascular thrombosis was more frequent or prominent in GBM than other central nervous system (CNS) malignancies and considered its prognostic significance in anaplastic astrocytoma (AA; WHO grade III), which lacks necrosis. Histologic sections were examined for thrombosis, necrosis and microvascular hyperplasia from each of 297 CNS tumors, including 103 GBMs, 46 AAs, 20 diffuse astrocytoma (DAs; WHO grade II), eight anaplastic oligodendrogliomas (AOs; WHO grade III), 20 oligodendrogliomas (ODs; WHO grade II), 49 metastatic carcinomas (METs), 31 primary central nervous system lymphomas (PCNSLs) and 20 medulloblastomas (MBs). Among newly diagnosed tumors, thrombosis was present in 92% of GBM resections, significantly greater than other types of CNS malignancies. Of tumors with thrombosis, GBMs had a higher frequency of affected vessels than AAs, DAs, AOs, ODs and MBs, but had a frequency similar to METs and PCNSLs. The sensitivity of thrombosis for the diagnosis of GBM in this set of tumors was 92% and the specificity was 91%. Intravascular thrombosis was uncommon in AAs and was only noted in stereotactic biopsies. This subset of patients had shorter survivals than those AAs without thrombosis. Thus, intravascular thrombosis is more frequent in GBM than other CNS malignancies. When present in AAs, it appears to indicate aggressive clinical behavior.
Keywords: astrocytoma, brain tumor, glioblastoma, hypoxia, necrosis, thrombosis
INTRODUCTION
Glioblastoma [GBM; World Health Organization (WHO) grade IV] is the highest grade astrocytoma and is associated with a dismal prognosis 1, 8, 15. The average survival of patients with GBM is 11–12 months, even in the setting of optimal neurosurgical resection and adjuvant therapy 13, 18, 34. GBM is distinguished histopathologically from anaplastic astrocytoma (AA; WHO grade III) by the presence of either necrosis or microvascular hyperplasia. Both features can usually be identified. Cells surrounding foci of necrosis—so‐called “pseudopalisading” cells—are hypoxic and secrete pro‐angiogenic factors that promote microvascular hyperplasia and tumor expansion, explaining the intimate co‐existence of these two pathologic features and their shared prognostic significance 3, 27.
While the presence of necrosis has long been recognized as one of the most powerful predictors of poor prognosis among the diffuse gliomas, mechanisms that give rise to necrosis and the ensuing hypoxia‐induced tumor progression have not been firmly established 2, 7, 17. One model suggests that the development of necrosis could be initiated or propagated by vaso‐occlusion following intravascular thrombosis within the neoplasm 4, 5, 27. Intravascular thrombosis can often be identified grossly during the resection of GBMs and neurosurgeons are trained to favor the diagnosis of GBM over other central nervous system (CNS) malignancies when distended and thrombosed veins are seen entering or exiting an intra‐axial neoplasm (23). Careful microscopic analysis of GBM specimens has demonstrated that a large percentage of these tumors will show some degree of intra‐tumoral thrombosis (5). However, the sensitivity and specificity of intravascular thrombosis for the diagnosis of GBM, as opposed to other primary CNS malignancies, have not been formally investigated.
Moreover, the finding of intra‐tumoral thrombosis could be diagnostically and prognostically significant. If intravascular thrombosis does indeed precede the development of necrosis and microvascular hyperplasia during the progression to GBM, its presence might be expected to be a morphologic marker of aggressive clinical behavior in AA (WHO grade III), which lacks necrosis and microvascular hyperplasia, but nearly always progresses to the GBM histology over time. Alternatively, intravascular thrombosis could be a surrogate marker of necrosis and microvascular hyperplasia in GBM. In order to address these issues, we examined 103 GBMs and 194 other CNS malignancies, including AAs, diffuse astrocytoma (DAs; WHO grade II), anaplastic oligodendrogliomas, (AOs; WHO grade III), oligodendrogliomas (ODs; WHO grade II), primary central nervous system lymphomas (PCNSLs), metastatic carcinomas (METs) and medulloblastomas (MBs) for the presence and degree of intravascular thrombosis. The sensitivity and specificity of thrombosis for the diagnosis of GBM were established and the prognostic significance of intravascular thrombosis in AA was evaluated.
MATERIALS AND METHODS
A retrospective histologic analysis was performed on 297 CNS malignancies, including 103 GBMs, 46 AAs, 20 DAs, eight AOs, 20 ODs, 49 METs, 31 PCNSLs and 20 MBs. Primary sites for METs included lung (24), breast (10), ovary (2), gastrointestinal (2), kidney (1), and 10 with primary sites that could not be definitively established. All cases were retrieved from the archives of the Department of Pathology at Emory University Hospital between the years 1999 and 2006. Cases were identified based on a computerized search of the diagnoses of interest. No other selection criteria were applied. A sufficient number of cases were obtained by retrieving them retrospectively from the most recent dates. All cases were reviewed to confirm the initial diagnosis before inclusion in the study.
