Abstract
αB-Crystallin (CryAB) is the most abundant small heat shock protein (HSP) constitutively expressed in cardiomyocytes. Gain- and loss-of-function studies demonstrated that CryAB can protect against myocardial ischemia/reperfusion injury. However, the role of CryAB or any HSPs in cardiac responses to mechanical overload is unknown. This study addresses this issue. Non-transgenic (NTG) mice and mice with cardiomyocyte-restricted transgenic overexpression of CryAB (TG) or with germ-line ablation of the CryAB/HSPB2 genes (KO) were subjected to transverse aortic constriction (TAC) or sham surgery. Two weeks later, cardiac responses were analyzed by fetal gene expression profiling, cardiac function analyses, and morphometry. Comparison among the 3 sham surgery groups reveals that CryAB overexpression is benign whereas the KO is detrimental to the heart as reflected by cardiac hypertrophy and malfunction at 10 weeks of age. Compared to NTG mice, TG mouse hearts showed significantly reduced NFAT transactivation and attenuated cardiac hypertrophic responses to TAC but unchanged cardiac function, whereas NFAT transactivation was significantly increased in cardiac and skeletal muscle of the KO mice at baseline and they developed cardiac insufficiency at 2 weeks after TAC. CryAB overexpression in cultured neonatal rat cardiomyocytes significantly attenuated adrenergic stimulation-induced NFAT transactivation and hypertrophic growth. We conclude that CryAB suppresses cardiac hypertrophic responses likely through attenuating NFAT signaling and CryAB and/or HSPB2 are essential for normal cardiac function.
Keywords: heat shock proteins, hypertrophy, the nuclear factors of activated T-cells (NFAT), myocyte-enriched calcineurin interacting protein-1 (MCIP1), heart function, fetal genes
αB-Crystallin (CryAB), also known as heat shock protein (HSP) B5, belongs to the small HSP (sHSP) subfamily. The CryAB gene and another sHSP (HSPB2) gene reside head-to-head in chromosome 9 in mice. Controlled by the shared bidirectional promoter, CryAB and HSPB2 are co-expressed in mammalian hearts.1, 2 CryAB is the most abundant sHSP in cardiomyocytes.1 The molecular chaperone properties of CryAB may entail prevention of stress-induced aggregation of denaturing proteins as well as trapping aggregation-prone proteins in large, soluble, multimeric reservoirs.3, 4
Studies on cardiac role(s) of HSPs including CryAB are focused on their effects on ischemic or oxidative forms of stress.5 Ex vivo perfused hearts from transgenic mice that ubiquitously overexpress CryAB tolerated ischemia/reperfusion (I/R) better.6 By contrast, CryAB/HSPB2 null mouse hearts displayed poorer functional recovery, a higher cell death rate,7 increased stiffness and hence poor relaxation of myocardium following I/R,8 compared to wild type controls. CryAB was found to interact with mitochondria.9 Mitochondrial permeability transition and calcium uptake were increased in cardiomyocytes from CryAB/HSPB2 null mice.10 Interestingly, HSP20 was recently shown to attenuate isoproterenol infusion induced cardiac remodeling.11 However, the potential role(s) of HSPs in general in cardiac response to mechanical stress has rarely been investigated. As a bona fide sHSP in the heart, CryAB associates with the cytoskeleton and contractile apparatus in cardiac myocytes.12 Both the cytoskeleton and the contractile apparatus play important roles and undergo dramatic remodeling in cardiac response to mechanical overload. Hence, CryAB may modulate this response.
Pressure overload as seen in hypertension and aortic stenosis is an important cause of congestive heart failure (CHF). Cardiac hypertrophy is the most powerful cardiac response to increased workload but increased cardiac mass has been more recently recognized as an independent risk factor for poor prognosis.13 Protein quality control (PQC) is accomplished by the collaboration between molecular chaperones and selective proteolysis in the cell.4 In terminally differentiated cardiomyocytes, PQC is undoubtedly essential for survival and normal function.4 Transgenic expression of human (cardio)myopathy linked missense (R120G) mutant CryAB compromises PQC in the heart and causes CHF.14-16 Multiple lines of evidence point to an increasingly attractive hypothesis that inadequacy in PQC plays an important role in cardiac remodeling and failure.17 Using both gain- and loss-of-function approaches, the present study has tested and proven the hypothesis that sHSP CryAB suppresses cardiac hypertrophic responses to pressure overload and delays progression to cardiac failure likely through inhibiting the calcineurin-NFAT signaling pathway.
Materials and Methods
An expanded Materials and Methods section can be found in online supplements.
Experimental animals
FVB/N transgenic mice with cardiomyocyte-restricted overexpression of CryAB (TG), the NFAT binding site-dependent luciferase reporter (NFAT-Luc) mice, and the CryAB/HSPB2 double knockout mice (KO) were previously characterized.14, 18, 19 The KO mice used here were derived from 8 generations of back-crossing of the original 129/Svj KO mice into the FVB/N background. Institutional guidelines were followed in the care and use of animals.
Transverse aortic constriction (TAC)
TAC or the sham surgery was performed on 8 weeks old male mice as previously described with minor modifications.20 A 29G needle (OD 0.33mm) was used as the mode of TAC. Sham control mice underwent the same procedure except for aortic constriction.
Transthoracic echocardiography (Echo)
Echo was performed using a high resolution Vevo 770™ Echo system with a 30MHz transducer (Visual Sonics, Toronto, Canada).
Left ventricular (LV) catheterization and pressure measurements
Close-chest LV pressure and its derivatives were recorded using a Powerlab data acquisition system (ADInstruments, Colorado Springs, CO) and a high-fidelity 1.4F Millar Mikro-Tip® catheter transducer (model SPR-835, Millar Instruments, TX) placed into the LV chamber via the right common carotid artery.
RNA analyses
RNA dot blot analysis and semi-quantitative reverse transcription PCR (RT-PCR) were done as described.21, 22
In vitro studies of cardiomyocyte hypertrophy
Neonatal rat cardiomyocyte (NRCM) culture and adenoviral infection were performed as described.23 Recombinant adenoviruses harboring a GFP-fused NFATc1 (Ad-NFAT-GFP) or a constitutively active form of mouse calcineurin Aα (Ad-CnAΔ) and Ad-WT-CryAB were described previously.15, 24-26 Concomitant infections of myocytes with Ad-NFAT-GFP and Ad-WT-CryAB were performed at MOIs of 100 and 50, respectively.24, 25 Twenty-four hours after Ad-NFAT-GFP infection, cells were treated with various adrenergic agonists or 100μM of L-ascorbic acid (AA) vehicle in serum-free medium for 48 hours. The profile areas of individual cardiomyocytes identified by positive staining of F-actin with Alexa Fluor-568 conjugated phalloidin (Molecular Probes, Eugene, OR) were measured from digitalized images using the Image-Pro Plus image analysis system (Media Cybernetics, Silver Springs, MD).
