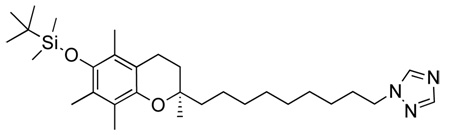
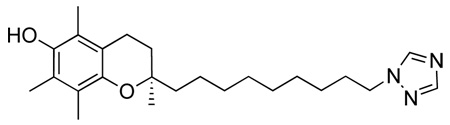
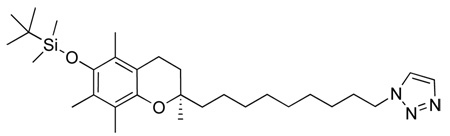
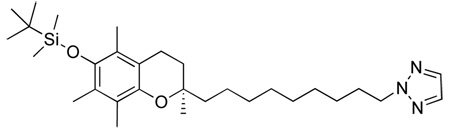
Abstract
The oxidative metabolism of tocopherols and tocotrienols by monooxygenases is a key factor in the plasma and tissue clearance of forms of vitamin E other than α-tocopherol. It is well known that a commonly ingested form of vitamin E, γ-tocopherol, has greatly reduced plasma half life (faster clearance) than α-tocopherol. The tocotrienols are metabolized even faster than γ-tocopherol. Both γ-tocopherol and α- and δ-tocotrienol possess intriguing biological activities that are different from α-tocopherol, making them potentially of interest for therapeutic use. Unfortunately, the fast clearance of non-α-tocopherols from animal tissues is a significant hurdle to maximizing their effect(s) as dietary supplements. We report here the design and synthesis of N-heterocycle-containing analogues of α-tocopherol that act as inhibitors of Cyp4F2, the key monooxygenase responsible for ω-hydroxylation of the side chain of tocols. In particular, an ω-imidazole containing compound, 1, [(R)-2-(9-(1H-imidazol-1-yl)nonyl)-2,5,7,8-tetramethylchroman-6-ol] had an ED50 for inhibition of γ-CEHC production from γ-tocopherol of ~ 1 nM when tested in HepG2 cells in culture. Furthermore, feeding of 1 to mice along with rapidly metabolized δ-tocopherol, resulted in a doubling of the δ-tocopherol / α-tocopherol ratio in liver (P < 0.05). Thus, 1 may be a useful adjuvant to the therapeutic use of non-α-tocopherols.
Keywords: tocopherol, tocotrienol, vitamin E, oxidative metabolism, CYP4F2, enzyme inhibition
1. Introduction
The tocopherols are understood to be the major lipid soluble antioxidants in mammalian membranes. They act as chain-breaking inhibitors of free radical peroxidation of unsaturated fatty acids. Despite the fact that the diets of most North Americans contain more γ-tocopherol than α-tocopherol, it is predominantly α-tocopherol that is retained in the body due to the actions of a specific binding and transfer protein known as the tocopherol transfer protein (TTP).1, 2 This protein is mostly expressed in liver tissue, binds α-tocopherol specifically,3, 4 and aids the secretion of the vitamin in lipoproteins such as VLDL that then carry the vitamin to remote tissues of the body. Forms of vitamin E other than α-tocopherol are poorly retained by the body and are rapidly metabolized to water-soluble compounds for excretion in urine or transported to bile.5–7
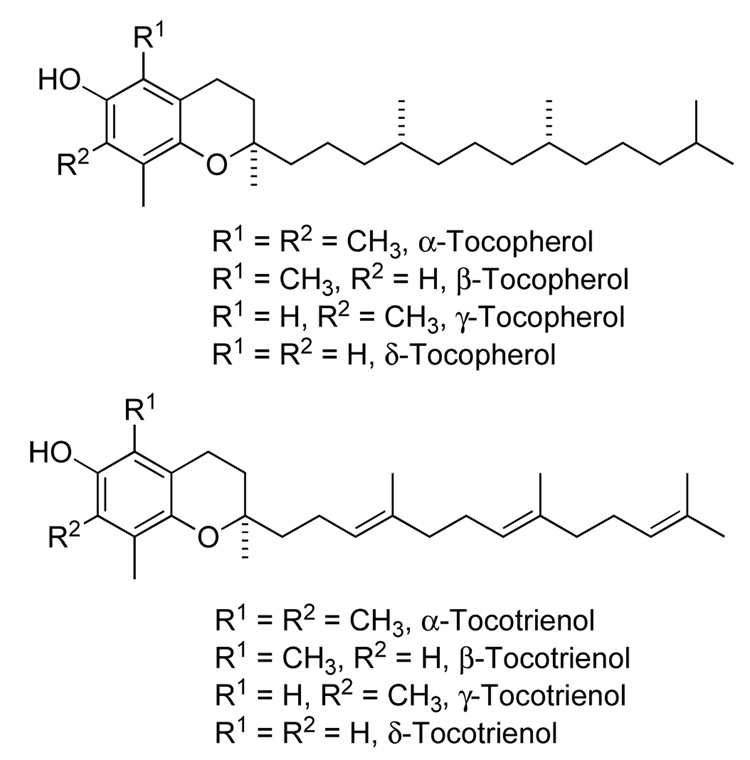
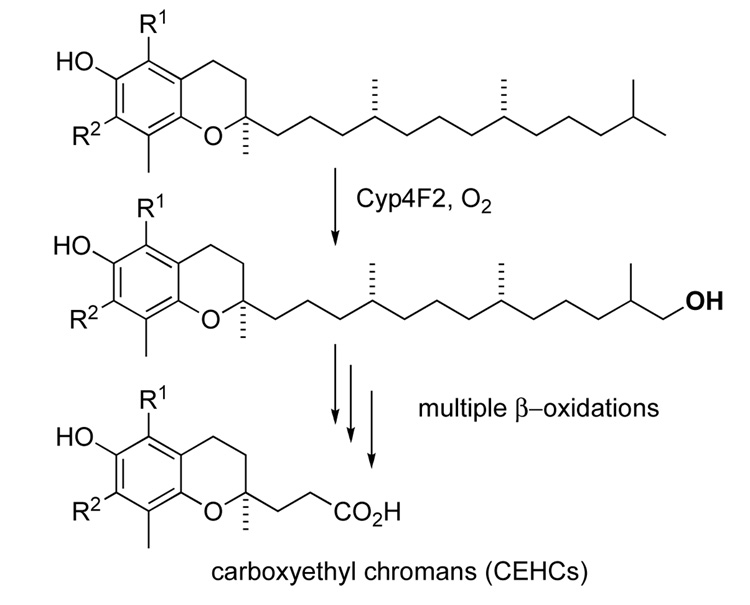
The metabolism of non-retained tocopherols (i.e. non-α-tocopherols and tocotrienols, see Figure 1) is initiated in human cells by a cytochrome P450 monooxygenase, Cyp4F2, and its orthologs in other species. The enzyme activity is referred to as tocopherol-ω-hydroxylase.8 This enzyme metabolizes all forms of vitamin E by placing a hydroxy group at the terminus of the side chain. This is illustrated in Figure 2 for tocopherols, but is the same for the tocotrienols.
Figure 1.
Structures of naturally occurring tocopherols and tocotrienols.
Figure 2.
Illustration of the monoxygenase-catalyzed primary ω-oxidation step in the metabolism of tocols (here shown for tocopherols) followed by repeated rounds of β-oxidation that results in the formation of carboxyethyl chromans.
Recently, it has been noted that γ-tocopherol and the tocotrienols have biological activities that are different that α-tocopherol. γ-Tocopherol is known to act as an anti-inflammatory, possibly by mechanisms different than α-tocopherol9–17 and to scavenge reactive nitrogen species such as peroxynitrite.18, 19 The tocotrienols have been demonstrated to have significantly different biological activities from the tocopherols20–22 including inhibition of cholesterol biosynthesis23–25, anti-cancer effects26–28, and more recently protection against glutamate-induced neurodegeneration in animal models of stroke.29–32
To take advantage of the promising biological activities of γ-tocopherol and the tocotrienols requires overcoming their short half-lives of retention in plasma and tissues.33 The chief reason for their poor bioavailability and short plasma half-lives is their rapid (in comparison to α-tocopherol) oxidative metabolism by the above-mentioned tocopherol-ω-hydroxylase. Any nutritional or chemotherapy involving the use of tocols other than α-tocopherol will suffer from the extremely fast metabolism of these forms of vitamin E. This makes it very difficult to raise plasma levels and thus tissue levels of, for instance, α- or δ-tocotrienol when taken orally. The promising biological activities observed for tocotrienols such as protection from glutamate dependent neurodegeneration in models of stroke would thus benefit enormously by elevating the circulating levels of tocotrienol by inhibiting its metabolism.
We describe here the design, synthesis and biological testing of α-tocopherol derivatives that act as specific inhibitors of tocopherol-ω-hydroxylase and greatly extend the biological half-lives of non-α-tocopherols and tocotrienols.