The criteria for tumor classification and grading were based on the World Health Organization Classification of Brain Tumors (15). In particular, GBM was diagnosed in the setting of a high grade infiltrating astrocytoma with either microvascular hyperplasia or necrosis, or both. A diagnosis of AA was established in the setting of an infiltrative astrocytoma that had more than one mitotic figures present, but lacked necrosis and microvascular hyperplasia. AO was diagnosed based on the presence of ≥6 mitoses per 10 high power field (HPF) or the presence of vascular proliferation or necrosis 10, 15. Tumors were classified as either primary or recurrent based on the absence or presence of a past medical history of a preceding brain tumor that had either progressed or recurred. Tumors were classified as either stereotactic biopsy or resection based on the type of procedure that was performed to obtain diagnostic tissue. The stereotactic biopsies were generally composed of two to five small needle cores, whereas resection specimens contained multiple pieces that were always larger than 1.0 cm in aggregate.
All tumor tissues were fixed in 10% buffered formalin, uniformly processed and sectioned at 8 µm. Our standard practice is to obtain two levels for each block for brain tumors. All frozen and permanent section slides for each case were examined. Each tumor was evaluated for the presence or absence of intravascular thrombosis, defined as vascular occlusion by an organized deposit of fibrin and platelets that generally distended the vascular lumen (see Figure 1). In those cases where thrombosis was present, the number of vessels with thrombus was counted within the entire specimen. The number of thrombosed vessels was also counted per 10 HPFs in triplicate for each tumor. In a set of five GBMs with abundant intravascular thrombosis, the diameter of involved vessels was measured in a total of 50 random vascular structures using an ocular micrometer. In this same set of tumors, we quantitated the percentage of thrombosed vessels that were arteries, veins or a component of microvascular proliferation. The presence of necrosis and microvascular hyperplasia was noted.
Figure 1.
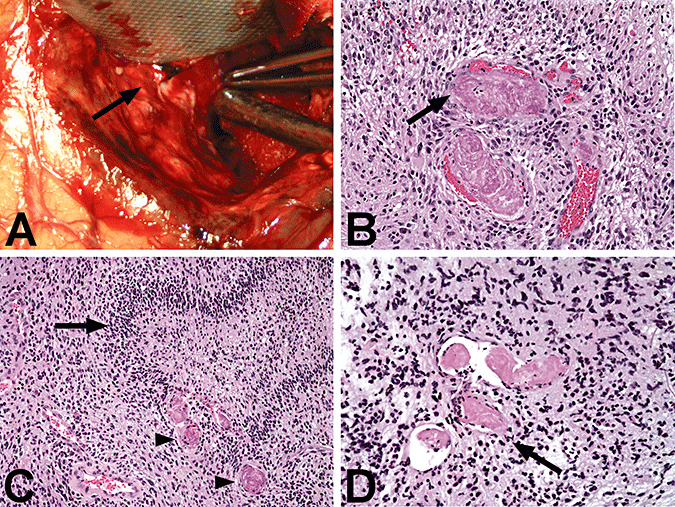
Intravascular thrombosis in central nervous system malignancies. A. Thrombosed vessels (arrow) can often be seen grossly during the neurosurgical resection of glioblastoma and is present here in the wall of the resection cavity. B. Intravascular thrombosis in glioblastoma (GBM). The vascular lumen is filled by an aggregate of platelets and fibrin that completely occludes and distends the vessel (arrow). C. Intravascular thrombosis (arrowheads) is often present in the regions of “pseudopalisading” necrosis (arrow) in GBM. D. Intravascular thrombosis (arrow) in AA is only rarely encountered and involves only a few vessels in those tumors that are affected.
Given our hypothesis that intravascular thrombosis may initiate or propagate necrosis or represent a surrogate marker of GBM, we were interested in determining whether intravascular thrombosis might be a feature associated with a poor prognosis in AAs. The survival of patients with newly diagnosed AA was determined through clinical records and the Social Security Death Index (http://ssdi.rootsweb.com).
Statistical analyses were performed using a two‐sided student's t‐test for comparisons of continuous variables and χ2‐tests for comparing proportions, with statistical significance defined as a P‐value <0.05.
RESULTS
We performed a histologic review of 297 CNS malignancies, including 103 GBMs, 46 AAs, 20 DAs, eight AOs, 20 ODs, 49 METs, 31 PCNSLs and 20 MBs. The demographic characteristics of patients in this study were similar to those with these diseases in the general population (Table 1).
Table 1.
Tumor types and patient demographics for included cases. Abbreviations: CNS = central nervous system; M = male; F = female; PCNSL = primary central nervous system lymphoma.