NFAT nuclear translocation assessment
Cells in chamber slides were fixed with 4% paraformaldehyde and stained with phalloidin and DAPI (for nuclei). The stained cardiomyocytes and their NFAT-GFP distribution were visualized with an inverted epi-fluorescence microscope (model IX71, Olympus, Melville, NY) and the images were captured and digitized. To calculate the percentage of NRCMs that have NFAT nuclear translocation, 10 microscopic fields (∼500 NFAT-GFP expressing NRCMs) per group were examined for the distribution of NFAT-GFP. In addition, the nuclear translocation of endogenous NFAT in myocardium was analyzed with nuclear fractionation and Western blotting for NFATc4.
Luciferase reporter assay
Luciferase activity in NFAT-Luc transgenic, NFAT-Luc::CryAB double transgenic, and NFAT-Luc::CryAB/HSPB2-null mice were measured using a Luciferase Assay Kit (Roche Diagnostics Corporation, Indianapolis, IN, USA), according to the manufacturer’s instructions.
Results
Up-regulation of CryAB in the Early Phase of Pressure-overloaded Cardiac Hypertrophy
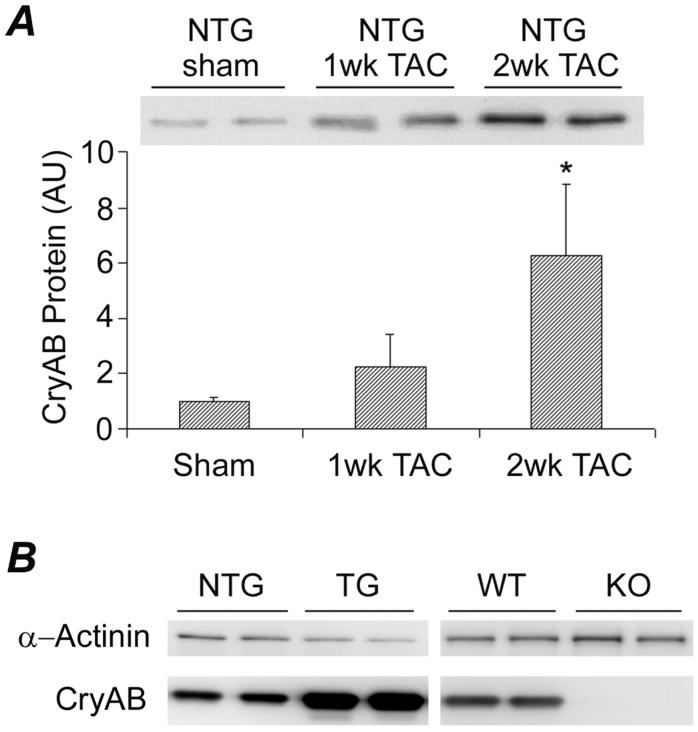
Our examination revealed that CryAB protein levels were gradually and significantly increased in the LV of wild type mice (NTG) during the first 2 weeks after TAC (Figure 1A). To investigate the pathophysiological significance of CryAB in cardiac responses to mechanical overload, we adopted both a gain- and loss-of-function approaches. For the gain-of-function, a cardiomyocyte-restricted CryAB overexpression mouse model (TG) was used. For the loss-of-function, the CryAB/HSPB2 double knockout mice (KO) were utilized. Compared with the NTG TAC group, LV myocardial CryAB remained to be overexpressed in the TG TAC but was absent in the KO TAC mice at 2 weeks after surgery (Figure 1B).
Figure 1.
CryAB protein expression in the left ventricle (LV). Age-matched male FVB/N mice with the indicated genotype were subjected to TAC or sham surgery. LV myocardium was collected at 1 or 2 weeks (wk) after TAC for protein analysis. A, A representative western blot image (upper part) and a bar graph summarizing densitometry data (lower part). Compared with sham and 1wk TAC, *: p<0.01. B, Representative western blot images for CryAB in the LV of the indicated groups at 2 wk after TAC. α-Actinin was probed as loading controls.
CryAB/HSPB2 KO but not CryAB Overexpression Produces Cardiomyopathy
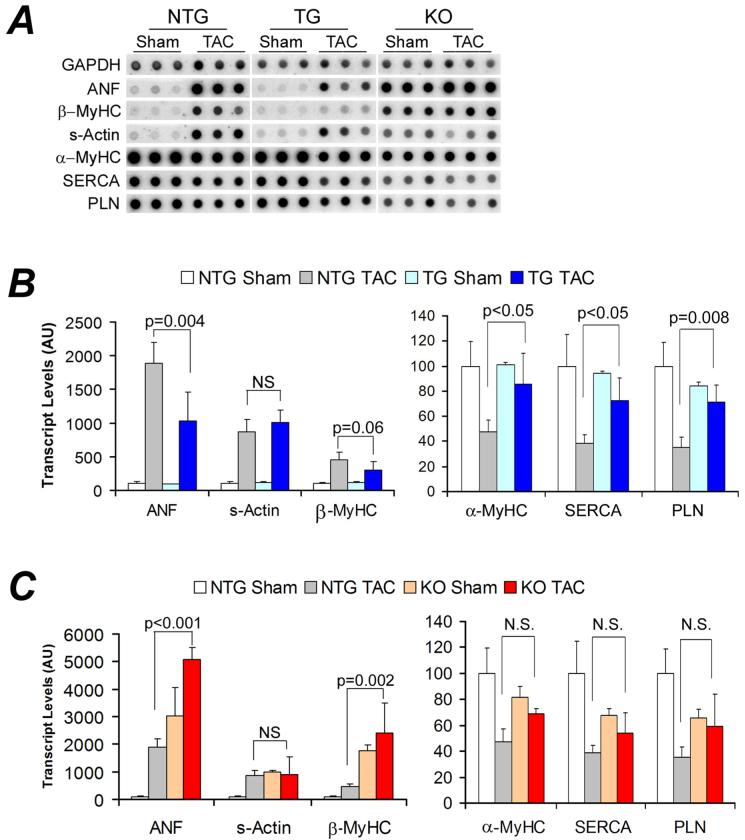
To test the effects of CryAB gain- and loss- of-function on cardiac responses to a minor stress condition, we compared cardiac fetal gene expression, cardiac mass, LV geometry, and cardiac function among the NTG, TG, and KO groups at 2 weeks after the sham surgery (i.e., 10 weeks of age). Echo, LV hemodynamics, cardiac mass (Supplemental Table 1), and the expression of the fetal gene program (Figure 2A, B) did not show any statistically significant difference between TG and NTG animals.
Figure 2.
Reactivation of the fetal gene program. Total RNA isolated from LV myocardium at 2 weeks after TAC or sham surgery was used for quantitative RNA dot blot analysis of the transcript levels of atrial natriuretic factor (ANF), β-myosin heavy chain (β-MyHC), skeletal α-actin (s-Actin), α-MyHC, sarcoplasmic endoplasmic reticulum calcium ATPase 2A (SERCA), phospholamban (PLN), and GAPDH with 32P-labeled transcript-specific oligonucleotide probes. The composed RNA dot blot images are shown in panel A. Each dot represents an individual animal. After normalized to the corresponding GAPDH signal, the mean intensity value of the NTG sham group was set at 100 arbitrary units (AU). The intensity signal of each individual dot was then normalized to the mean of the NTG sham. Comparison among the three sham control groups is presented in the online supplementary Figure 1. ANF, s-Actin, and β-MyHC transcripts were significantly increased whereas α-MyHC, SERCA, and PLN were significantly decreased in the NTG TAC (p<0.01). Compared with the NTG TAC group, ANF and β-MyHC up-regulation and the down-regulation of α-MyHC, SERCA, and PLN were substantially attenuated in the TG group (B) whereas the up-regulation of ANF and β-MyHC was significantly enhanced in the KO group (C).