2. Results and Discussion
2.1 Synthesis
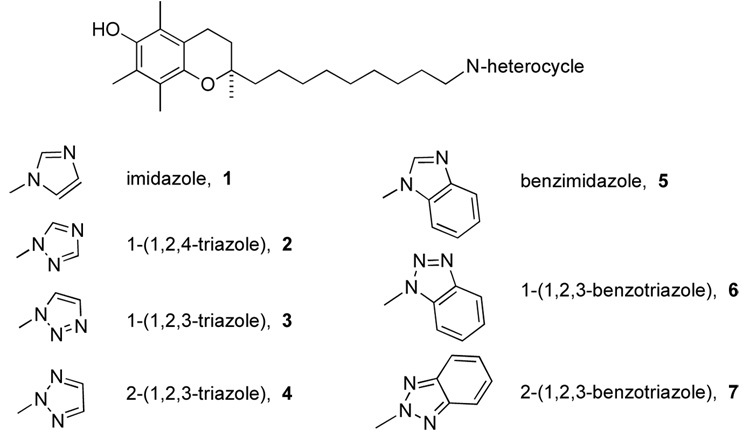
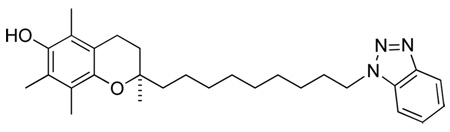
Figure 3 shows the compounds prepared; imidazole, triazole, and benzimidazole derivatives of α-tocopherol, where the isoprenyl side chain has been modified to incorporate the nitrogen heterocycle that is expected to acts as a ligand at the heme-iron atom of the Cyp4F2.
Figure 3.
Structures of the N-heterocycle-containing chromanols prepared in this work.
The use of nitrogen heterocycles as ligands at heme iron atoms has been used previously in the design of many agrichemical fungicides.34–36 There are also some antifungal human therapeutics37, 38 including the imidazoles clotrimazole, miconazole, ketoconazole, and triazoles such as fluconazole. All of these compounds act as heme ligands (and thus inhibitors) of the fungal sterol 14[a]-demethylase also known as Cyp51.37, 38 An analogous design principle has been used in the development of inhibitors of retinoic acid metabolism by Cyp2C8 and Cyp26.39–43 These compounds appear now to have utility in the treatment in retinoid responsive cancers.44
The inhibition of tocopherol metabolism is known to occur using the methylenedioxy-containing lignan sesamin 8, 45–49 but this requires micromolar concentrations of sesamin for complete inhibition in cell culture assays versus less than 0.25 µM for compound 1. Furthermore, sesamin is known to inhibit other enzymes such as fatty acid delta-5-desaturase.50
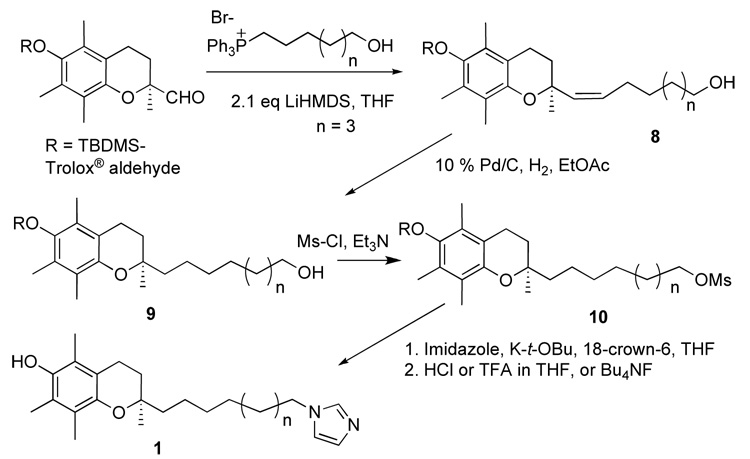
In general the syntheses followed the first steps of a method we reported earlier51, 52 to form a chromanol that replaces the phytyl side chain with a C9 alkyl extension terminating in an alcohol group. The alcohol is derivatized to the mesylate then displaced with N-hetereocyclic anions to give compounds 1–7. The process is illustrated below for compound 1.
Of the compounds prepared, only the smaller imidazole and triazoles showed inhibitory activity, while the larger benzimidazole 5 and benzotriazoles 6 and 7 had no activity. This can be understood by considering the shape of the active site for CYP4F2 that tends to perform ω-hydroxylations on long chain substrates such a leukotrienes (LTB4) and arachidonic acid.8, 53–55 We prepared the benzimidazole and benzotriazoles because CYP4F2 also shows ethoxycoumarin O-deethylase activity,53 suggesting that larger ring systems might also be acceptable substrates in this series of tocopherol analogues. For compounds 5–7 the benzimidazoles and benzotriazoles may be too large to be bound in the active site, or when bound they have difficulty offering a N-atom as a ligand to the heme iron.
2.2 Biological Assessment
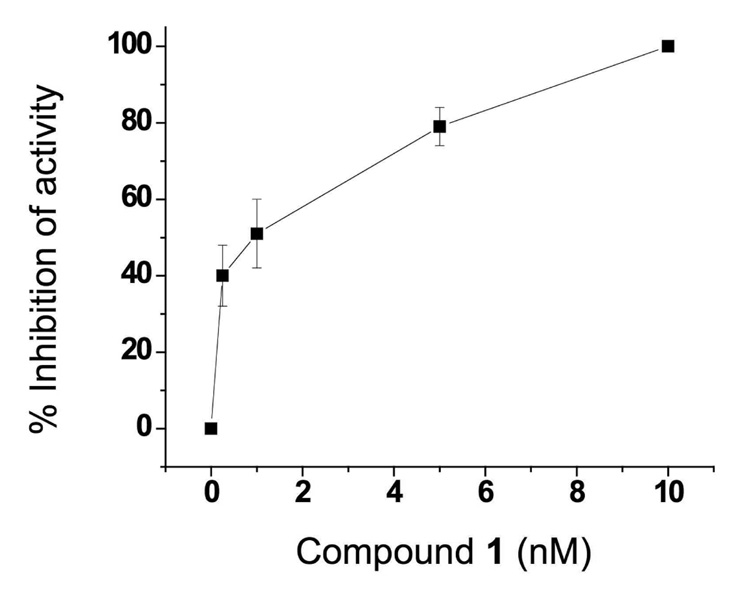
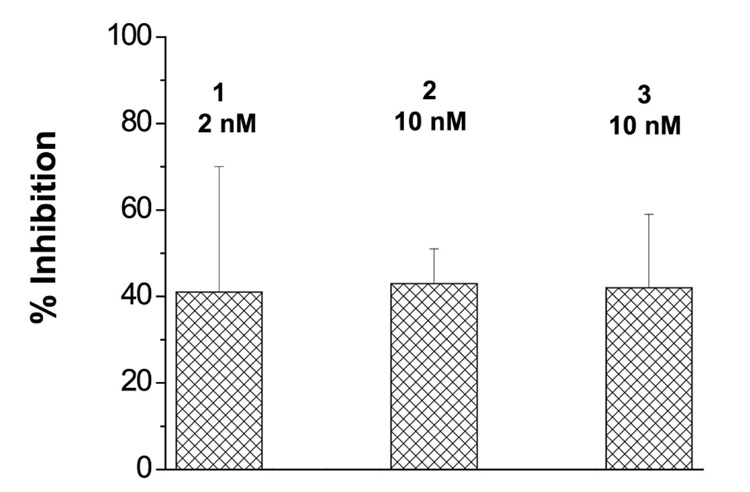
Compound 1–7 were tested in cultured HepG2 liver cells by following the metabolism of γ-tocopherol. Reduction in concentration of 3’- and 5’-carboxychromanol metabolites in the culture media following incubation of the cells with γ-tocopherol and various concentrations of one of compounds 1–7 was illustrative of inhibition of the first step of oxidative metabolism (ω-hydroxylation). Cells were pre-incubated with the inhibitor for 4 hr, then the media replaced with fresh media containing 25 µM γ-tocopherol and the inhibitor. After 48 hr of incubation the media was analyzed for γ-CEHC. The imidazole analogue 1 showed complete inhibition of γ-tocopherol metabolism at concentrations as low as 250 nanomolar (nM). After titrating this dose lower we obtained an ED50 value – effective dose for 50% inhibition – of approximately 1.0 nM (Figure 4). Complete inhibition by 1 was also obtained when cells were exposed to the inhibitor only during a 6 hr pre-incubation period. There was no effect of 1 on total cell protein, and all monolayers appeared normal in morphology. The 1,2,4-triazole 2 had reduced inhibition with 42% inhibition at 20 nM and the 1,2,3-triazole 3 showed only partial inhibition (~ 36%) at 1.0 µM concentration. None of the benzimidazole or benzotriazole derivatives showed any inhibition of γ-tocopherol metabolism in this assay. These results demonstrate that 1 is a remarkably potent inhibitor of human tocopherol ω-hydroxylase as expressed in human hepatoblastoma cultures.
Figure 4.
Inhibition of metabolism of γ-tocopherol in HepG2 cultures by imidazole-containing chromanol, 1.