| CNS Malignancy | Total number | Age range (median), years | M : F |
|---|---|---|---|
| Glioblastoma | 103 | 16–89 (59) | 1.7:1 |
| Anaplastic Astrocytoma | 46 | 9–89 (51) | 1.5:1 |
| Diffuse Astrocytoma | 20 | 24–73 (41) | 3:1 |
| Anaplastic Oligodendroglioma | 8 | 41–74 (44) | 1:3 |
| Oligodendroglioma | 20 | 22–66 (43.5) | 1:1.6 |
| Metastatic carcinoma | 49 | 42–85 (57) | 1:1.5 |
| PCNSL | 31 | 35–83 (63) | 1:1.1 |
| Medulloblastoma | 20 | 4–38 (9) | 1.9:1 |
Glioblastoma
The 103 GBMs included 88 that were primary (the patient's initial diagnosis) and 15 that were post‐treatment recurrences. Cases included 72 resections and 31 stereotactic biopsies from 65 men and 38 women, with ages ranging from 16 to 89 years (median, 59 years). Out of the 88 primary cases, 60 were resections and 28 were biopsies. Among primary GBM resection specimens, intravascular thrombosis was identified in 92% and the number of vessels involved by thrombosis ranged from one to 60 (mean ± SEM, 21.0 ± 2.2 vessels) (1.8 ± 0.2 vessels/10HPF) (Table 2) (Figure 1). This same set of tumors showed necrosis in 98% and microvascular hyperplasia in 97%. Among stereotactic biopsies of primary GBMs, 57% showed intravascular thrombosis, while 86% showed necrosis and 89% showed microvascular hyperplasia. The number of vessels involved in those stereotactic biopsies that had thrombosis was lower than resection specimens (range, 1–18 vessels; mean ± SEM, 5.0 ± 1.0). Of the primary GBM specimens (biopsies and resections) with thrombosis, 99% had necrosis and 99% had microvascular hyperplasia. Intravascular thrombosis was found in the frozen section slides of 45% of primary GBM resections and 14% of primary biopsies. Only 33% of post‐treatment, recurrent GBMs showed intravascular thrombosis (Table 3). Necrosis was found in 93% recurrent GBMs and microvascular hyperplasia was identified in 93%. The presence of thrombosis in microvascular proliferation accounted for 40% of the total and thrombosis of stable vessels accounted for 60%. Among stable vessels, thrombosis occurred more frequently in veins (70%) than in arteries (30%). The diameter of thrombosed vessels ranged from 20 to 400 µm with a mean ± SEM of 87.4 ± 11.4 µm.
Table 2.
Intravascular thrombosis in CNS malignancies. Only resection specimens from newly diagnosed neoplasms are shown. Abbreviations: CNS = central nervous system; HPF = high power field; PCNSL = primary central nervous system lymphoma.
| CNS Malignancy | Number of primary resection specimens | Intravascular thrombosis (%) | Number of involved vessels mean ± SEM (range) | Mean number of involved vessels/10HPF (range) |
|---|---|---|---|---|
| Glioblastoma | 60 | 92 | 21.0 ± 2.2 (1–60) | 1.8 ± 0.2 (0.3–6.0) |
| Anaplastic Astrocytoma | 11 | 0* | 0 | 0 |
| Diffuse Astrocytoma | 4 | 0* | 0 | 0 |
| Anaplastic Oligodendroglioma | 4 | 25* | 6 | 6 (0.6) |
| Oligodendroglioma | 8 | 0* | 0 | 0 |
| Metastatic carcinoma | 49 | 10* | 16.4 ± 8.9 (2–50) | 1.6 ± 0.9 (0.5–5) |
| PCNSL | 31 | 6* | 15 ± 5 (7–20) | 1.3 ± 0.3 (1.0–1.5) |
| Medulloblastoma | 20 | 15* | 10 ± 2.9 (5–20) | 0.8 ± 0.2 (0.3–1.0) |
P < 0.05, χ2‐test, with comparison with glioblastoma.
Table 3.
Comparison of intravascular thrombosis in primary (newly diagnosed) resections, primary biopsies, and recurrent tumors for GBM and AA.
| Glioblastoma (GBM) (n) | Intravascular thrombosis (%) | Anaplastic astrocytoma (AA) (n) | Intravascular thrombosis (%) | |
|---|---|---|---|---|
| Primary resections | 60 | 92 | 11 | 0 |
| Primary biopsies | 28 | 57* | 25 | 16 |
| Recurrent tumors | 15 | 33* | 10 | 10† |
P < 0.05, χ2‐test, with comparison with GBM resections.
Non‐significant difference; P > 0.05, χ2‐test, with comparison with AA resections.
Anaplastic astrocytoma
A total of 46 AAs were reviewed, consisting of 36 primary and 10 recurrent tumors. Tumors were from 28 men and 18 women with ages ranging from 9 to 89 years (median, 51 years) and included 12 resections and 34 biopsies. Intravascular thrombosis was identified in 16% (4/25) of primary AA biopsies (Figure 1) (Table 3). No thrombosis was identified in the 11 AA primary resections (0%). Among the four primary AA biopsies with thrombosis, the number of involved vessels ranged from two to six (mean ± SEM, 3.5 ± 1.0) (0.6 ± 0.2 vessels/10HPF). Intravascular thrombosis was found in the frozen section slides of 6% of primary AA biopsies, but not in any primary AA resections (0/11). One case (10%) of recurrent AAs showed intravascular thrombosis and only one vessel was involved in this case. By definition, no necrosis or microvascular hyperplasia was identified in AAs.
Diffuse astrocytoma
A total of 20 DAs were reviewed, which were all primary tumors. Tumors were from 15 men and 5 women with ages ranging from 24 to 73 years (median, 41 years) and included four resections and 16 biopsies. No intravascular thrombosis was identified in these 20 cases (0%).
