Abstract
The conserved protein kinase Chk1 is a player in the defense against DNA damage and replication blocks. The current model is that after DNA damage or replication blocks, ATRMec1 phosphorylates Chk1 on the non-catalytic C-terminal domain. However, the mechanism of activation of Chk1 and the function of the Chk1 C terminus in vivo remains largely unknown. In this study we used an in vivo assay to examine the role of the C terminus of Chk1 in the response to DNA damage and replication blocks. The conserved ATRMec1 phosphorylation sites were essential for the checkpoint response to DNA damage and replication blocks in vivo; that is, that mutation of the sites caused lethality when DNA replication was stalled by hydroxyurea. Despite this, loss of the ATRMec1 phosphorylation sites did not change the kinase activity of Chk1 in vitro. Furthermore, a single amino acid substitution at an invariant leucine in a conserved domain of the non-catalytic C terminus restored viability to cells expressing the ATRMec1 phosphorylation site-mutated protein and relieved the requirement of an upstream mediator for Chk1 activation. Our findings show that a single amino acid substitution in the C terminus, which could lead to an allosteric change in Chk1, allows it to bypass the requirement of the conserved ATRMec1 phosphorylation sites for checkpoint function.
Cell cycle checkpoints coordinate the maintenance of genomic integrity with cell division. The checkpoints that monitor replication fork integrity and DNA damage lesions sensed outside of DNA replication use similar mechanisms, and sometimes some of the same proteins, to delay cell cycle transitions, and in addition play an important role in the stability of replication forks and in DNA repair (1, 2).
The conserved protein kinase Chk1 is a player in the defense against DNA damage and replication blocks in metazoans. Chk1 is activated in budding yeast when damage is sensed outside the context of a replication fork. In this case the checkpoint proteins in addition to the kinase Chk1 and the mediator/signal amplifier protein Rad9 inhibit spindle elongation and chromosome segregation in response to DNA damage sensed in late S or G2. One effector of Chk1 in this response is the securin Pds1, which is stabilized by phosphorylation in response to DNA damage (3–6). Although the Chk1/Rad9 DNA damage checkpoint arm is not required during replication blocks in the budding yeast, we have shown that Chk1 is activated when replication is slowed down by the ribonucleotide reductase inhibitor hydroxyurea (HU).3 We have also shown that cells lacking the checkpoint kinase Dun1 are dependent on the Chk1/Rad9 arm for survival of replication blocks elicited by HU (7, 8). Studies addressing the in vivo roles of mammalian Chk1 have been difficult due to the fact that Chk1 is essential for early development of mouse embryos (9). Therefore, yeast cells lacking Dun1 provide a valuable model to explore the role of Chk1 in vivo in the recovery from replication blocks and the response to DNA damage generated outside of S phase.
The Chk1 proteins consist of two primary domains, the well conserved N-terminal kinase domain and the less conserved non-catalytic C-terminal domain (10, 11). The C-terminal domains of the Chk1 orthologues are phosphorylated after DNA damage. The C-terminal domain of vertebrate Chk1 has also been suggested to play an inhibitory role in the kinase activity of the protein (12). Kinases are regulated by allosteric changes caused by post-translational modifications, by protein interactions (mitogen-activated protein kinases and protein kinase A (PKA)), by modulating access to substrates via subcellular localization (IκB kinase, mitogen-activated protein kinase/extracellular signal-regulated kinase kinase (MEK)), and by binding of inhibitors (cyclin-dependent kinases, PKA) (13–15). However, the mechanism of activation of Chk1 and the function of the Chk1 C terminus remains largely unknown.
We used our in vivo assay in the genetically tractable model system (Saccharomyces cerevisiae) and cells lacking Dun1 to examine the role of Chk1 in the response to DNA damage and replication blocks. Using this assay we explored the role of phosphorylation and conserved domains of Chk1 in its regulation. We found that the conserved ATRMec1 phosphorylation sites (equivalent to Ser-317 and Ser-345 in human Chk1) of Chk1 are required for both the response to DNA damage and replication blocks. We also identified residues on the same face of a predicted α-helix in the GD domain of the C terminus of Chk1 that when changed result in a kinase that is constitutively modified in the absence of a checkpoint signal. In particular, a single amino acid substitution at a conserved leucine in the GD domain resulted in a constitutively active kinase that did not require the conserved ATR phosphorylation sites of Chk1 for checkpoint activation and function.