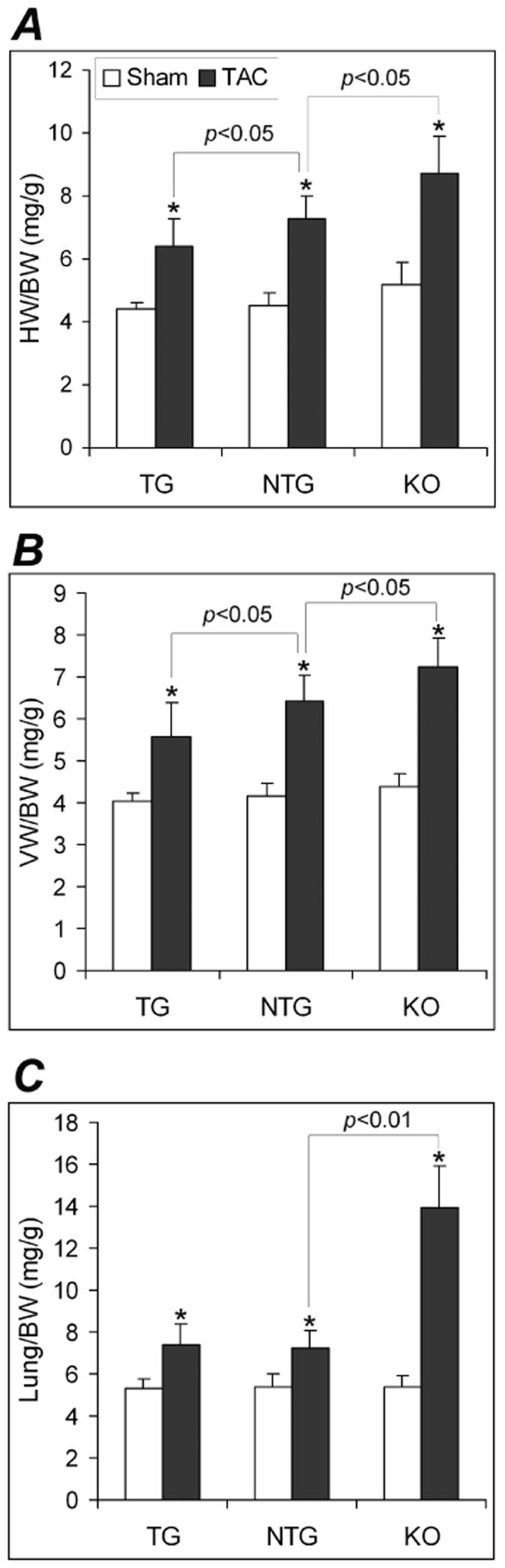
However, KO mice showed distinct changes at all the levels examined. Echo revealed a significant increase in LV diastolic posterior wall thickness (LVPWd) but no changes in the LV chamber dimension, fractional shortening (FS), and ejection fraction (EF, Supplemental Table 1). Consistent with the Echo findings, KO mice displayed a moderate but statistically significant increase in heart weight/body weight ratios (HW/BW) in absence of significant changes in the average body weight compared with the NTG group. There was no evidence of organ congestion at this time point (Supplemental Table 1). Functionally, although all parameters for LV systolic function or contractile function did not differ among the 3 groups at 10 weeks, LV diastolic function was compromised in the KO mice, as reflected by depressed minimum dP/dt (-dP/dtmax) and elevated LV end-diastolic pressure (LVEDP, Supplemental Table 1). In a separate cohort, we observed statistically significant decreases in FS and EF in the 18-week-old KO mice at the unstressed baseline condition (Supplemental Table 2). These findings demonstrate that the absence of CryAB/HSPB2 induces abnormal cardiac growth and defective myocardial relaxation.
The re-activation of the fetal gene program was striking in the LV of the KO mice (Figure 2A, Supplementary Figure 1). At 10 weeks of age, these mice displayed significant increases in the transcript levels of ANF, β-MyHC, and skeletal actin (s-Actin) and a tendency of decreases in α-MyHC, SERCA, and PLN. This genetic reprogramming persisted and was aggravated at 20 weeks (Supplementary Figure 2).
CryAB Overexpression Attenuates the Early Cardiac Response to Pressure Overload
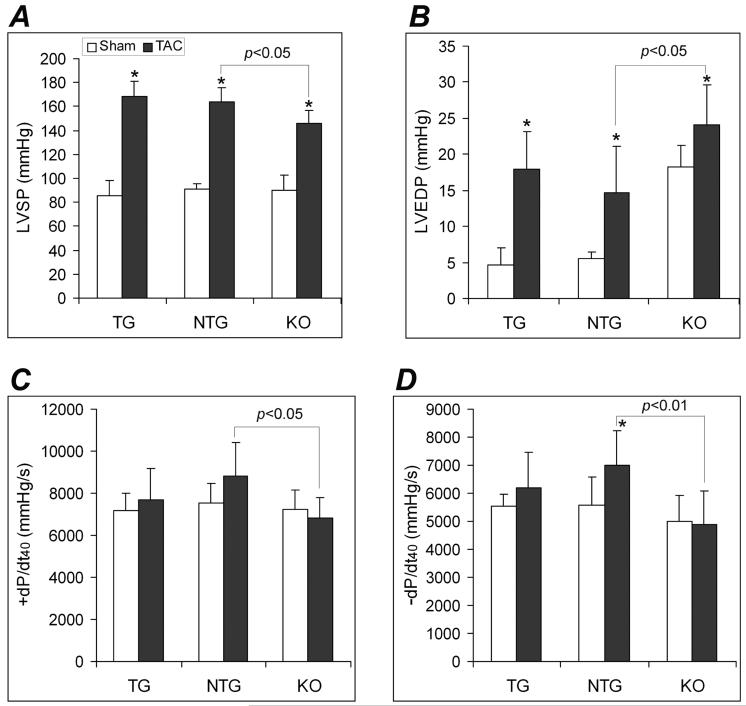
Previous reports and the baseline characterizations done in this study have shown that the cardiac-restricted CryAB overexpression does not produce an abnormal phenotype.14 This study delineates whether CryAB overexpression is beneficial or detrimental in a mechanical overload condition. Two weeks after TAC, NTG mice displayed classical pressure overloaded hypertrophic responses, including fetal gene program re-activation, increases in LVPWd, and increases in HW/BW and VW/BW ratios (Figures 2, 3, 4), compared with NTG sham controls. These changes were significantly attenuated in the TG TAC mice although the LV systolic peak pressure (LVSP) was comparable between NTG TAC and TG TAC groups (Figure 5A). Compared with NTG TAC mice, gravimetric data showed 15% less ventricular hypertrophy in the TG TAC hearts (Figure 3). The induction of fetal genes by TAC was significantly attenuated in the TG hearts as well. The TG TAC mice showed ANF upregulation but this was significantly less than that in the NTG TAC hearts. MyHC isoform switch (from α to β) and the significant down-regulation of the genes involved in calcium handling (SERCA and PLN) in the LV were observed in NTG TAC mice. However, these changes were significantly less in the TG TAC group (Figure 2A, 2B).