Anaplastic oligodendroglioma
A total of eight AOs were reviewed. These tumors were resected (7) or biopsied (1) from two men and six women with ages ranging from 41 to 74 years (median, 44 years). There were five primary and three recurrent tumors. Intravascular thrombosis was identified in 25% of primary resections (1/4). This single AO with thrombosis had classic morphology and showed 1p/19q loss by fluorescence in situ hybridization. No thrombosis was identified in the primary biopsies (0%). In the primary resection specimen with thrombosis, the number of total involved vessels was six (0.6 vessels/10 HPF). Both necrosis and vascular proliferation were present in addition to thrombosis. Two vessels showed intravascular thrombosis in the frozen section slides from this case. In the seven cases without thrombosis, two had necrosis and five had vascular proliferation. One of the recurrent AOs showed intravascular thrombosis and four vessels (0.3/10 HPF) were involved.
Oligodendroglioma (WHO grade II)
A total of 20 ODs were reviewed, consisting of 15 primary and five recurrent tumors. Tumors were from eight men and 12 women with ages ranging from 22 to 66 years (median, 43.5 years) and included 13 resections and seven biopsies. No intravascular thrombosis was identified in these cases.
Metastatic carcinoma
The 49 METs were from 19 men and 30 women ranging in age from 42 to 85 years (median, 57 years). None of these tumors had been previously resected or treated with radiation. All specimens were derived from surgical resections rather than stereotactic biopsies. Intravascular thrombosis was identified in 10% of METs. The number of vessels involved ranged from two to 50 (mean ± SEM, 16.4 ± 8.9 vessels) (1.6 ± 0.9 vessels/10HPF). Necrosis and microvascular hyperplasia were identified in 96% and 8% of cases, respectively. Among METs with thrombosis, necrosis was present in 100%, while microvascular hyperplasia was not present in any (0%). Intravascular thrombosis was present in the frozen section slides of 4% of METs.
Primary central nervous system lymphomas
Thirty‐one PCNSLs were reviewed. These tumors came from 15 men and 16 women with an age range of 35–83 years (median, 63 years). None were recurrent or post‐treatment tumors. Intravascular thrombosis was identified in 6% of cases and, among them, the number of vessels involved ranged from seven to 20 (mean ± SEM, 15.0 ± 5.0 vessels) (1.3 ± 0.3 vessels/10HPF). Necrosis and microvascular hyperplasia were identified in 35% and 6% of cases, respectively. Among those tumors with thrombosis, 100% had necrosis, while none contained microvascular hyperplasia (0%). Intravascular thrombosis was present in the frozen section slides of 6 % of PCNSL.
Medulloblastoma
A total of 20 MBs were reviewed that occurred in 13 men and seven women with ages ranging from 4 to 38 (median, 9 years). All MBs were derived from surgical resection rather than stereotactic biopsy. None of these were recurrences or post‐treatment tumors. Intravascular thrombosis was identified in 15% of cases. The number of vessels involved ranged from 5 to 20 (mean ± SEM, 10.0 ± 2.9 vessels) (0.8 ± 0.2 vessels/10HPF). Necrosis and microvascular hyperplasia were identified in 25% and 35% of cases, respectively. Among those tumors with thrombosis, 33% had necrosis and 66% had microvascular hyperplasia. Intravascular thrombosis was not present in the frozen section slides of any MB (0/20).
Correlation
GBMs showed the highest frequency of intravascular thrombosis among CNS malignancies (Table 2). For the sake of valid comparison, we based our statistical analysis on resection specimens of primary (non‐recurrent, non‐treated) tumors. Intravascular thrombosis was present in 92% of GBMs, 0% of AAs, 0% of DAs, 25% of AOs, 0% of ODs, 10% of METs, 6% of PCNSLs and 15% of MBs. χ2‐tests revealed that the frequency of thrombosis in GBM was statistically greater than that in other tumor types (P < 0.05).
Among those resection specimens with thrombosis, GBMs had significantly more affected vessels (1.8 ± 0.2 vessels/10HPF) than MBs (0.8 ± 0.2 vessels/10HPF), AOs (0.6 vessels/10HPF), ODs (0), AAs (0), DAs (0), but had a similar number of involved vessels as METs (1.6 ± 0.9 vessels/10HPF) and PCNSLs (1.3 ± 0.3 vessels/10HPF).
The sensitivity of intravascular thrombosis for the diagnosis GBM among the primary resection specimens included in this study was 92% and the specificity was also 91%. The positive predictive value of intravascular thrombosis for the diagnosis of GBM was 83% and the negative predictive value was 96%.
We were interested in determining if intravascular thrombosis in AA was associated with a more aggressive clinical behavior, as it has been suggested that thrombosis may precede or promote the development of necrosis and microvascular hyperplasia during the progression from AA to GBM (27). Five of the AAs in this investigation had thrombosis, whereas 41 did not. The clinical features of patients with AA with and without thrombosis are shown in Table 4. The patients with AAs with thrombosis were slightly older than those without and there were more female patients. The MRI studies of patients with AA with thrombosis demonstrated cerebral hemispheric masses centered in the frontal (3) or parietal (2) lobes that were hyperintense on T2 or fluid attenuation inversion recovery (FLAIR) sequences, with mild or moderate levels of contrast enhancement (Figure 2A). No “ring‐like” contrast‐enhancement with central necrosis was seen in these five cases. We compared the survivals of patients with newly diagnosed AA with (n = 4) and without thrombosis (n = 32). Of the four patients with AAs that showed intravascular thrombosis, all four (100%) died within 1 year of diagnosis (mean survival 6.3 months) (Table 5). The one patient with a recurrent AA that showed thrombosis had been previously treated with radiation therapy. This patient is alive at last follow‐up (17 months). Among the 32 patients with newly diagnosed AA that did not show thrombosis, six died within 1 year (19%; χ2‐test, P < 0.05) with a mean follow‐up time of 45 months.