2.3 Effect of 1 on tissue enrichment in δ-tocopherol in mice in vivo
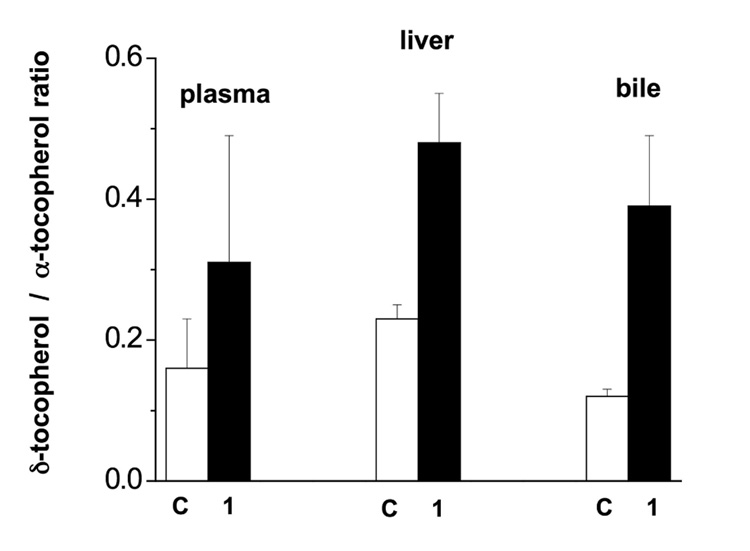
α-Tocopherol is a poor substrate for tocopherol ω-hydroxylase, thus as expected there was no evidence that α-tocopherol status was affected by feeding δ-tocopherol or 1. Therefore, data in Figure 5 are presented as the δ-tocopherol / α-tocopherol ratio, where an increase in the ratio reflects enrichment in δ-tocopherol relative to α-tocopherol, a meaningful endpoint.
Figure 5.
Effect of 1 on the tissue enrichment in δ-tocopherol in mice. Data are means and standard deviation. C = control; 1 = (R)-2-(9-(1H-imidazol-1-yl)nonyl)-2,5,7,8-tetramethylchroman-6-ol.
Feeding of 1 to mice resulted in a doubling in the δ-tocopherol / α-tocopherol ratio in mouse liver (P < 0.05), an expected outcome if 1 inhibited the metabolism of δ-tocopherol (a good tocopherol ω-hydroxylase substrate) but not α-tocopherol, a poor substrate. In addition, the corresponding ratio in bile was tripled in mice fed 1 (P<0.05), indicating that at least some of the unmetabolized δ-tocopherol was being secreted into bile.
These results clearly demonstrate that 1 is effective in inhibiting tocopherol ω-hydroxylase activity in vivo and in increasing tissue concentrations of tocopherols that are good substrates of that enzyme.
2.4 Cytochrome P450 selectivity of imidazole and triazole compounds: Effect on CYP3A4 activity
Ideally the novel imidazole and triazole tocopherol ω-hydroxylase inhibitors would be specific for Cyp4F2 activity, with no effect on other cytochrome P450 enzymes. The major human liver cytochrome P450 is CYP3A4, and its model activity is the 6-hydroxylation of testosterone. The effect of 1, 2, and 3 on testosterone 6-hydroxylase activity was determined in HepG2/C3A cell culture, and in commercially available insect cell microsomes expressing only recombinant human CYP3A4 (BD-Gentest, Woburn, MA). All three compounds inhibited testosterone-6-hydroxylase activity in HepG2/C3A cultures as shown in Figure 6. 1 was more potent than either of the triazole compounds 2 or 3.
Figure 6.
Differing inhibition of CYP3A4 (testosterone-6-hydroxylase) activity by compounds 1, 2, and 3, in HepG2/C3A cultures.
2.5 Inhibition of CYP3A4 in microsomes
When tested with CYP3A4 microsomes, 4µM 1 inhibited testosterone-6-hydroxylase activity by 90 percent, as shown in Figure 7. The results of studies on CYP3A4 activity, as assessed using testosterone-6-hydroxylase as model activity, show that none of the three compounds tested were specific for tocopherol ω-hydroxylase activity, but rather also inhibited CYP3A4 activity as assessed in two different experimental systems. While not ideal, this situation appears analogous to that of many antifungal imidazole and triazole compounds (e.g. ketoconazole) that are therapeutically effective in humans while also exhibiting anti-CYP3A4 activity unrelated to their therapeutic action.
Figure 7.
Inhibition of CYP3A4 (testosterone-6-hydroxylase) activity by compound 1 in insect cell microsomes.
3. Summary
The N-heterocyclic derivatives of α-tocopherol prepared in this study were designed as potential P450 enzyme inhibitors based on the well-established use of N-heterocycles as competitive inhibitors of these monooxygenases in agrochemical and therapeutic fungicides. We were successful in preparing (R)-2-(9-(1H-imidazol-1-yl)nonyl)-2,5,7,8-tetramethylchroman-6-ol, 1, which is an excellent inhibitor of CYP4F2 (ED50 ~ 1 nM in HepG2 cells) and raised the level of δ-tocopherol in mice when 1 was provided at 500 mg per kg of diet that also contained δ-tocopherol. Our preliminary investigation into the P450 isoform specificity showed that 1 had considerable activity against the major human P450 isoform in liver CYP3A4, but not nearly to the same extent as CYP4F2. Thus, compound 1 can be considered a possible drug candidate that might allow the therapeutic use of tocols that have shown promise in treating disease. This is especially true of the tocotrienols that have very short lifetimes in plasma.
4. Experimental
4.1 Materials
All starting materials for synthesis were purchased from Sigma-Aldrich (Oakville, Ontario) and used without any further purification. (S)-Trolox was a kind gift of Dr. Thomas Netscher, DSM Nutritional Products, Basel, Switzerland. Solvents were purchased from Caledon and, where indicated, were dried under argon prior to use. Dichloromethane (CH2Cl2), hexane, and triethylamine (Et3N) were distilled from calcium hydride (CaH2). Tetrahydrofuran (THF) was dried by distillation over sodium and benzophenone. Dry methanol (MeOH) was obtained by distillation from magnesium and a catalytic amount of iodine.
4.2 Analytical Methods
Preparative chromatography was carried out on silica gel (200–300Å mesh) with the indicated solvent systems. Analytical thin layer chromatography (TLC) was performed on 0.25 mm pre-coated silica gel 60Å F-254 plates (Merck). Visualization of the TLC plates was achieved using an ultraviolet (UV) lamp at 254 nm and exposure to iodine vapour, or immersion in 4% H2SO4 in methanol followed by heating.
NMR spectra were recorded using a Bruker Advance DPX-300 Digital FT-NMR spectrometer at 300 MHz (1H) and 75 MHz (13C). Deuterated chloroform (99.8% pure, Cambridge Isotope Laboratories, Inc.) was used as the solvent unless otherwise noted with the internal reference of residual chloroform (1H = 7.24 ppm, 13C = 77.0 ppm). Chemical shifts are reported in ppm (δ) (multiplicity, number of protons, assignment, and coupling constant in Hz). Multiplicity is designated using the following abbreviations: s (singlet), d (doublet), t (triplet), q (quartet), m (multiplet), br (broad). Mass spectra (MS) were recorded on a Carlo Erba/Kratos GC/MS Concept 1S double focusing mass spectrometer interfaced to a Kratos DART acquisition system and a SUN SPARC workstation. Samples were introduced through a direct inlet system and ions were generated using electron impact (EI) at 70 eV and are reported as m/z values for the parent peak and major fragment ions.