Figure 3.
Gravimetric analyses of cardiac hypertrophy at 2 weeks after TAC. A, Changes in the heart weight (HW)/body weight (BW) ratio. B, Changes in the ventricular weight (VW)/BW ratio. C, Changes in the Lung/BW ratio. Compared with the sham control of the same genotype, *: p<0.01.
Figure 4.
Echocardiography analysis of LV morphometry and function at 2 weeks after TAC or sham surgery. LVPWd: LV diastolic posterior wall thickness; EF: the ejection fraction; FS: fractional shortening; compared to the sham control of the same genotype, *: p<0.01.
Figure 5.
Changes in LV pressure at 2 weeks after TAC or sham surgery. A, Changes in the LV systolic peak pressure (LVSP). B, Changes in the LV end diastolic pressure (LVEDP). C and D, Changes in the LV pressure rising (+dP/dt40, C) and declining velocities (-dP/dt40, D) at the LV pressure being 40mmHg. Compared with the same genotype sham control, *: p<0.05, 0.01.
Despite less hypertrophic responses in TG TAC mice, neither Echo nor hemodynamic assessments showed any statistically significant difference in LV function between the TG TAC and the NTG TAC groups (Figures 4, 5). It should be noted that NTG mice had not yet shown significant decreases in major LV function parameters (e.g., FS, EF, dP/dt40, -dP/dt40) at 2 weeks after TAC.
CryAB Overexpression Attenuates Hypertrophic Growth of Cultured Cardiomyocytes
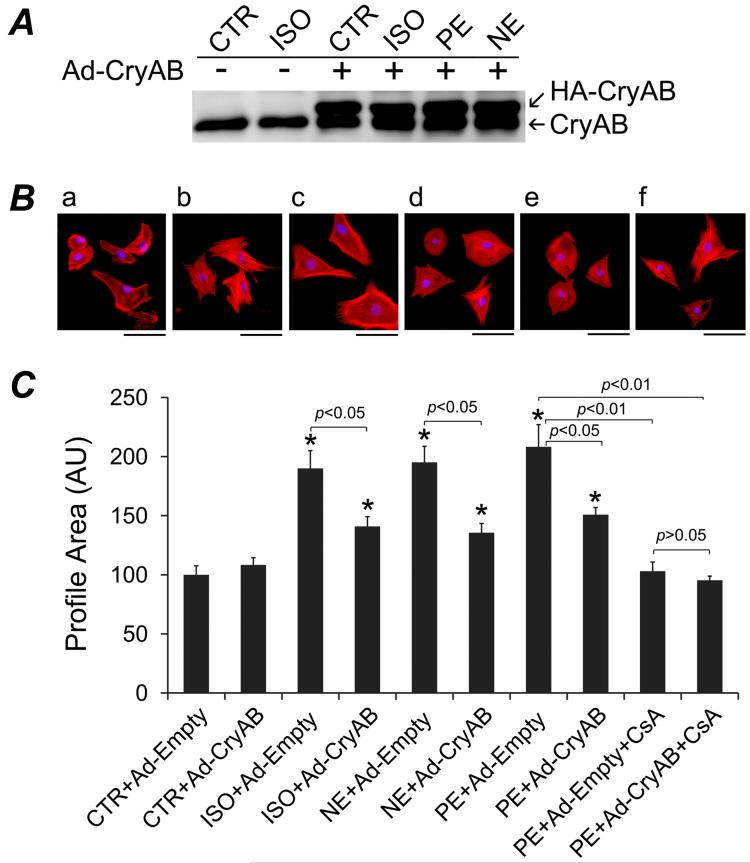
To test whether the hypertrophy suppression effects of CryAB is cardiomyocyte-autonomous, we induced CryAB overexpression via adenoviral vectors in cultured NRCMs and tested its effects on pharmacologically induced cardiomyocyte hypertrophic growth. Compared with the endogenous CryAB of the control viral (Ad-Empty) infected cells, Ad-CryAB infection at the MOI used here overexpressed CryAB protein by a factor of ∼3, (Figure 6A), which is less than the levels of overexpression in the TG mice (∼5 folds). The treatment of norepinephrine (NE, 2 μM), phenylephrine (PE, 30 μM), or isoproterenol (ISO, 2 μM) induced significant increases in the profile area of NRCMs but the increases were significantly attenuated by CryAB overexpression (Figure 6B, 6C). Consistent with previous reports,27, 28 the calcineurin inhibitor cyclosporine A (CsA; 500 ng/ml) significantly suppressed NE-induced NRCM hypertrophy; but the combination of CryAB overexpression and CsA treatment did not show additional suppression (Figure 6B, 6C). Similar results were obtained over PE-induced hypertrophy (data not shown). These data suggest that CryAB suppresses hypertrophy likely through the same pathway as CsA does.
Figure 6.
CryAB overexpression suppresses hypertrophy of cultured neonatal rat cardiomyocytes (NRCMs). Twenty-four hours after being plated, NRCMs were infected with Ad-CryAB or Ad-Empty for 24 hours and then treated with isoproterenol (ISO), norepinephrine (NE), phenylepinephrine (PE), or vehicle control (CTR, ascorbic acid)), in combination with or without cyclosporine A (CsA) for 48 hours in serum-free media. A, Western blot analysis of CryAB overexpression at 48 hours after Ad-CryAB infection. The transgenic CryAB contains a HA tag (HA-CryAB) and therefore migrates slower than the endogenous CryAB. Changes in myocyte profile area are shown in panel B and C. The cells in chamber slides were fixed and double-stained with Alexa Fluor-568 conjugated phalloidin (red) and DAPI (blue). Digital images were captured at the same settings for subsequent profile area measurements. B, Representative fluorescence micrographs of NRCMs treated with: (a) CTR + Ad-Empty; (b) CTR + Ad-CryAB; (c) PE + Ad-Empty; (d) PE + Ad-CryAB; (e) PE + Ad-Empty + CsA; (f) PE + Ad-CryAB + CsA. C, A summary of NRCMs profile area changes after the indicated treatments. Approximately 60 cells evenly from 3 duplicates per group were measured. Mean + SE are presented. Compared to the CTR+Ad-Empty group, *: p < 0.01.
Absence of CryAB/HSPB2 is Deleterious in Cardiac Pressure Overload
Because of the proximity between the CryAB and the HSPB2 genes in mouse genome, HSPB2 is accidentally ablated when targeting the CryAB gene.18 The resultant CryAB/HSPB2 null mouse is the only mouse model currently available for CryAB loss-of-function studies. Compared with the NTG TAC group, KO TAC mice showed significantly lower LVSP (Figure 5A) but statistically greater HW/BW and VW/BW ratios indicating more hypertrophy (Figure 3A, 3B). Consistently, transcriptional up-regulation of ANF and β-MyHC was also significantly greater in the KO TAC mouse hearts (Figure 2C). Compared with the NTG TAC group, Echo showed statistically smaller EF and FS in the KO TAC group (Figure 4B, C). Since LVSP significantly differed between KO TAC and NTG TAC groups (Figure 5A), +dP/dt40 and - dP/dt40 were analyzed and compared. The absolute values of LV +dP/dt40 and -dP/dt40 were significantly smaller in the KO TAC group than the NTG TAC group (Figure 5C, 5D). LVEDP were elevated in all 3 TAC groups but the elevation was greater in the KO TAC group than the NTG TAC. Consistent with left heart failure in KO TAC mice, Lung/BW ratios (Figure 3C) but not kidney/BW (data not shown) were markedly increased in KO TAC mice compared with both the KO sham and the NTG TAC groups.