Table 4.
Patient demographics and specimen types for AAs with intravascular thrombosis and AAs without intravascular thrombosis. Abbreviations: AA = anaplastic astrocytoma; M = male; F = female.
| AA with intravascular thrombosis (n = 5) | AA without intravascular thrombosis (n = 41) | |
|---|---|---|
| Age range (median) | 30–89 (65.5) | 9–89 (51.5) |
| M : F | 1:4 | 1.9:1 |
| Biopsy : resection | 4:1 | 1.7:1 |
| Primary : recurrence | 4:1 | 3.6:1 |
| Neuroimaging | T2‐hyperintense, mild to moderate contrast enhancement | T2‐hyperintense, mild to moderate contrast enhancement |
Figure 2.
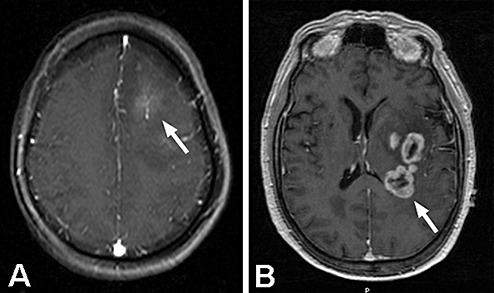
A. Post‐contrast, axial MRI of a patient with anaplastic astrocytoma (AA) that showed microscopic evidence of intravascular thrombosis in the biopsy specimen. The MRI shows mild contrast‐enhancement of the neoplasm (arrow) with surrounding T1‐hypointensity, typical of AA. B. Post‐contrast, axial MRI of a patient with glioblastoma, demonstrating the pattern of “ring‐enhancement” surrounding central necrosis that is characteristic of this disease (arrow).
Table 5.
Comparison of patient survival at one year between patients with newly diagnosed AA with and without thrombosis. Abbreviations: IVT = intravascular thrombosis.
| Anaplastic astrocytoma | Total number | Dead at 1 year (%) | Alive at most recent follow‐up |
|---|---|---|---|
| Cases with IVT | 4 | 4/4 (100%) | 0/4; mean survival, 6.3 months |
| Cases without IVT | 32 | 6/32 (19%)* | 26/32; mean follow‐up, 45 months |
P < 0.05, χ2‐test, with comparison with anaplastic astrocytoma with thrombosis.
DISCUSSION
Previous studies have suggested that vaso‐occlusion and thrombosis may precede the development of necrosis during the biological progression of lower grade astrocytomas to GBM (27). However, there has been no systematic study of the frequency, specificity or diagnostic significance of thrombosis in GBM as compared with other CNS malignancies. In the present study, we investigated a diverse group of malignant brain tumors for the histologic presence of intravascular thrombosis and found that it was much more frequent and prominent in GBM than other tumor types. The sensitivity of thrombosis for the diagnosis of GBM was 92% among primary resection specimens in this study, similar to the value of 94% noted in a previous study (5). Thrombosis was less frequently noted in stereotactic biopsies of GBM (57%), most likely because of sampling considerations, and in resection specimens derived from post‐treatment recurrences (33%) for unknown reasons. Other CNS malignancies, including AA, DA, AO, OD, MET, PCNSL and MB, did not show a high frequency of thrombosis, leading to a relatively high specificity (91%) of intravascular thrombosis for the diagnosis of GBM in this set of malignancies.
The relationship between human malignancy and pathologic coagulation has been recognized since the observations of trousseau in the 18th century 22, 24, 37. Compared with the general population, patients with cancer have a fourfold increased risk of developing deep venous thrombosis (DVT). Patients with GBM have among the highest incidence of systemic thrombosis—nearly 30% develop DVTs or pulmonary embolism within a year (35). Less well appreciated is the strong tendency for intravascular thrombosis to develop within GBMs, which we have clearly demonstrated in the present study (27). Neurosurgeons have been taught that the intra‐operative finding of distended, thrombosed blood vessels within a cerebral hemispheric mass favors the diagnosis of GBM over other brain tumors (23). Neuropathologists, however, have only rarely commented on this finding. Some early studies suggested that thrombosis might lead directly to microvascular hyperplasia through active vascular reorganization and recanalization (33). Others noted that thrombosis is typically located centrally in GBM, near coagulative necrosis, and is geographically separate from microvascular hyperplasia, which is more peripheral 28, 31.