4.3 Synthesis
Details on the synthesis of long chain alcohol 9 have been provided elsewhere.52
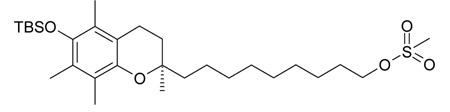
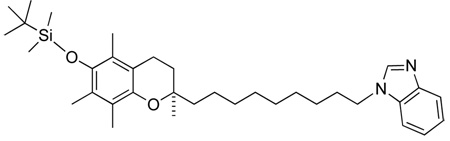
(R)-9-(6-tert-butyl-dimethyl-silanyloxy)-2,5,7,8-tetramethylchroman-2-yl)nonyl methanesulfonate, 10
500 mg (1.08 mmol) of (R)-9-(6-tert-butyl-dimethyl-silanyloxy)-2,5,7,8-tetramethylchroman-2-yl)nonanol 9 was dissolved in dry DCM (10 ml) under argon atmosphere, followed by the addition of triethylamine (224 µl), MsCl (mesylchloride) (125 µl) and a catalytic amount of DMAP (dimethylaminopyridine) at 0 °C. After 15 min the cooling bath was removed and the reaction stirred at r.t for 1.5 h. TLC monitoring showed complete conversion in 100% DCM as mobile phase. Extraction with water and DCM afforded crude product that was obtained following evaporation of the solvent under reduced pressure. Column chromatography on silica (100% DCM) gave pure (7) in high yields (following high vacuum). (90%, 526 mg, 0.97 mmol) TLC: Rf= 0.50 (100% DCM)
1H-NMR (CDCl3); δ 4.24 (t, 2H, C9′-CH2, J=6.408), 3.02 (s, 3H, SCH3), 2.57 (t, 2H, O-CH2, J=6.782), 2.12 (s, 3H, Ar-CH3), 2.09 (s, 3H, Ar-CH3), 2.07 (s, 3H, Ar-CH3), 1.80 (m, 1H)
13C-NMR (CDCl3); δ 145.90, 144.07, 125.85, 123.53, 122.66, 117.51, 74.46, 70.19, 39.58, 37.39, 31.55, 30.11, 29.49, 29.37, 29.14, 29.03, 26.12, 25.42, 23.83, 23.60, 20.91, 18.62, 14.34, 13.41, 11.96, −3.34
MS[EI+] m/z 540 (M+, 3%), 444 (13.4%), 97 (56.2%), 57 (100%)
HRMS (EI): calculated for C29H52O5SSi: 540.33047 Found 540.32732
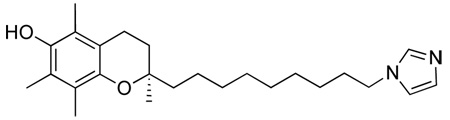
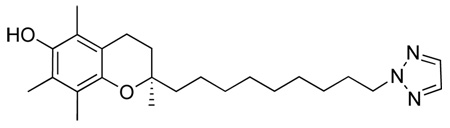
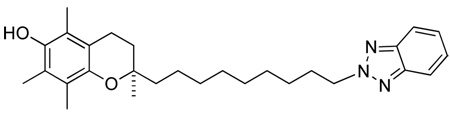
(R)-2-(9-(1H-imidazol-1-yl)nonyl)-2,5,7,8-tetramethylchroman-6-ol, 1
A solution of (R)-9-(6-ter-butyl-dimethyl-silanyloxy)-2,5,7,8-tetramethylchroman-2-yl)nonyl methansulfonate, 10 (500 mg, 0.924 mmol) in dry ACN (5 ml) was added dropwise at room temperature under argon to a suspension of imidazole (126 mg, 1.849 mmol), KOtBu (207 mg, 1.849 mmol) and 18-crown-6-ether (25 mg, 0.0924 mmol) in dry ACN (10 ml). The mixture was refluxed (90°C) under argon atmosphere for 15 h. After cooling to room temperature, the resulting white precipitate was filtered off and the remaining organic phase acidified to pH 6 using 10% HCl. The reaction was then diluted with DCM (50 ml), washed several times with H2O (3 × 30 ml), dried with Na2SO4, decanted, and concentrated under reduced pressure to give a brown oil. The crude product was purified by column chromatography (DCM to DCM/MeOH 100:1.5) to yield a light yellow oil (260 mg, 71%). On inspection of this product it was clear that not only had the imidaozle added to the chain terminus as expected, but the silyl protecting group had also been removed.
1H-NMR (CDCl3); δ 7.48 (s, 1H), 7.07 (s, 1H), 6.91 (s, 1H), 5.30 (s, 1H), 3.91 (t, 2H, J=7.1 Hz), 2.63 (t, 2H, J=~6 Hz), 2.24 (s, 3H), 2.19 (s, 3H), 2.15 (s, 3H), 1.89-1.69 (m, 4H), 1.66-1.53 (m, 2H), 1.49-1.41 (m, 2H), 1.311 (br m, 10H), 1.27 (s, 3H)
13C-NMR (CDCl3); δ 145.32, 136.90, 128.91, 122.75, 122.20, 120.21, 118.84, 117.13, 74.35, 53.53, 47.10, 39.53, 31.68, 31.04, 30.11, 29.50, 29.38, 29.09, 26.53, 23.91, 23.62, 20.86, 12.78, 11.89, 11.83.
MS[EI+] For C25H38N2O2; m/z 398 (M+, 79%), 387 (13%), 235 (100%), 203 (10%), 179 (9%), 165 (26%). 137 (13%), 123 (10%), 96 (11%).
HRMS (EI): calculated for C25H38N2O2: 398.29333. Found 398.29336
(R)-1-(9-(6-(tert-butyldimethylsilyloxy)-2,5,7,8-tetramethylchroman-2-yl)nonyl)-1H-1,2,4-triazole
Clear colourless oil. Rf 0.4 (EtOAc). Purified by silica gel column chromatography using 100% Et2O.
1H-NMR (CDCl3); δ 8.04 (s, 1H), 7.94 (s, 1H), 4.15 (t, 2H, J=7Hz), 2.56 (t, 2H, J=6.5 Hz), 2.11 (s, 3H), 2.08 (s, 3H), 2.07 (s, 3H), 1.83 (m, 2H), 1.76 (m, 2H, J=6.6 Hz), 1.61-1.49 (m, 2H), 1.42 (m, 2H), 1.28 (m, 10H), 1.23 (m, 3H), 1.06 (s, 9H), 0.13 (s, 6H)
13C-NMR (CDCl3); δ 151.85, 145.90, 144.06, 125.81, 123.49, 122.63, 117.47, 74.42, 49.69, 39.54, 31.55, 30.07, 29.77, 29.44, 29.32, 28.99, 26.43, 26.12, 23.83, 23.56, 20.90, 18.60, 14.34, 13.41, 11.96, −3.33.
MS[EI+] For C30H51N3O2Si; m/z 513 (M+, 37%), 221 (11%), 205 (24%), 149 (12%), 138 (10%), 129 (14%).
HRMS (EI): calculated for C30H51N3O2Si: 513.37506. Found 513.37517
(R)-2-(9-(1H-1,2,4-triazol-1-yl)nonyl)-2,5,7,8-tetramethylchroman-6-ol, 2
Off white solid. m.p. 114–115 °C Rf 0.31 (EtOAc). Purified by silica gel column chromatography using 100% Et2O.
1H-NMR (CDCl3); δ 8.03 (s, 1H), 7.93 (s, 1H), 5.13 (s, 1H), 4.14 (t, 2H, J=7Hz), 2.59 (t, 2H, J=6.5 Hz), 2.16 (s, 3H), 2.11 (s, 3H), 2.10 (s, 3H), 1.86 (m, 2H), 1.76 (m, 2H, J=6.6 Hz), 1.59-1.49 (m, 2H), 1.43 (m, 2H), 1.28 (m, 10H), 1.23 (m, 3H).
13C-NMR (CDCl3); δ 151.73, 144.49, 144.74, 142.76, 122.47, 117.28, 74.43, 49.75, 39.44, 31.58, 30.04, 29.76, 29.43, 29.30, 28.99, 26.42, 23.84, 23.55, 20.78, 12.42, 11.81.
MS[EI+] For C24H37N3O2; m/z 399 (M+, 68%), 236 (16%), 205 (12%), 203 (13%), 165 (100%), 121 (11%).
HRMS (EI): calculated for C24H37N3O2: 399.28858. Found 399.28924
(R)-1-(9-(6-(tert-butyldimethylsilyloxy)-2,5,7,8-tetramethylchroman-2-yl)nonyl)-1H-1,2,3-triazole
A solution of (R)-9-(6-ter-butyl-dimethyl-silanyloxy)-2,5,7,8-tetramethylchroman-2-yl)nonyl methansulfonate(110 mg, 0.200 mmol) in dry ACN (2 ml) was added dropwise at room temperature under argon to a suspension of 1H-1,2,3-triazole (27 mg, 0.400 mmol), KOtBu (45 mg, 0.400 mmol) and 18-crown-6-ether (5 mg, 0.020 mmol) in dry ACN (4 ml). The mixture was refluxed (90°C) under argon atmosphere for 15 h. Upon cooling to room temperature, the resulting white precipitate was filtered off and the remaining organic phase acidified to pH 6 using 10% HCl. The reaction was then diluted with DCM (25 ml), washed several times with H2O (3 × 20 ml), dried with Na2SO4, decanted, and concentrated under vacuum to give a yellow oil. The crude material was purified by column chromatography (DCM to Et2O) affording the product as a clear, colourless oil. (42 mg, 40%) Rf = 0.42 (Et2O)
1H-NMR (CDCl3); δ 7.71 (s, 1H), 7.54 (s, 1H), 4.39 (t, 2H, J=7.2 Hz), 2.56 (t, 2H, J=6.7 Hz), 2.11 (s, 3H), 2.08 (s, 3H), 2.07 (s, 3H), 1.92 (m, 2H), 1.79 (m, 2H, J=6.9 Hz), 1.65-1.49 (m, 2H), 1.45 (m, 2H), 1.32-1.28 (m, 10H), 1.23 (s, 3H), 1.06 (s, 9H), 0.132 (s, 6H).