Interestingly, the fetal gene program was markedly re-activated in the KO sham control group and TAC induced pressure overload did not produce a more pronounced fetal gene re-activation (Figure 2C).
CryAB Suppresses NFAT Signaling in vivo and in vitro
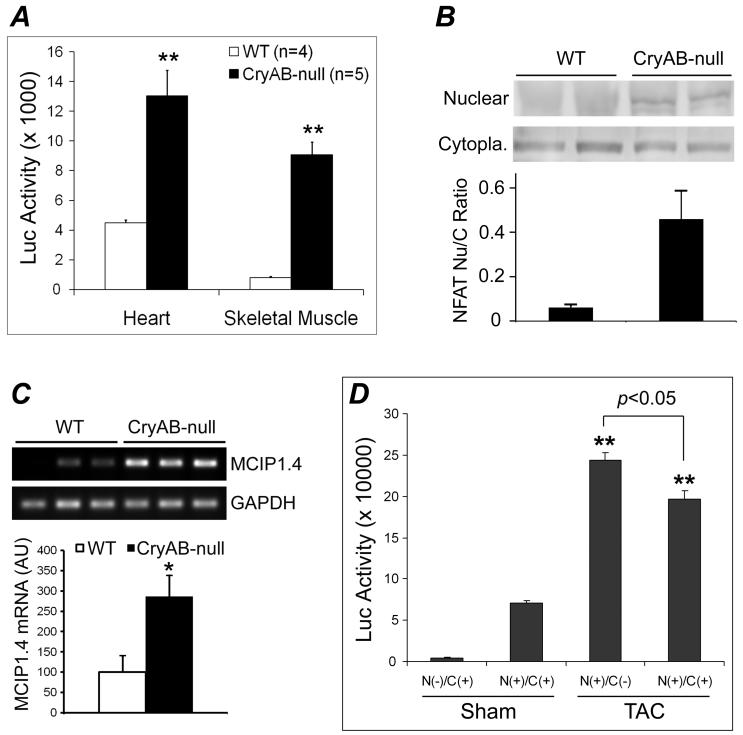
To investigate the potential mechanisms underlying the suppression of hypertrophic responses by CryAB, we conducted both in vivo and cell culture experiments to determine the influence of CryAB on the NFAT signaling pathway, a well established signaling pathway for pathological cardiac growth. To determine the in vivo potential effect of CryAB loss-of-function on NFAT signaling, NFAT-Luc reporter mice were cross-bred with the KO mice and the luciferase activities in ventricular myocardium and in the soleus muscle were measured at 18 weeks. Compared with wild type littermates, luciferase activities were significantly increased in both cardiac and skeletal muscle of KO mice (Figure 7A). Consistent with the reporter assays, increases in NFATc4 nuclear to cytoplasmic ratios were detected in the KO hearts (Figure 7B). Moreover, myocyte-enriched calcineurin interacting protein-1.4 (MCIP1.4) is a bona fide NFAT target gene.29, 30 MCIP1.4 transcript levels were significantly higher in KO hearts than the wild type controls (Figure 7C). These findings indicate that CryAB and/or HSPB2 suppress NFAT signaling at the baseline condition.
Figure 7.
CryAB suppresses NFAT transactivation in mice. A, NFAT-Luc transgenic mice were cross-bred with the KO (CryAB-null) mice and the luciferase (Luc) activities in myocardium and the soleus muscle of CryAB-null and wild type littermates (WT) were measured. Compared with WT, **: p < 0.01. B, Western blot analyses of NFATc4 in the nuclear and the cytoplasmic (Cytopla.) fractions of myocardium from WT and KO mice. A representative set of images are shown at the top and the nuclear to cytoplasmic NFAT ratios derived from the densitometry of the Western blot images are summarized in the bar graph. C, RT-PCR analyses of myocardial MCIP1.4 expression in KO mice. *: p < 0.05, KO vs WT. D, The NFAT-Luc mice were cross-bred with the CryAB TG mice and the resulting littermate mice with the indicated genotypes were subjected to TAC or sham surgery at 12 weeks. LV myocardial luciferase activities were assessed at 2 weeks after the surgery. N(-): NFAT-Luc Ntg; N(+): NFAT-Luc Tg; C(-): CryAB Ntg; C(+): CryAB Tg. Mean+SD; n = 4 mice/group; compared with either sham groups, **: p < 0.01.
To illustrate further the in vivo effect of CryAB on NFAT signaling, NFAT-Luc reporter mice were cross-bred with CryAB TG mice and the resultant littermates were subjected to TAC at 12 weeks of age. NFAT activation at 2 weeks after TAC was significantly attenuated by CryAB overexpression (Figure 7D).
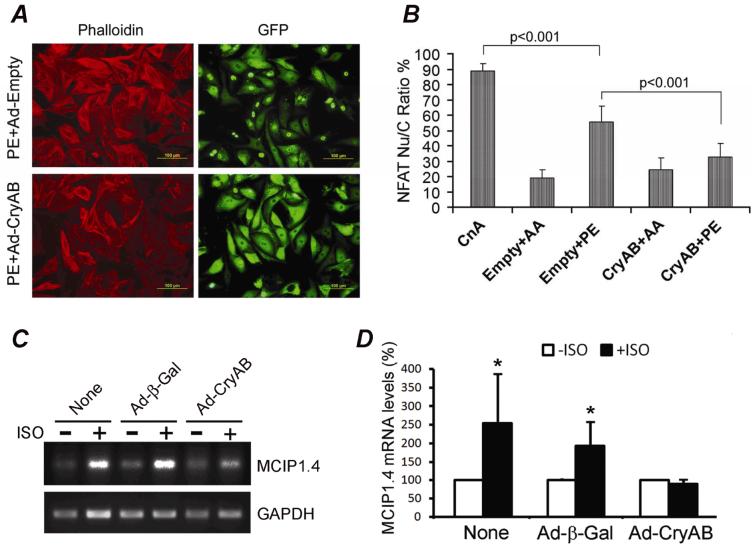
NFAT nuclear translocation is a critical step of NFAT activation. To test whether the in vivo effect of CryAB on NFAT transactivation (Figure 7) is cardiomyocyte-autonomous, we determined the effects of CryAB overexpression on hypertrophy agonist induced NFAT nuclear translocation and NFAT target gene expression in cultured cardiomyocytes. Consistent with previous reports,31, 32 GFP-tagged NFATc1 expressed in cultured NRCMs existed predominantly in the cytoplasm in a serum-free culture condition; but it showed significant nuclear translocation upon expression of a constitutively active form of CnAΔ or exposure to an α1-adrenergic agonist (PE). Overexpression of CryAB markedly reduced PE-induced nuclear translocation of NFAT-GFP (Figure 8A, 8B). Furthermore, adrenergic stimulation-induced MCIP1.4 expression was markedly attenuated by CryAB overexpression (Figure 8C, 8D).