The current WHO Classification of Brain Tumors mentions intravascular thrombosis as one of many diverse histologic findings in its definition of GBM (15). However, diagnostic criteria for GBM currently include necrosis or microvascular hyperplasia within a high grade astrocytoma, but not thrombosis. It is possible that the potential diagnostic and prognostic significance of thrombosis has been unrecognized or masked because of the nearly constant coexistence of necrosis, one of the most powerful markers of poor prognosis in diffuse gliomas 7, 17. In the present study, for example, 99% of newly diagnosed GBMs with thrombosis also had necrosis. To date, thrombosis has not been systematically investigated as an independent prognostic marker among the diffuse gliomas 9, 14, 25.
Multiple factors likely contribute to the high degree of intravascular thrombosis in GBM, including abnormal blood flow within a distorted vasculature, increased interstitial pressure, dysregulation of pro‐ and anti‐ coagulant factors, and increased access of plasma clotting factors to tumoral tissue because of leakiness or breakdown of the blood‐brain barrier 29, 30. One of the body's most potent prothrombotic proteins, tissue factor, is highly upregulated in astrocytomas and its expression correlates with tumor grade 11, 12. Genetic events that occur during the transition from AA to GBM, such as PTEN loss and the epidermal grow factor receptor overexpression, have been shown to upregulate tissue factor 22, 26. Together, these events likely promote a prothrombotic environment in GBM that is not present to the same degree in other CNS malignancies.
Until recently, the presence of thrombosis within GBM has been viewed as an uninteresting epiphenomenon and largely neglected from a biological perspective. The view that intravascular thrombosis could be mechanistically important to biologic progression of astrocytomas received more attention once it was recognized that hypoxia is a critical driving force in tumor biology 32, 36. Vaso‐occlusion secondary to thrombosis could lead directly to the development of hypoxia, necrosis and the resultant hypoxia‐induced microvascular hyperplasia that defines GBM histologically 4, 27 (Figure 3). One study identified intravascular thrombosis within a subset of “pseudopalisading” cells surrounding necrosis, and suggested that these hypercellular configurations arose from the hypoxic migration of tumor cells away from central vaso‐occlusion and toward a viable vasculature 5, 36. These pseudopalisades are hypoxic, express hypoxia inducible factor‐1α, and secrete hypoxia‐inducible pro‐angiogenic factors including VEGF and IL‐8 6, 20, 36. The resulting wave of peripheral tumor migration and angiogenesis leads to the neuro‐imaging finding of a rapidly growing, contrast‐enhancing mass and to the aggressive clinical behavior of GBM.
Figure 3.
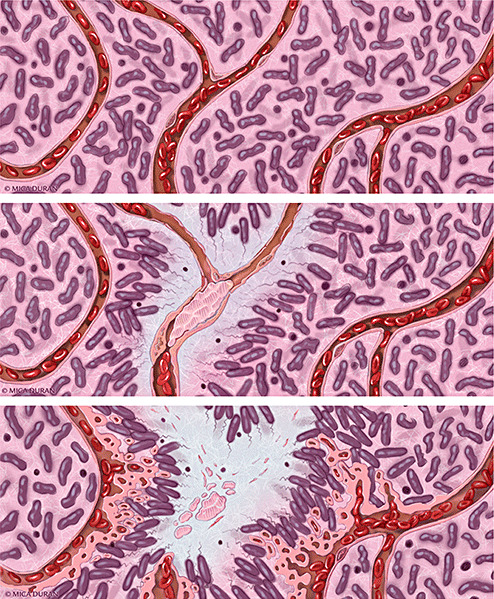
Schematic representation of a proposed model of progression of anaplastic astrocytoma (AA; WHO grade III) to glioblastoma (upper panel). In AA, tumor cells infiltrate through central nervous system parenchyma, receiving oxygen and nutrients from intact native blood vessels (middle panel). A vascular insult occurs, causing endothelial injury, vascular leakiness and intravascular thrombosis. Thrombotic vaso‐occlusion leads to tumor cell migration away from hypoxia and toward a viable vasculature, creating a peripherally moving wave that is seen microscopically as pseudopalisading cells (lower panel). The zone of hypoxia and central necrosis expands, while hypoxic tumor cells of pseudopalisades secrete pro‐angiogenic factors (VEGF, IL‐8) that induce microvascular proliferation in adjacent regions, supporting an accelerated outward expansion of tumor. Illustration by Mica Duran.
If intravascular thrombosis does precede the development of necrosis and microvascular hyperplasia, it would be expected that a subset of AAs (WHO grade III) would have intravascular thrombosis, but not necrosis or microvascular hyperplasia (by definition). We found that 11% of the AAs in this study (including primary and recurrent tumors, biopsies and resections) showed histologic evidence of intravascular thrombosis. The degree of thrombosis in these AAs was low, generally with only a few affected vessels. Neuroimaging of AAs with thrombosis showed T2‐ or FLAIR‐hyperintensity with mild to moderate enhancement following the administration of contrast, but no “ring‐enhancing” patterns of enhancement suggestive of central necrosis and the diagnosis of GBM (19).