13C-NMR (CDCl3); δ 145.90, 144.06, 133.73, 125.82, 123.52, 123.10, 122.64, 117.50, 74.44, 50.18, 39.55, 31.55, 30.33, 30.07, 29.44, 29.31, 28.97, 26.45, 26.11, 23.82, 23.57, 20.90, 18.60. 14.33, 13.41, 11.96, −3.33
MS[EI+] For C30H51N3O2Si; m/z 513 (M+, 17%), 205 (10%), 129 (10%), 82 (100%)
HRMS (EI): calculated for C30H51N3O2Si: 513.37506. Found 513.37517
(R)-2-(9-(1H-1,2,3-triazol-1-yl)nonyl)-2,5,7,8-tetramethylchroman-6-ol, 3
A solution of tetrabutylammonium fluoride (TBAF) (1M in THF, 250 µL) was added dropwise via syringe to a stirred solution of (R)-1-(9-(6-(tert-butyldimethylsilyloxy)-2,5,7,8-tetramethylchroman-2-yl)nonyl)-1H-1,2,3-triazole (65 mg, 0.126 mmol) in dry THF (5 ml). The mixture was stirred at room temperature for 30 min until starting material was not detected by TLC. The reaction was then quenched with 500 µL of 1N HCl and diluted with 25 ml of ether, to which an additional 10 ml of H2O was added. The water phase was extracted again with ether (2 × 25 ml), dried over anhydrous Na2SO4, filtered, and concentrated under reduced pressure. The crude product was chromatographed on silica gel (Et2O) to give the pure product (TLC Rf = 0.49, EtOAc) as an off-white/yellow solid, m.p. 103–106 °C. (35 mg, 70%)
1H-NMR (CDCl3); δ 7.71 (s, 1H), 7.54 (s, 1H), 4.55 (s, 1H), 4.39 (t, 2H, J=7.2 Hz), 2.62 (t, 2H, J=6.7 Hz), 2.18 (s, 3H), 2.13 (s, 3H), 2.12 (s, 3H), 1.91 (m, 2H), 1.79 (m, 2H, J=6.9 Hz), 1.65-1.51 (m, 2H), 1.48-1.41 (m, 2H), 1.32-1.28 (m, 10H), 1.24 (s, 3H).
13C-NMR (CDCl3); δ 145.55, 144.65, 133.72, 123.12, 122.52, 121.24, 118.71, 117.32, 74.45, 50.20, 39.45, 31.55, 30.33, 30.04, 29.43, 29.29, 28.97, 26.44, 23.82, 23.55, 20.76, 12.29, 11.80, 11.35.
MS[EI+] For C24H37N3O2 399.6 (M+, 68%), 236 (20%) 205 (12%), 203 (21%), 165 (100%), 121 (11%)
HRMS (EI): calculated for C24H37N3O2: 399.28858. Found 399.28812
(R)-2-(9-(6-(tert-butyldimethylsilyloxy)-2,5,7,8-tetramethylchroman-2-yl)nonyl)-2H-1,2,3-triazole
Clear colourless oil. Rf=0.41 (DCM). Purified by sílica gel column chromatography using 100% DCM to 100% Et2O.
1H-NMR (CDCl3); δ 7.60 (s, 2H), 4.46 (t, 2H, J=7.1 Hz), 2.57 (t, 2H, J=6.8 Hz), 2.12 (s, 3H), 2.10 (s, 3H), 2.08 (s, 3H), 1.98 (m, 2H, J=6.7 Hz), 1.74 (m, 2H, J=7.1 Hz), 1.62-1.52 (m, 2H), 1.50-1.42 (m, 2H), 1.37-1.29 (m, 10H), 1.24 (s, 3H), 1.07 (s, 9H), 0.143 (s, 6H).
13C-NMR (CDCl3); δ 145.91, 144.07, 133.79, 125.84, 123.50, 122.67, 117.49, 74.45, 54.88, 39.59, 31.55, 30.11, 29.75, 29.47, 29.35, 29.02, 26.47, 26.13, 23.83, 23.61, 20.91, 18.62, 14.34, 13.41, 11.96, −3.2
MS[EI+] For C30H51N3O2Si; m/z 513 (M+, 83%), 332 (12%), 317 (10%), 279 (20%), 221 (20%), 220, (21%), 168 (44%), 128 (12%).
HRMS (EI): calculated for C30H51N3O2Si: 513.37506. Found 513.37446
(R)-2-(9-(2H-1,2,3-triazol-2-yl)nonyl)-2,5,7,8-tetramethylchroman-6-ol, 4
Off-white solid, m.p. 61–62 °C. Rf = 0.60 (EtOAc) Purified by sílica gel column chromatography using 100% Et2O
1H-NMR (CDCl3); δ 7.61 (s, 2H), 4.45 (t, 2H, J=7.3 Hz), 4.42 (br s, 1H), 2.62 (t, 2H, J=6.8 Hz), 2.18 (s, 3H), 2.13 (s, 6H), 2.04-1.94 (m, 2H), 1.88-1.73 (m, 2H), 1.66-1.49 (m, 2H),1.28 (brs, 10H), 1,24 (s, 3H).
13C-NMR (CDCl3); δ 144.58, 133.80, 122.58, 121.18, 118.65, 117.34, 74.47, 54.89, 39.42, 31.56, 30.05, 29.75, 29.43, 29.30, 29.00, 26.45, 23.83, 23.56, 20.77, 12.26, 11.79, 11.32.
MS[EI+] For C24H37N3O2; m/z 399 (M+, 36%), 231 (10%), 165 (78%), 122 (20%), 106 (21%), 79 (100%).
HRMS (EI): calculated for C24H37N3O2: 399.28858. Found 399.28895
(R)-1-(9-(6-(tert-butyldimethylsilyloxy)-2,5,7,8-tetramethylchroman-2-yl)nonyl)-1H-benzo[d]imidazole
Clear colourless oil. Rf 0.45 (EtOAc). Purified by silica gel column chromatography using DCM:Et2O (3:2).
1H-NMR (CDCl3); δ 7.91 (s, 1H), 7.85 (m, 1H), 7.44-7.41 (m, 1H), 7.35-7.28 (m, 2H), 4.19 (t, 2H, J=7.1 Hz), 2.58 (t, 2H, J=6.5 Hz), 2.14 (s, 3H), 2.11 (s, 3H), 2–10 (s, 3H), 1.94-1.84 (m, 2H), 1.82-1.75 (m, 2H), 1.67-1.51 (m, 2H), 1.53-1.43 (m, 2H), 1.34-1.30 (m, 10H), 1.26 (s, 3H), 1.09 (s, 9H), 0.159 (s, 6H).
13C-NMR (CDCl3); δ 145.93, 144.09, 143.90, 142.94, 133.85, 125.84, 123.53, 122.78, 122.67, 122.00, 120.40, 117.52, 109.68, 74.45, 45.10, 39.57, 31.58, 30.10, 29.49, 29.39, 29.12, 26.83, 26.15, 23.86, 23.59, 20.93, 18.63, 14.37, 13.45, 12.00, −3.30
MS[EI+] For C35H54N2O2Si; m/z 562 (M+, 11%), 258 (13%), 230 (17%), 229 (14%), 220 (28%), 205 (100%), 201 (11%), 189 (22%), 187 (19%), 136 (25%), 124 (16%), 122 (20%).
HRMS (EI): calculated for C35H54N2O2Si: 562.39546. Found 562.39774
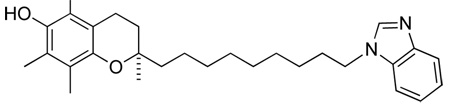
(R)-2-(9-(1H-benzo[d]imidazol-1-yl)nonyl)-2,5,7,8-tetramethylchroman-6-ol, 5
Light yellow oil, Rf = 0.31 (EtOAc). Purified by silica gel column chromatography using DCM:Et2O (3:2)
1H-NMR (CDCl3); δ 7.90 (s, 1H), 7.84-7.81 (m, 1H), 7.44-7.40 (m, 1H), 7.33-7.29 (m, 2H), 5.15 (br s, 1H), 4.17 (t, 2H, J=7.1 Hz), 2.62 (t, 2H, J=6.5 Hz), 2.20 (s, 3H), 2.15, (s, 3H), 2.13 (s, 3H), 1.91-1.85 (m, 2H), 1.81-1.71 (m 2H), 1.66-1.54 (m, 2H), 1.52-1.39 (m, 2H), 1.33-1.28 (m, 10H), 1.24 (s, 3H).
13C-NMR (CDCl3); δ 145.48, 144.77, 143.77, 142.89, 133.80, 122.80, 122.48, 122.04, 121.60, 120.36, 119.06, 117.29, 109.68, 74.43, 45.12, 39.42, 31.58, 30.05, 29.81, 29.44, 29.35, 29.09, 26.80, 23.86, 23.56, 20.78, 12.41, 11.81, 11.47
MS[EI+] For C29H40N2O2; m/z 448 (M+, 1%), 437 (1%), 286 (11%), 243 (17%), 229 (25%), 205 (23%), 145 (12%), 131 (35%) 118 (28%), 86 (64%), 84 (100%)
HRMS (EI): calculated for C29H40N2O2: 448.30898. Found 448.30867
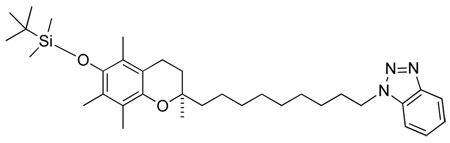
(R)-1-(9-(6-(tert-butyldimethylsilyloxy)-2,5,7,8-tetramethylchroman-2-yl)nonyl)-1H-benzo[d][1,2,3]triazole
Clear colourless oil. Rf 0.19 (DCM). Purified by silica gel column chromatography using 100% DCM to 100% Et2O.