Figure 8.
CryAB overexpression suppresses NFAT transactivation in cultured NRCMs. Overexpression of NFAT-GFP, HA-tagged CryAB, and CnAΔ (CnA) was mediated by respective adenoviral infection. Ad-Empty (Empty) or Ad-β-Gal was used as control viral infection. A, Representative micrographs of NRCMs to show NFAT-GFP distribution. B, A summary of changes in NFAT-GFP nuclear translocation which is semi-quantitatively reflected by the percentage of NRCMs with nuclear NFAT-GFP over all cardiomyocytes with NFAT-GFP expression. C and D, RT-PCR analyses of MCIP1.4. Cultured NRCMs infected with the indicated adenoviruses for 48 hours were treated with ISO (+ISO) or vehicle control (-ISO) for 24 hours in serum-free media. Total RNA was then isolated from the cells for RT-PCR. A representative gel image is shown in panel C. The MCIP1.4 signal of each sample is normalized to its GAPDH signal and the mean of each vehicle control group is set as 100%. The semi-quantitative data are summarized in panel D. P<0.05, +ISO vs -ISO.
Discussion
PQC in the cell assists proper folding of nascent proteins, keeps normal matured proteins from denaturing and misfolding, removes terminally misfolded proteins.4 Molecular chaperones play critical roles in each of these processes and thereby protect the cell. Even under physiological conditions, the heart is constantly under tremendous stress. Various insults from pathological conditions, such as hypertension and myocardial ischemia, inevitably increase the stress on cardiomyocytes. The stress conceivably poses a significant challenge to PQC in cardiomyocytes. For example, increases in protein synthesis in cardiomyocytes characteristic of cardiac hypertrophy require PQC to work harder because approximately 30% of newly synthesized polypeptides are degraded before they become mature proteins.33 HSPs are an important family of molecular chaperones and therefore are essential to the cell in dealing with various stress conditions by participation in PQC. However, until the present study the (patho)physiological significance of HSPs, especially the sHSPs, in cardiac responses to hemodynamic overload has not been demonstrated.
In cardiomyocytes, CryAB is a bona fide constitutively expressed sHSP. Here, we report that cardiac CryAB protein expression can be induced by pressure overload. Moreover, we have investigated the (patho)physiological role of CryAB in early cardiac responses to pressure overload and in the modulation of a pivotal hypertrophic signaling pathway. Our data show that CryAB/HSPB2 deficiency activates the NFAT signaling and induces cardiac hypertrophic responses at the unstressed or minimal stress conditions and exacerbates cardiac malfunction upon pressure overload, whereas CryAB overexpression significantly attenuates pressure overloaded hypertrophic responses and associated NFAT activation in mouse hearts. Our further experiments revealed that CryAB overexpression suppressed adrenergic stimulation induced nuclear translocation of NFAT and the expression of a bona fide NFAT target gene (Figure 8) and attenuated adrenergic stimulation induced hypertrophy (Figure 6) in cultured cardiomyocytes. CryAB overexpression failed to further suppress hypertrophic growth when the calcineurin-NFAT pathway is blocked by CsA (Figure 6). These new findings demonstrate that CryAB negatively regulates pressure overload cardiac hypertrophic responses likely through inhibiting NFAT signaling in cardiomyocytes.
To determine the necessity of CryAB/HSPB2 for the heart to respond to stress, we subjected the KO mice to sham and TAC surgery. Compared with wild type mice (NTG), KO mice responded considerably differently to these procedures. At 2 weeks after the sham surgery which is a relatively milder stress condition, KO mice displayed marked reactivation of the fetal gene program (Figure 2A, Supplementary Figure 1), concentric cardiac hypertrophy as evidenced by increased LV wall thickness and HW/BW ratio, and LV diastolic malfunction as indicated by changes in minimum dP/dt and LVEDP (Supplementary Table 1). These data suggest CryAB and/or HSPB2 are required to maintain normal cardiac function in response to a general stress.
This is somewhat surprising because it was reported that young CryAB/HSPB2 KO mice under an unstressed condition do not show discernible cardiac abnormalities in expression of the fetal gene program, myocardial histology, and echocardiography.7 Although increased HW/BW ratio was observed in 4 months old CryAB/HSPB2 KO mice by Morrison et al it was attributed to a decrease in BW.7 We did not observe a statistically significant difference in BW between NTG and KO sham groups. This is consistent with previous reports which have shown normal growth curves in the KO mice until 30∼40 weeks of age.8, 18 It is noted that the previously reported study characterized mixed gender mice in a 129/Svj isogenic background whereas the present study used all male and FVB inbred mice.
Compared with the NTG TAC group, the KO TAC group showed greater reactivation of ANF and β-MyHC (Figure 2), greater cardiac hypertrophy (Figure 3, 4A), lower LVSP and +/- dP/dt40 (Figure 5), lower EF and FS (Figure 4), and higher LVEDP (Figure 5B) and Lung/BW ratio (Figure 3C). These indicate that the absence of CryAB/HSPB2 renders cardiac responses to TAC-induced LV pressure overload more pathological. Taken together, the loss-of-function studies demonstrated that CryAB and/or HSPB2 are essential to maintaining normal cardiac function in a hemodynamic overload condition.
In the present study, a gradual but significant upregulation of CryAB protein was observed in wild type mouse hearts under pressure overload (Figure 1). This up-regulation is likely a compensatory response of the heart to deal with increased PQC burden posted by pressure overload cardiac hypertrophy. However, the reactive CryAB increase does not appear to be adequate because constitutively forced overexpression of CryAB, as shown in the TG group, significantly attenuated TAC-induced NFAT transactivation (Figure 7D), reactivation of the fetal gene program (Figure 2A), and cardiac hypertrophy (Figure 3, 4A). Notably, both Echo and hemodynamics demonstrated that the attenuation of cardiac hypertrophy by CryAB overexpression did not compromise cardiac function under the pressure overload condition. This is consistent with recent reports.34, 35 This also suggests that at least a fraction of the hypertrophic response might be caused by the hypertrophic growth per se and is dispensable if the increased burden on PQC is diluted by molecular chaperones.
Notably, using a well-established NFAT reporter assay as well as monitoring NFAT nuclear translocation and the expression of a bona fide NFAT target gene MCIP1.4 (Figure 7A∼C), we have found for the first time that NFAT is activated in the heart (and skeletal muscle) of CryAB/HSPB2 null mice. This is consistent with the development of cardiac hypertrophy and malfunction observed in the present study (Supplementary Table 2) and the previously reported skeletal myopathy in the null mice under the baseline condition.18 Since Both CryAB and HSPB2 are ablated in the KO mice, we cannot pin-point which sHSP mediates the observed function based solely on the data from the KO mice. However, the highly complementary in vivo and in vitro effects of CryAB gain-of-function on both hypertrophic responses and NFAT activation indicate that loss of CryAB is at least in part responsible for the phenotypes that we observed in the KO mice. Therefore, the evidence strongly supports that CryAB inhibits the NFAT signaling and suppresses cardiac hypertrophic responses. The further mechanism underlying this inhibition remains to be delineated. It was recently shown that Mrj, a member of the HSP40 family interacts with class II HDACs and NFAT to repress NFAT transactivation.32 Hence, CryAB might directly interact with NFAT and prevent its nuclear translocation. From the PQC point of view, CryAB which has previously been shown to inhibit aggregation of abnormal proteins,36 may protect cardiomyocytes from being damaged by misfolded or damaged proteins under both physiological and pathological conditions. Therefore, the observed effect of CryAB on NFAT could also be secondary to its protection against stress.