Importantly, we found that all four patients (100%) with newly diagnosed AAs that contained thrombosis had survivals less than 1 year, significantly shorter than the usual for this disease. Most survival analyses report median overall survivals between 24 and 48 months for AAs 9, 16, 21. Among the 32 patients that had AA without thrombosis, only six died within 1 year (19%), which is more characteristic of AA behavior. One possibility is that the AAs with thrombosis were actually undersampled GBMs in which the biopsy needle missed the necrosis or microvascular hyperplasia that was present in the tumor. This possibility should be seriously considered, as thrombosis was only seen in the stereotactic biopsies of AA in this study and was not seen in the AA resection specimens, suggesting that these small biopsies might indeed represent GBMs that were incompletely biopsied. If this is true, the histologic finding of thrombosis might serve as a surrogate marker for necrosis or microvascular hyperplasia in GBM and this possibility deserves further investigation. Another explanation is that these AAs with thrombosis were truly AAs, without necrosis or vascular proliferation, and that thrombosis is a marker of aggressive behavior in this type of tumor, potentially indicating the imminent development of necrosis and progression to GBM. In either case, thrombosis seems to indicate rapid progression. These same findings could be relevant to ODs as well; however our current study did not have a sufficient number of AOs to draw any firm conclusions.
This small study of intravascular thrombosis in AAs was not constructed as a univariate or multivariate analysis of prognostic markers. Our clinical follow‐up is not complete and other factors important to survival, such as patient age, clinical performance status and treatment, were not appropriately controlled. Nonetheless, the short survivals noted in this small subset of patients with AA suggests that intravascular thrombosis may be a marker of aggressive behavior and should be studied in a larger, more comprehensive investigation of prognostic markers in AA.
ACKNOWLEDGMENTS
The authors would like to thank Mica Duran for her excellent illustrations in this manuscript. This project was supported in part by US Public Health Service National Institutes of Health (NIH) award NS053727 (DJB).
REFERENCES
- 1. CBTRUS (2005). Statistical Report: Primary Brain Tumors in the United States, 1998–2002. The Central Brain Tumor Registry of the United States: Hinsdale, IL. [Google Scholar]
- 2. Barker FG 2nd, Davis RL, Chang SM, Prados MD (1996) Necrosis as a prognostic factor in glioblastoma multiforme. Cancer 77:1161–1166. [DOI] [PubMed] [Google Scholar]
- 3. Brat DJ, Mapstone TB (2003) Malignant glioma physiology: cellular response to hypoxia and its role in tumor progression. Ann Intern Med 138:659–668. [DOI] [PubMed] [Google Scholar]
- 4. Brat DJ, Van Meir EG (2004) Vaso‐occlusive and prothrombotic mechanisms associated with tumor hypoxia, necrosis, and accelerated growth in glioblastoma. Lab Invest 84:397–405. [DOI] [PubMed] [Google Scholar]
- 5. Brat DJ, Castellano‐Sanchez AA, Hunter SB, Pecot M, Cohen C, Hammond EH et al (2004) Pseudopalisades in glioblastoma are hypoxic, express extracellular matrix proteases, and are formed by an actively migrating cell population. Cancer Res 64:920–927. [DOI] [PubMed] [Google Scholar]
- 6. Brat DJ, Bellail AC, Van Meir EG (2005) The role of interleukin‐8 and its receptors in gliomagenesis and tumoral angiogenesis. Neuro-oncol 7:122–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Burger PC, Green SB (1987) Patient age, histologic features, and length of survival in patients with glioblastoma multiforme. Cancer 59:1617–1625. [DOI] [PubMed] [Google Scholar]
- 8. Burger PC, Scheithauer BW, Vogel FS (2002) Surgical Pathology of the Nervous System and Its Coverings. Churchill Livingstone: New York. [Google Scholar]
- 9. Daumas‐Duport C, Scheithauer B, O'Fallon J, Kelly P (1988) Grading of astrocytomas. A simple and reproducible method. Cancer 62:2152–2165. [DOI] [PubMed] [Google Scholar]
- 10. Giannini C, Scheithauer BW, Weaver AL, Burger PC, Kros JM, Mork S et al (2001) Oligodendrogliomas: reproducibility and prognostic value of histologic diagnosis and grading. J Neuropathol Exp Neurol 60:248–262. [DOI] [PubMed] [Google Scholar]
- 11. Guan M, Jin J, Su B, Liu WW, Lu Y (2002) Tissue factor expression and angiogenesis in human glioma. Clin Biochem 35:321–325. [DOI] [PubMed] [Google Scholar]
- 12. Hamada K, Kuratsu J, Saitoh Y, Takeshima H, Nishi T, Ushio Y (1996) Expression of tissue factor in glioma. Noshuyo Byori 13:115–118. [PubMed] [Google Scholar]