1H-NMR (CDCl3); δ 8.08 (d, 1H, J=7.3 Hz), 7.52 (m, 2H), 7.38 (m, 1H), 4.65 (t, 2H, J=7.1 Hz), 2.57 (t, 2H, J=6.7 Hz), 2.12 (s, 3H), 2.10 (s, 3H), 2.08 (s, 3H), 2.03 (m, 2H), 1.92-1.72 (m, 2H), 1.65-1.52 (m, 6H), 1.49-1.36 (br m, 6H), 1.28 (s, 3H), 1.07 (s, 9H), 0.144 (s, 6H).
13C-NMR (CDCl3); δ 145.91, 144.08, 132.97, 127.11, 125.82, 123.74, 123.51, 122.65, 120.03, 117.50, 109.34, 74.45, 48.23, 39.57, 31.56, 30.08, 29.70, 29.47, 29.33, 29.04, 26.72, 26.14, 23.84, 23.58, 20.92, 18.62, 14.36, 13.43, 11.97, −3.31.
MS[EI+] For C34H53N3O2Si; m/z 563 (M+, 14%), 506 (3%), 259 (6%), 250 (5%), 221 (5%), 205 (4%), 174 (5%), 149 (16%).
HRMS (EI): calculated for C34H53N3O2Si: 563.39071. Found 563.39020
(R)-2-(9-(1H-benzo[d][1,2,3]triazol-1-yl)nonyl)-2,5,7,8-tetramethylchroman-6-ol, 6
Light yellow oil, TLC Rf = 0.55 (Et2O). Purified by sílica gel column chromatography using 100% DCM to 100% Et2O
1H-NMR (CDCl3); δ 8.08 (d, 1H, J=8.3 Hz), 7.52 (m, 2H), 7.38 (dt, 1H, J1=7.3 Hz, J2=0.9 Hz), 5.31 (s, 1H), 4.65 (t, 2H, J=7.1 Hz), 2.61 (t, 2H, J=6.8 Hz), 2.18 (s, 3H), 2.14 (s, 3H), 2.12 (s, 3H), 2.06-1.97 (m, 2H), 1.86-1.73 (m, 2H, J=6.8 Hz),1.65-1.51 (m, 2H), 1.47-1.35 (bm, 6H), 1.27 (bs, 6H), 1.24 (s, 3H).
13C-NMR (CDCl3); δ 145.51, 144.64, 132.96, 127.13, 123.77, 122.54, 121.22, 120.03, 118.69, 117.33, 108.34, 74.45, 48.25, 39.46, 31.55, 30.04, 29.69, 29.44, 29.30, 29.03, 26.71, 23.81, 23.56, 20.77, 15.27, 12.29, 11.80, 11.35
MS[EI+] For C28H39N3O2 m/z 449 (M+, 41%), 438 (32%), 286 (13%), 205 (12%), 203 (17%), 165 (60%),146 (13%), 137 (12%), 132 (21%), 120 (28%).
HRMS (EI): calculated for C28H39N3O2: 449.30423. Found 449.30393
(R)-2-(9-(6-(tert-butyldimethylsilyloxy)-2,5,7,8-tetramethylchroman-2-yl)nonyl)-2H-benzo[d][1,2,3]triazole
Clear colourless oil. Rf 0.56 (DCM). Purified by sílica gel column chromatography using 100% DCM to 100% Et2O.
1H-NMR (CDCl3); δ 7.90 (ddd, 2H, J1 (apparent)=9.6 Hz, J2(apparent)=3.1 Hz), 7.40 (ddd, 2H, J1 (apparent)=9.6 Hz, J2(apparent)=3.1 Hz), 2.57 (t, 2H, J= 6.7 Hz), 4.75 (t, 2H, J=7.1 Hz), 2.57 (t, 2H, J=6.2 Hz), 2.15 (obscured m, 2H, J=7.2 Hz), 2.13 (s, 3H), 2.13 (s, 3H), 2.10 (s, 3H), 1.92-1.72 (m, 2H, J=7.2 Hz), 1.66-1.50 (m, 2H), 1.47-1.38 (br m, 6H), 1.30 (br s, 6H), 1.24 (s, 3H).
13C-NMR (CDCl3); δ 145.92, 144.30, 126.13, 125.84, 123.50, 122.68, 117.97, 117.49, 74.45, 56.65, 39.59, 31.56, 30.09, 29.48, 29.32, 29.03, 26.57, 26.14, 23.83, 23.60, 20.93, 18.62, 14.35, 13.43, 11.97, −3.31.
MS[EI+] For C34H53N3O2Si; m/z 563 (M+, 14%), 428 (13%), 307 (1-%), 243 (11-%), 229 (12%), 205 (30%), 167 (14%), 149 (69%), 132 (10%), 131 (17%), 120 (22%)
HRMS (EI): calculated for C34H53N3O2Si: 563.39071. Found 563.38909
(R)-2-(9-(2H-benzo[d][1,2,3]triazol-2-yl)nonyl)-2,5,7,8-tetramethylchroman-6-ol, 7
Light yellow oil, Rf = 0.26 (DCM). Purified by sílica gel column chromatography using 100% DCM to 100% Et2O
1H-NMR (CDCl3); δ 7.90 (ddd, 2H, J1 (apparent)=9.6 Hz, J2(apparent)=3.1 Hz), 7.40 (ddd, 2H, J1(apparent)=9.6 Hz, J2(apparent)=3.1 Hz), 4.74 (t, 3H, J=7.1 Hz), 2.62 (t, 2H, J=6.7 Hz), ~ 2.14 (obscured m, 2H), 2.18 (s, 3H), 2.13 (s, 3H), 2.12 (s, 3H), 1.87-1.71 (m, 2H, J=6.8 Hz), 1.67 (s, 1H), 1.65-1.53 (m, 2H), 1.50-1.35 (br m, 6H), 1.28 (br m, 6H), 1.24 (s, 3H)
13C-NMR (CDCl3); δ 145.55, 144.56, 144.27, 126.16, 122.59, 121.14, 118.61, 117.94, 117.35, 74.47, 56.65, 39.41, 31.56, 30.07, 30.02, 29.43, 29.26, 29.01, 26.53, 23.83, 23.55, 20.76, 12.26, 11.79, 11.32.
MS[EI+] For C28H39N3O2; m/z 449 (M+, 46%), 399 (13%), 165 (100%), 120 (16%)
HRMS (EI): calculated for C28H39N3O2: 449.30423 Found 449.30397
4.4 Biological Assessment
Cell Culture
Effect of 1–7 on metabolism of γ-tocopherol in HepG2/C3A hepatoblastoma cultures was assessed as previously described 56. Briefly, compounds 1–7 and γ-tocopherol stock solutions were prepared in ethanol. Confluent monolayers of HepG2/C3A cells (a human hepatoblastoma cell line) were pre-incubated with varying concentrations of 1–7 (or ethanol) for 4 hrs, followed by addition of media containing γ-tocopherol (25 µM final concentration in media), a good substrate of tocopherol hydroxylase and replacement of the test compound (or ethanol). After 48 hrs, culture media was collected and analyzed for ω-oxidation products of γ-tocopherol (the 3’- and 5’-carboxychromanols) by GC-MS, using d9-3’-α-carboxychromanol (α-CEHC) as internal standard.8 In a separate design, confluent monolayers were incubated with 1 µM compound 1 for 6 hr, then for 24 hr with media containing 25 µM γ-tocopherol, but without replacing compound 1.
In order to assess the effect of 1 on CYP3A4 activity, confluent HepG2 cultures were pre-incubated 4 hr with inhibitor (or ethanol control), followed by addition of 50 µM testosterone. After 48 hr incubation, 6-hydroxytestosterone (as the trimethylsilyl ether) was determined in culture media by GC-MS after extraction with ethyl acetate. Data are presented as percent inhibition (means and standard deviation).
Microsomal assays
Insect cell microsomes expressing only recombinant human CYP3A4 (BD-Gentest, Woburn, MA), 20 pmol P450, were incubated 60 min with 200 µM testosterone (50% methanol stock) in the presence or absence of 4 µM 1 and 1 mM NADPH, 0.1 mM potassium phosphate buffer, pH 7.4, 0.25% bovine serum albumin (total reaction volume 0.5 ml). Following the incubation the reaction mixture was deproteinated with 1.0 ml cold ethanol, extracted with hexane / methyl tert-butyl ether (7:1) and analyzed for 6-OH-testosterone and membrane cholesterol as described above. Data is expressed as nmol product per nmol membrane cholesterol.