The role(s) of HSPs in the cardiac hypertrophic responses to pressure overload has not been described, whereas hypertension and cardiac hypertrophy are important antecedent factors for the development of CHF. Both the up-regulation of CryAB in familial hypertrophic cardiomyopathy and the down-regulation of CryAB in failing human hearts have been reported.1 Therefore, by investigating gain-of-function of CryAB and loss-of-function of CryAB/HSPB2, this study has significantly expanded our understanding of the pathophysiological significance of sHSPs and thereby the importance of PQC in the heart. The findings will help elucidate the potential therapeutic benefits of sHSP in heart disease.
Supplementary Material
Acknowledgements
Dr. X. Wang is an Established Investigator of the American Heart Association (AHA). This work was supported in part by NIH grants RO1HL072166 and R01HL085629 and AHA grants 235099N and 740025N (to X.W.) and AHA Fellowship grants 0510069Z (to A.R.K.K), 0625738Z (to H.S.), and 0620032Z (to H.Z.) and by the MD/PhD Program of University of South Dakota. We appreciate the generosity of Dr. Jeffery Molkentin of Cincinnati Children’s Hospital Medical Center (Cincinnati, OH) in providing the NFAT-Luc reporter mice and the NFAT-GFP and CnAΔ recombinant adenoviruses. We also thank Rebecca Redetzky for her assistance in hemodynamic data acquisition.
Footnotes
Disclosures
None
Kumarapeli - CryAB suppresses hypertrophy
References
- 1.Kumarapeli AR, Wang X. Genetic modification of the heart: chaperones and the cytoskeleton. J Mol Cell Cardiol. 2004;37:1097–1109. doi: 10.1016/j.yjmcc.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 2.Taylor RP, Benjamin IJ. Small heat shock proteins: a new classification scheme in mammals. J Mol Cell Cardiol. 2005;38:433–444. doi: 10.1016/j.yjmcc.2004.12.014. [DOI] [PubMed] [Google Scholar]
- 3.Perng MD, Muchowski PJ, van Den IP, Wu GJ, Hutcheson AM, Clark JI, Quinlan RA. The cardiomyopathy and lens cataract mutation in alpha B-crystallin alters its protein structure, chaperone activity, and interaction with intermediate filaments in vitro. J Biol Chem. 1999;274:33235–33243. doi: 10.1074/jbc.274.47.33235. [DOI] [PubMed] [Google Scholar]
- 4.Wang X, Robbins J. Heart failure and protein quality control. Circ Res. 2006;99:1315–1328. doi: 10.1161/01.RES.0000252342.61447.a2. [DOI] [PubMed] [Google Scholar]
- 5.Fan GC, Ren X, Qian J, Yuan Q, Nicolaou P, Wang Y, Jones WK, Chu G, Kranias EG. Novel cardioprotective role of a small heat-shock protein, Hsp20, against ischemia/reperfusion injury. Circulation. 2005;111:1792–1799. doi: 10.1161/01.CIR.0000160851.41872.C6. [DOI] [PubMed] [Google Scholar]
- 6.Ray PS, Martin JL, Swanson EA, Otani H, Dillmann WH, Das DK. Transgene overexpression of alphaB crystallin confers simultaneous protection against cardiomyocyte apoptosis and necrosis during myocardial ischemia and reperfusion. FASEB J. 2001;15:393–402. doi: 10.1096/fj.00-0199com. [DOI] [PubMed] [Google Scholar]
- 7.Morrison LE, Whittaker RJ, Klepper RE, Wawrousek EF, Glembotski CC. Roles for alphaB-crystallin and HSPB2 in protecting the myocardium from ischemia-reperfusion-induced damage in a KO mouse model. Am J Physiol Heart Circ Physiol. 2004;286:H847–855. doi: 10.1152/ajpheart.00715.2003. [DOI] [PubMed] [Google Scholar]
- 8.Golenhofen N, Redel A, Wawrousek EF, Drenckhahn D. Ischemia-induced increase of stiffness of alpha B-crystallin/HSPB2-deficient myocardium. Pflugers Arch. 2006;451:518–525. doi: 10.1007/s00424-005-1488-1. [DOI] [PubMed] [Google Scholar]
- 9.Maloyan A, Sanbe A, Osinska H, Westfall M, Robinson D, Imahashi K, Murphy E, Robbins J. Mitochondrial dysfunction and apoptosis underlie the pathogenic process in alpha-B-crystallin desmin-related cardiomyopathy. Circulation. 2005;112:3451–3461. doi: 10.1161/CIRCULATIONAHA.105.572552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kadono T, Zhang XQ, Srinivasan S, Ishida H, Barry WH, Benjamin IJ. CRYAB and HSPB2 deficiency increases myocyte mitochondrial permeability transition and mitochondrial calcium uptake. J Mol Cell Cardiol. 2006;40:783–189. doi: 10.1016/j.yjmcc.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 11.Fan GC, Yuan Q, Song G, Wang Y, Chen G, Qian J, Zhou X, Lee YJ, Ashraf M, Kranias EG. Small heat-shock protein Hsp20 attenuates beta-agonist-mediated cardiac remodeling through apoptosis signal-regulating kinase 1. Circ Res. 2006;99:1233–1242. doi: 10.1161/01.RES.0000251074.19348.af. [DOI] [PubMed] [Google Scholar]
- 12.Golenhofen N, Arbeiter A, Koob R, Drenckhahn D. Ischemia-induced association of the stress protein alpha B-crystallin with I-band portion of cardiac titin. J Mol Cell Cardiol. 2002;34:309–319. doi: 10.1006/jmcc.2001.1513. [DOI] [PubMed] [Google Scholar]
- 13.Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med. 1990;322:1561–1566. doi: 10.1056/NEJM199005313222203. [DOI] [PubMed] [Google Scholar]
- 14.Wang X, Osinska H, Klevitsky R, Gerdes AM, Nieman M, Lorenz J, Hewett T, Robbins J. Expression of R120G-alphaB-crystallin causes aberrant desmin and alphaB-crystallin aggregation and cardiomyopathy in mice. Circ Res. 2001;89:84–91. doi: 10.1161/hh1301.092688. [DOI] [PubMed] [Google Scholar]
- 15.Chen Q, Liu JB, Horak KM, Zheng H, Kumarapeli AR, Li J, Li F, Gerdes AM, Wawrousek EF, Wang X. Intrasarcoplasmic amyloidosis impairs proteolytic function of proteasomes in cardiomyocytes by compromising substrate uptake. Circ Res. 2005;97:1018–1026. doi: 10.1161/01.RES.0000189262.92896.0b. [DOI] [PubMed] [Google Scholar]