- 13. Homma T, Fukushima T, Vaccarella S, Yonekawa Y, Di Patre PL, Franceschi S, Ohgaki H (2006) Correlation among pathology, genotype, and patient outcomes in glioblastoma. J Neuropathol Exp Neurol 65:846–854. [DOI] [PubMed] [Google Scholar]
- 14. Kernohan JW, Mabon RF, Svien HJ, Adson AW (1949) A simplified classification of gliomas. Proc Staff Meet Mayo Clin 24:71–75. [PubMed] [Google Scholar]
- 15. Louis D, Ohgaki H, Weistler O, Cavenee W (2007) WHO Classification of Tumours of the Central Nervous System. IARC Press, Lyon. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Miller SJ, Rangwala F, Williams J, Ackerman P, Kong S, Jegga AG et al (2006) Large‐scale molecular comparison of human schwann cells to malignant peripheral nerve sheath tumor cell lines and tissues. Cancer Res 66:2584–2591. [DOI] [PubMed] [Google Scholar]
- 17. Nelson JS, Tsukada Y, Schoenfeld D, Fulling K, Lamarche J, Peress N (1983) Necrosis as a prognostic criterion in malignant supratentorial, astrocytic gliomas. Cancer 52:550–554. [DOI] [PubMed] [Google Scholar]
- 18. Ohgaki H, Kleihues P (2005) Population‐based studies on incidence, survival rates, and genetic alterations in astrocytic and oligodendroglial gliomas. J Neuropathol Exp Neurol 64:479–489. [DOI] [PubMed] [Google Scholar]
- 19. Osborn AG (2004) Diagnostic Imaging. Amirsys: Salt Lake City. [Google Scholar]
- 20. Plate KH, Breier G, Weich HA, Risau W (1992) Vascular endothelial growth factor is a potential tumour angiogenesis factor in human gliomas in vivo. Nature 359:845–848. [DOI] [PubMed] [Google Scholar]
- 21. Prados MD, Seiferheld W, Sandler HM, Buckner JC, Phillips T, Schultz C et al (2004) Phase III randomized study of radiotherapy plus procarbazine, lomustine, and vincristine with or without BUdR for treatment of anaplastic astrocytoma: final report of RTOG 9404. Int J Radiat Oncol Biol Phys 58:1147–1152. [DOI] [PubMed] [Google Scholar]
- 22. Rak J, Yu JL, Luyendyk J, Mackman N (2006) Oncogenes, trousseau syndrome, and cancer‐related changes in the coagulome of mice and humans. Cancer Res 66:10643–10646. [DOI] [PubMed] [Google Scholar]
- 23. Raza SM, Lang FF, Aggarwal BB, Fuller GN, Wildrick DM, Sawaya R (2002) Necrosis and glioblastoma: a friend or a foe? A review and a hypothesis. Neurosurgery 51:2–12; discussion 12–13. [DOI] [PubMed] [Google Scholar]
- 24. Rickles FR, Falanga A (2001) Molecular basis for the relationship between thrombosis and cancer. Thromb Res 102:V215–V224. [DOI] [PubMed] [Google Scholar]
- 25. Ringertz N (1950) Grading of gliomas. Acta Pathol Microbiol Scand 27:51–64. [PubMed] [Google Scholar]
- 26. Rong Y, Post DE, Pieper RO, Durden DL, Van Meir EG, Brat DJ (2005) PTEN and hypoxia regulate tissue factor expression and plasma coagulation by glioblastoma. Cancer Res 65:1406–1413. [DOI] [PubMed] [Google Scholar]
- 27. Rong Y, Durden DL, Van Meir EG, Brat DJ (2006) Pseudopalisading' necrosis in glioblastoma: a familiar morphologic feature that links vascular pathology, hypoxia, and angiogenesis. J Neuropathol Exp Neurol 65:529–539. [DOI] [PubMed] [Google Scholar]
- 28. Russell DS, Rubinstein LJ (1959) Pathology of Tumors of the Nervous System. Edward Arnold: London. [Google Scholar]
- 29. Sawaya R, Ramo OJ, Shi ML, Mandybur G (1991) Biological significance of tissue plasminogen activator content in brain tumors. J Neurosurg 74:480–486. [DOI] [PubMed] [Google Scholar]
- 30. Sawaya R, Yamamoto M, Ramo OJ, Shi ML, Rayford A, Rao JS (1995) Plasminogen activator inhibitor‐1 in brain tumors: relation to malignancy and necrosis. Neurosurgery 36:375–380; discussion 380–381. [DOI] [PubMed] [Google Scholar]
- 31. Scherer HJ (1935) Gliomstudien III. Angioplastiche Gliome. Virchows Arch 294:823. [Google Scholar]
- 32. Semenza GL (2002) HIF‐1 and tumor progression: pathophysiology and therapeutics. Trends Mol Med 8:S62–S67. [DOI] [PubMed] [Google Scholar]
- 33. Storring FK, Duguid JB (1954) The vascular formations in glioblastoma. J Pathol Bacteriol 68:231–233. [DOI] [PubMed] [Google Scholar]
- 34. Stupp R, Mason WP, Van Den Bent MJ, Weller M, Fisher B, Taphoorn MJ et al (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352:987–996. [DOI] [PubMed] [Google Scholar]
- 35. Walsh DC, Kakkar AK (2001) Thromboembolism in brain tumors. Curr Opin Pulm Med 7:326–331. [DOI] [PubMed] [Google Scholar]
- 36. Zagzag D, Zhong H, Scalzitti JM, Laughner E, Simons JW, Semenza GL (2000) Expression of hypoxia‐inducible factor 1alpha in brain tumors: association with angiogenesis, invasion, and progression. Cancer 88:2606–2618. [PubMed] [Google Scholar]
- 37. Zwicker JI, Furie BC, Furie B (2007) Cancer‐associated thrombosis. Crit Rev Oncol Hematol 62:126–136. [DOI] [PubMed] [Google Scholar]