Animal studies
Two groups of three male FVB/N mice were fed a commercial (Prolab 1000, Agway, Syracuse, NY) ground chow feed enriched with δ-tocopherol (6000 mg per kg diet), with or without 500 mg per kg 1. Compound 1 (in ethanol) and δ-tocopherol were premixed in Enova diglyceride oil (ADM Lao LLC, Decatur, IL), which was then added dropwise to the ground chow while mixing. After 10 days on diet, mice were fasted for 5 hrs, euthanized, and tocopherol concentrations determined in plasma, liver and bile, using GC-MS and d9-α-tocopherol as internal standard.8 Liver samples (50 mg) were extracted using hexane-isopropanol (3:2), and plasma and bile aliquots were extracted with hexane / methyl tert-butyl ether after protein precipitation with ethanol. All animal procedures were approved by the Institutional Animal Care and Use Committee of Cornell University.
Scheme 1.
General synthetic method for the synthesis of N-heterocycle-containing chromanols, exemplified for 1, (R)-2-(9-(1H-imidazol-1-yl)nonyl)-2,5,7,8-tetramethylchroman-6-ol.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Manor D, Morley S. The alpha-tocopherol transfer protein. Vitam Horm. 2007;76:45–65. doi: 10.1016/S0083-6729(07)76003-X. [DOI] [PubMed] [Google Scholar]
- 2.Traber MG, Arai H. Molecular mechanisms of vitamin E transport. Ann. Rev. Nutr. 1999;19:343–355. doi: 10.1146/annurev.nutr.19.1.343. [DOI] [PubMed] [Google Scholar]
- 3.Panagabko C, Morley S, Hernandez M, Cassolato P, Gordon H, Parsons R, Manor D, Atkinson J. Ligand specificity in the CRAL-TRIO protein family. Biochemistry. 2003;42:6467–6474. doi: 10.1021/bi034086v. [DOI] [PubMed] [Google Scholar]
- 4.Hosomi A, Arita M, Sato Y, Kiyose C, Ueda T, Igarashi O, Arai H, Inoue K. Affinity for alpha tocopherol transfer protein as a determinant of the biological activities of vitamin E analogs. FEBS Lett. 1997;409:105–108. doi: 10.1016/s0014-5793(97)00499-7. [DOI] [PubMed] [Google Scholar]
- 5.Swanson JE, Ben RN, Burton GW, Parker RS. Urinary excretion of 2,7, 8-trimethyl-2-(beta-carboxyethyl)-6-hydroxychroman is a major route of elimination of gamma-tocopherol in humans. J Lipid Res. 1999;40:665–671. [PubMed] [Google Scholar]
- 6.Parker RS, Sontag TJ, Swanson JE, McCormick CC. Discovery, characterization, and significance of the cytochrome P450 omega-hydroxylase pathway of vitamin E catabolism. Ann N Y Acad Sci. 2004;1031:13–21. doi: 10.1196/annals.1331.002. [DOI] [PubMed] [Google Scholar]
- 7.Brigelius-Flohe R, Traber MG. Vitamin E: function and metabolism. FASEB J. 1999;13:1145–1155. [PubMed] [Google Scholar]
- 8.Sontag TJ, Parker RS. Cytochrome P450 omega-hydroxylase pathway of tocopherol catabolism. Novel mechanism of regulation of vitamin E status. J Biol Chem. 2002;277:25290–25296. doi: 10.1074/jbc.M201466200. [DOI] [PubMed] [Google Scholar]
- 9.Samandari E, Visarius T, Zingg JM, Azzi A. The effect of gamma-tocopherol on proliferation, integrin expression, adhesion, and migration of human glioma cells. Biochem Biophys Res Commun. 2006;342:1329–1333. doi: 10.1016/j.bbrc.2006.02.110. [DOI] [PubMed] [Google Scholar]
- 10.Mazlan M, Sue Mian T, Mat Top G, Zurinah Wan Ngah W. Comparative effects of alpha-tocopherol and gamma-tocotrienol against hydrogen peroxide induced apoptosis on primary-cultured astrocytes. J Neurol Sci. 2006 doi: 10.1016/j.jns.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 11.Campbell SE, Stone WL, Lee S, Whaley S, Yang HS, Qui M, Goforth P, Sherman D, McHaffie D, Krishnan K. Comparative effects of RRR-alpha- and RRR-gamma-tocopherol on proliferation and apoptosis in human colon cancer cell lines. Bmc Cancer. 2006;6 doi: 10.1186/1471-2407-6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu JHY, Indrawan AP, Ward NC, Clarke MW, Hodgson JM, Proudfoot JM, Puddey IB, Croft KD. Mixed tocopherol supplementation rich in gamma-tocopherol inhibits LTB4 synthesis in human neutrophils: Results from a controlled human intervention trial. Free Radical Research. 2005;39:S84–S84. [Google Scholar]
- 13.Devaraj S, Jialal I. Failure of vitamin E in clinical trials: Is gamma-tocopherol the answer? Nutrition Reviews. 2005;63:290–293. doi: 10.1111/j.1753-4887.2005.tb00143.x. [DOI] [PubMed] [Google Scholar]
- 14.Wagner KH, Kamal-Eldin A, Elmadfa I. Gamma-tocopherol - An underestimated vitamin? Ann Nutr Metab. 2004;48:169–188. doi: 10.1159/000079555. [DOI] [PubMed] [Google Scholar]
- 15.Jiang Q, Wong J, Fyrst H, Saba JD, Ames BN. gamma-Tocopherol or combinations of vitamin E forms induce cell death in human prostate cancer cells by interrupting sphingolipid synthesis. Proc Natl Acad Sci U S A. 2004;101:17825–17830. doi: 10.1073/pnas.0408340102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grammas P, Hamdheydari L, Benaksas EJ, Mou S, Pye QN, Wechter WJ, Floyd RA, Stewart C, Hensley K. Anti-inflammatory effects of tocopherol metabolites. Biochem Biophys Res Commun. 2004;319:1047–1052. doi: 10.1016/j.bbrc.2004.05.082. [DOI] [PubMed] [Google Scholar]
- 17.Jiang Q, Christen S, Shigenaga MK, Ames BN. gamma-tocopherol, the major form of vitamin E in the US diet, deserves more attention. Am J Clin Nutr. 2001;74:714–722. doi: 10.1093/ajcn/74.6.714. [DOI] [PubMed] [Google Scholar]
- 18.Wolf G. Gamma tocopherol: an efficient protector of lipids against nitric oxide initiated peroxidative damage. Nutrition Reviews. 1997;55:376–378. doi: 10.1111/j.1753-4887.1997.tb01566.x. [DOI] [PubMed] [Google Scholar]
- 19.Christen S, Woodall AA, Shigenaga MK, Southwell-Keely PT, Duncan MW, Ames BN. gamma-tocopherol traps mutagenic electrophiles such as NO(X) and complements alpha-tocopherol: physiological implications. Proc Natl Acad Sci U S A. 1997;94:3217–3222. doi: 10.1073/pnas.94.7.3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sen CK, Khanna S, Rink C, Roy S. Tocotrienols: the emerging face of natural vitamin E. Vitam Horm. 2007;76:203–261. doi: 10.1016/S0083-6729(07)76008-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sen CK, Khanna S, Roy S. Tocotrienols in health and disease: The other half of the natural vitamin E family. Mol Aspects Med. 2007 doi: 10.1016/j.mam.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Theriault A, Chao JT, Wang Q, Gapor A, Adeli K. Tocotrienol: A review of its therapeutic potential. Clin. Biochem. 1999;32:309–319. doi: 10.1016/s0009-9120(99)00027-2. [DOI] [PubMed] [Google Scholar]
- 23.Parker RA, Pearce BC, Clark RW, Gordon DA, Wright JJ. Tocotrienols regulate cholesterol production in mammalian cells by post-transcriptional suppression of 3-hydroxy-3-methylglutaryl-coenzyme A reductase. J Biol Chem. 1993;268:11230–11238. [PubMed] [Google Scholar]
- 24.Pearce BC, Parker RA, Deason ME, Dischino DD, Gillespie E, Qureshi AA, Volk K, Wright JJ. Inhibitors of cholesterol biosynthesis. 2. Hypocholesterolemic and antioxidant activities of benzopyran and tetrahydronaphthalene analogues of the tocotrienols. J Med Chem. 1994;37:526–541. doi: 10.1021/jm00030a012. [DOI] [PubMed] [Google Scholar]
- 25.Pearce BC, Parker RA, Deason ME, Qureshi AA, Wright JJ. Hypocholesterolemic activity of synthetic and natural tocotrienols. J Med Chem. 1992;35:3595–3606. doi: 10.1021/jm00098a002. [DOI] [PubMed] [Google Scholar]
- 26.Nesaretnam K, Stephen R, Dils R, Darbre P. Tocotrienols inhibit the growth of human breast cancer cells irrespective of estrogen receptor status. Lipids. 1998;33:461–469. doi: 10.1007/s11745-998-0229-3. [DOI] [PubMed] [Google Scholar]