- 16.Rajasekaran NS, Connell P, Christians ES, Yan LJ, Taylor RP, Orosz A, Zhang XQ, Stevenson TJ, Peshock RM, Leopold JA, Barry WH, Loscalzo J, Odelberg SJ, Benjamin IJ. Human alphaB-crystallin mutation causes oxido-reductive stress and protein aggregation cardiomyopathy in mice. Cell. 2007;130:427–439. doi: 10.1016/j.cell.2007.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang X, Su H, Ranek MJ. Protein quality control and degradation in cardiomyocytes. J Mol Cell Cardiol. 2008;45:11–27. doi: 10.1016/j.yjmcc.2008.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brady JP, Garland DL, Green DE, Tamm ER, Giblin FJ, Wawrousek EF. AlphaB-crystallin in lens development and muscle integrity: a gene knockout approach. Invest Ophthalmol Vis Sci. 2001;42:2924–2934. [PubMed] [Google Scholar]
- 19.Wilkins BJ, Dai YS, Bueno OF, Parsons SA, Xu J, Plank DM, Jones F, Kimball TR, Molkentin JD. Calcineurin/NFAT coupling participates in pathological, but not physiological, cardiac hypertrophy. Circ Res. 2004;94:110–118. doi: 10.1161/01.RES.0000109415.17511.18. [DOI] [PubMed] [Google Scholar]
- 20.Rockman HA, Ross RS, Harris AN, Knowlton KU, Steinhelper ME, Field LJ, Ross J, Jr., Chien KR. Segregation of atrial-specific and inducible expression of an atrial natriuretic factor transgene in an in vivo murine model of cardiac hypertrophy. Proc Natl Acad Sci U S A. 1991;88:8277–8281. doi: 10.1073/pnas.88.18.8277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kumarapeli AR, Horak KM, Glasford JW, Li J, Chen Q, Liu J, Zheng H, Wang X. A novel transgenic mouse model reveals deregulation of the ubiquitin-proteasome system in the heart by doxorubicin. FASEB J. 2005;19:2051–2053. doi: 10.1096/fj.05-3973fje. [DOI] [PubMed] [Google Scholar]
- 22.Su H, Huang W, Wang X. The COP9 signalosome negatively regulates proteasome proteolytic function and is essential to transcription. Int J Biochem Cell Biol. 2008 doi: 10.1016/j.biocel.2008.07.008. article in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dong X, Liu J, Zheng H, Glasford JW, Huang W, Chen QH, Harden NR, Li F, Gerdes AM, Wang X. In situ dynamically monitoring the proteolytic function of the ubiquitin-proteasome system in cultured cardiac myocytes. Am J Physiol Heart Circ Physiol. 2004;287:H1417–1425. doi: 10.1152/ajpheart.01233.2003. [DOI] [PubMed] [Google Scholar]
- 24.Liu Y, Cseresnyes Z, Randall WR, Schneider MF. Activity-dependent nuclear translocation and intranuclear distribution of NFATc in adult skeletal muscle fibers. J Cell Biol. 2001;155:27–39. doi: 10.1083/jcb.200103020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Braz JC, Bueno OF, Liang Q, Wilkins BJ, Dai YS, Parsons S, Braunwart J, Glascock BJ, Klevitsky R, Kimball TF, Hewett TE, Molkentin JD. Targeted inhibition of p38 MAPK promotes hypertrophic cardiomyopathy through upregulation of calcineurin-NFAT signaling. J Clin Invest. 2003;111:1475–1486. doi: 10.1172/JCI17295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.De Windt LJ, Lim HW, Taigen T, Wencker D, Condorelli G, Dorn GW, 2nd, Kitsis RN, Molkentin JD. Calcineurin-mediated hypertrophy protects cardiomyocytes from apoptosis in vitro and in vivo: An apoptosis-independent model of dilated heart failure. Circ Res. 2000;86:255–263. doi: 10.1161/01.res.86.3.255. [DOI] [PubMed] [Google Scholar]
- 27.Taigen T, De Windt LJ, Lim HW, Molkentin JD. Targeted inhibition of calcineurin prevents agonist-induced cardiomyocyte hypertrophy. Proc Natl Acad Sci U S A. 2000;97:1196–1201. doi: 10.1073/pnas.97.3.1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pu WT, Ma Q, Izumo S. NFAT transcription factors are critical survival factors that inhibit cardiomyocyte apoptosis during phenylephrine stimulation in vitro. Circ Res. 2003;92:725–731. doi: 10.1161/01.RES.0000069211.82346.46. [DOI] [PubMed] [Google Scholar]
- 29.Yang J, Rothermel B, Vega RB, Frey N, McKinsey TA, Olson EN, Bassel-Duby R, Williams RS. Independent signals control expression of the calcineurin inhibitory proteins MCIP1 and MCIP2 in striated muscles. Circ Res. 2000;87:E61–68. doi: 10.1161/01.res.87.12.e61. [DOI] [PubMed] [Google Scholar]
- 30.Ni YG, Berenji K, Wang N, Oh M, Sachan N, Dey A, Cheng J, Lu G, Morris DJ, Castrillon DH, Gerard RD, Rothermel BA, Hill JA. Foxo transcription factors blunt cardiac hypertrophy by inhibiting calcineurin signaling. Circulation. 2006;114:1159–1168. doi: 10.1161/CIRCULATIONAHA.106.637124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hunton DL, Lucchesi PA, Pang Y, Cheng X, Dell’Italia LJ, Marchase RB. Capacitative calcium entry contributes to nuclear factor of activated T-cells nuclear translocation and hypertrophy in cardiomyocytes. J Biol Chem. 2002;277:14266–14273. doi: 10.1074/jbc.M107167200. [DOI] [PubMed] [Google Scholar]
- 32.Dai YS, Xu J, Molkentin JD. The DnaJ-related factor Mrj interacts with nuclear factor of activated T cells c3 and mediates transcriptional repression through class II histone deacetylase recruitment. Mol Cell Biol. 2005;25:9936–9948. doi: 10.1128/MCB.25.22.9936-9948.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schubert U, Anton LC, Gibbs J, Norbury CC, Yewdell JW, Bennink JR. Rapid degradation of a large fraction of newly synthesized proteins by proteasomes. Nature. 2000;404:770–774. doi: 10.1038/35008096. [DOI] [PubMed] [Google Scholar]
- 34.Dorn GW., 2nd. Containing hypertrophy with a PICOT fence. Circ Res. 2006;99:228–230. doi: 10.1161/01.RES.0000236795.57759.45. [DOI] [PubMed] [Google Scholar]
- 35.Depre C, Wang Q, Yan L, Hedhli N, Peter P, Chen L, Hong C, Hittinger L, Ghaleh B, Sadoshima J, Vatner DE, Vatner SF, Madura K. Activation of the cardiac proteasome during pressure overload promotes ventricular hypertrophy. Circulation. 2006;114:1821–1828. doi: 10.1161/CIRCULATIONAHA.106.637827. [DOI] [PubMed] [Google Scholar]
- 36.Wang X, Klevitsky R, Huang W, Glasford J, Li F, Robbins J. AlphaB-crystallin modulates protein aggregation of abnormal desmin. Circ Res. 2003;93:998–1005. doi: 10.1161/01.RES.0000102401.77712.ED. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.