- 27.Sylvester PW, Shah SJ. Mechanisms mediating the antiproliferative and apoptotic effects of vitamin E in mammary cancer cells. Front Biosci. 2005;10:699–709. doi: 10.2741/1565. [DOI] [PubMed] [Google Scholar]
- 28.Sylvester PW, Shah SJ, Samant GV. Intracellular signaling mechanisms mediating the antiproliferative and apoptotic effects of g-tocotrienol in neoplastic mammary epithelial cells. J Plant Physiol. 2005;162:803–810. doi: 10.1016/j.jplph.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 29.Khanna S, Roy S, Slivka A, Craft TKS, Chaki S, Rink C, Notestine MA, DeVries AC, Parinandi NL, Sen CK. Neuroprotective properties of the natural vitamin E alpha-tocotrienol. Stroke. 2005;36:E144–E152. doi: 10.1161/01.STR.0000181082.70763.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khanna S, Roy S, Ryu H, Bahadduri P, Swaan PW, Ratan RR, Sen CK. Molecular basis of vitamin E action: tocotrienol modulates 12-lipoxygenase, a key mediator of glutamate-induced neurodegeneration. J Biol Chem. 2003;278:43508–43515. doi: 10.1074/jbc.M307075200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sen CK, Khanna S, Roy S. Tocotrienol - The natural vitamin E to defend the nervous system? Vitamin E And Health. 2004;volume 1031:127–142. doi: 10.1196/annals.1331.013. [DOI] [PubMed] [Google Scholar]
- 32.Sen CK, Khanna S, Roy S, Packer L. Molecular basis of vitamin E action - Tocotrienol potently inhibits glutamate-induced pp60(c-Src) kinase activation and death of HT4 neuronal cells. J Biol Chem. 2000;275:13049–13055. doi: 10.1074/jbc.275.17.13049. [DOI] [PubMed] [Google Scholar]
- 33.Patel V, Khanna S, Roy S, Ezziddin O, Sen CK. Natural vitamin E alpha-tocotrienol: retention in vital organs in response to long-term oral supplementation and withdrawal. Free Radic Res. 2006;40:763–771. doi: 10.1080/10715760600672491. [DOI] [PubMed] [Google Scholar]
- 34.Roberts T, Hutson D, editors. Metabolic Pathways of Agrichemicals. Cambridge, UK: Royal Society of Chemistry; 1999. [Google Scholar]
- 35.Tomlin C, editor. The Pesticide Manual. Farnham, Surrey, UK: Britich Crop Protection Council; 2003. [Google Scholar]
- 36.Dehne H-W, Gisi H, Kuck K, Russell P, Lyr H, editors. Modern Fungicides and Antifungal Compounds IV. Farnham, Surrey, UK: British Crop Protection Council; 2004. [Google Scholar]
- 37.Georgopapadakou NH. Antifungals: mechanism of action and resistance, established and novel drugs. Curr Opin Microbiol. 1998;1:547–557. doi: 10.1016/s1369-5274(98)80087-8. [DOI] [PubMed] [Google Scholar]
- 38.Lamb D, Kelly D, Kelly S. Molecular aspects of azole antifungal action and resistance. Drug Resist Updat. 1999;2:390–402. doi: 10.1054/drup.1999.0112. [DOI] [PubMed] [Google Scholar]
- 39.Njar VC, Nnane IP, Brodie AM. Potent inhibition of retinoic acid metabolism enzyme(s) by novel azolyl retinoids. Bioorg Med Chem Lett. 2000;10:1905–1908. doi: 10.1016/s0960-894x(00)00391-7. [DOI] [PubMed] [Google Scholar]
- 40.Njar VC. Cytochrome p450 retinoic acid 4-hydroxylase inhibitors: potential agents for cancer therapy. Mini Rev Med Chem. 2002;2:261–269. doi: 10.2174/1389557023406223. [DOI] [PubMed] [Google Scholar]
- 41.Wu C, Njar V, Brodie A, Borenstein M, Nnane I. Quantification of a novel retinoic acid metabolism inhibitor, 4-(1H-imidazol-1-yl)retinoic acid (VN/14-1RA) and other retinoids in rat plasma by liquid chromatography with diode-array detection. J Chromatogr B Analyt Technol Biomed Life Sci. 2004;810:203–208. doi: 10.1016/j.jchromb.2004.07.028. [DOI] [PubMed] [Google Scholar]
- 42.Huynh CK, Brodie AM, Njar VC. Inhibitory effects of retinoic acid metabolism blocking agents (RAMBAs) on the growth of human prostate cancer cells and LNCaP prostate tumour xenografts in SCID mice. Br J Cancer. 2006;94:513–523. doi: 10.1038/sj.bjc.6602971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Njar VC, Gediya L, Purushottamachar P, Chopra P, Vasaitis TS, Khandelwal A, Mehta J, Huynh C, Belosay A, Patel J. Retinoic acid metabolism blocking agents (RAMBAs) for treatment of cancer and dermatological diseases. Bioorg Med Chem. 2006;14:4323–4340. doi: 10.1016/j.bmc.2006.02.041. [DOI] [PubMed] [Google Scholar]
- 44.Bruno RD, Njar VC. Targeting cytochrome P450 enzymes: a new approach in anti-cancer drug development. Bioorg Med Chem. 2007;15:5047–5060. doi: 10.1016/j.bmc.2007.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yamashita K, Ikeda S, Iizuka Y, Ikeda I. Effect of sesaminol on plasma and tissue alpha-tocopherol and alpha-tocotrienol concentrations in rats fed a vitamin E concentrate rich in tocotrienols. Lipids. 2002;37:351–358. doi: 10.1007/s11745-002-0902-6. [DOI] [PubMed] [Google Scholar]
- 46.Parker RS, Sontag TJ, Swanson JE. Cytochrome P4503A-dependent metabolism of tocopherols and inhibition by sesamin. Biochem Biophys Res Commun. 2000;277:531–534. doi: 10.1006/bbrc.2000.3706. [DOI] [PubMed] [Google Scholar]
- 47.Parker RS, Sontag TJ, Swanson JE, McCormick CC. Discovery, characterization, and significance of the cytochrome p450 omega-hydroxylase pathway of vitamin E catabolism. In: Kelly F, Meydani M, Packer L, editors. Vitamin E And Health. Volume 1031. York, NY: NYAS; 2004. pp. 13–21. [DOI] [PubMed] [Google Scholar]
- 48.Swanson JE, You CS, Parker RS. Tocopherol metabolism by Caco-2 cells and inhibition by sesamin and the polyphenols genistein and resveratrol. Faseb Journal. 2004;18:A160–A160. [Google Scholar]
- 49.Yamashita K, Nohara Y, Katayama K, Namiki M. Sesame seed lignans and gamma-tocopherol act synergistically to produce vitamin E activity in rats. J Nutr. 1992;122:2440–2446. doi: 10.1093/jn/122.12.2440. [DOI] [PubMed] [Google Scholar]
- 50.Shimizu S, Akimoto K, Shinmen Y, Kawashima H, Sugano M, Yamada H. Sesamin is a potent and specific inhibitor of delta 5 desaturase in polyunsaturated fatty acid biosynthesis. Lipids. 1991;26:512–516. doi: 10.1007/BF02536595. [DOI] [PubMed] [Google Scholar]
- 51.Lei HS, Atkinson J. Synthesis of phytyl- and chroman-derivatized photoaffinity labels based on alpha-tocopherol. J Org Chem. 2000;65:2560–2567. doi: 10.1021/jo000029l. [DOI] [PubMed] [Google Scholar]
- 52.Nava P, Cecchini M, Chirico S, Gordon H, Morley S, Manor D, Atkinson J. Preparation of fluorescent tocopherols for use in protein binding and localization with the alpha-tocopherol transfer protein. Bioorg Med Chem. 2006;14:3721–3736. doi: 10.1016/j.bmc.2006.01.053. [DOI] [PubMed] [Google Scholar]
- 53.Kikuta Y, Kusunose E, Kusunose M. Characterization of human liver leukotriene B(4) omega-hydroxylase P450 (CYP4F2) J Biochem. 2000;127:1047–1052. doi: 10.1093/oxfordjournals.jbchem.a022696. [DOI] [PubMed] [Google Scholar]
- 54.Kikuta Y, Miyauchi Y, Kusunose E, Kusunose M. Expression and molecular cloning of human liver leukotriene B4 omega-hydroxylase (CYP4F2) gene. DNA Cell Biol. 1999;18:723–730. doi: 10.1089/104454999315006. [DOI] [PubMed] [Google Scholar]
- 55.Stec DE, Roman RJ, Flasch A, Rieder MJ. Functional polymorphism in human CYP4F2 decreases 20-HETE production. Physiol Genomics. 2007;30:74–81. doi: 10.1152/physiolgenomics.00003.2007. [DOI] [PubMed] [Google Scholar]
- 56.You CS, Sontag TJ, Swanson JE, Parker RS. Long-chain carboxychromanols are the major metabolites of tocopherols and tocotrienols in A549 lung epithelial cells but not HepG2 cells. J Nutr. 2005;135:227–232. doi: 10.1093/jn/135.2.227. [DOI] [PubMed] [Google Scholar]