EXPERIMENTAL PROCEDURES
Chk1 Mutant Alleles—All mutant chk1 alleles were constructed by sequential PCR. The template for both reactions was a plasmid containing the wild-type CHK1. First, a PCR was carried out using a primer containing the desired mutation and a primer upstream of the BamHI in CHK1. This PCR product was then used as a “mega primer” along with a primer from the polylinker for the second PCR. The product of the second PCR was digested with BamHI and SacI and used to replace the corresponding fragment in pML107.1 (5), which carries an HA-tagged wild-type CHK1. All mutations were confirmed by sequencing.
Yeast Strains—The yeast strains used in this study are listed in Table 1. Strains were generated using standard genetic techniques. Integration of mutant chk1 alleles in cdc13-1 or dun1Δ cells was accomplished by cloning the C-terminal PshAI-SacI fragment of the chk1 alleles into integrating vector pRS406 (16) digested with SmaI-SacI. The resulting partial chk1 constructs were linearized by MfeI and then transformed into a cdc13-1 or dun1Δ strain. Plasmid integration resulted in a genetic exchange at the CHK1 locus consisting of a full-length mutant chk1 allele and a 3′-truncated (nonfunctional) copy of the endogenous wild-type allele. The expected genome changes were confirmed by diagnostic PCR followed by restriction enzyme digestion or sequencing.
TABLE 1.
Yeast strains used in this study
| Strains | Genotype | Source |
|---|---|---|
| Y300 | MATa ade2-1 trp1-1 ura3-1 leu2-3,112 his 3-11,15 can1-100 | Ref. 26 |
| Y801 | As Y300 chk1Δ::HIS3 | Ref. 5 |
| YJP8 | As Y300 chk1Δ::HIS3 dun1Δ::HIS3 | Ref. 7 |
| Y578 | As Y300 dun1Δ::HIS3 | Ref. 27 |
| YHC300 | As Y578 chk1Δ::URA3 | This study |
| YHC302r | As Y578 chk1-ΔGD::URA3 | This study |
| YHC305 | As Y578 chk1-G503D::URA3 | This study |
| YHC306 | As Y578 chk1-L506R::URA3 | This study |
| YHC308 | As Y578 chk1-ΔTKF::URA3 | This study |
| YHC312 | As Y578 chk1-F419A::URA3 | This study |
| YHC332 | As Y578 chk1-3AQ::URA3 | This study |
| YHC334 | As Y578 chk1-L506R, 3AQ::URA3 | This study |
| YHC335 | As Y578 chk1-T421A::URA3 | This study |
| YHC336 | As Y578 chk1-L506R, T421A::URA3 | This study |
| YJP321 | As Y578 rad9Δ::HIS3 | Ref. 7 |
| YJP1129 | As Y578 chk1-L506R::URA3 rad9Δ::HIS3 | This study |
| YJP1130 | As Y578 chk1-L506R, 3AQ::URA3 rad9Δ::HIS3 | This study |
| Y438 | As Y300 rad9Δ::HIS3 | Ref. 5 |
| YJP219 | As Y300 chk1Δ::URA3 rad9Δ::HIS3 | This study |
| Y581 | As Y300 mec1Δ::HIS3 trp1-1::GAP-RNR1-TRP1 | Ref. 27 |
| Y816 | As Y300 cdc13-1 | Ref. 5 |
| Y818 | As Y300 cdc13-1 chk1Δ::HIS3 | Ref. 5 |
| Y811 | As Y300 cdc13-1 chk1Δ::HIS3 PDS1-3XHA::URA3 | Ref. 5 |
| YHC200 | As Y816 chk1Δ::URA3 | This study |
| YHC202 | As Y816 chk1-ΔGD::URA3 | This study |
| YHC205 | As Y816 chk1-G503D::URA3 | This study |
| YHC206 | As Y816 chk1-L506R::URA3 | This study |
| YHC208 | As Y816 chk1-ΔTKF::URA3 | This study |
| YHC212 | As Y816 chk1-F419A::URA3 | This study |
| YHC232 | As Y816 chk1-3AQ::URA3 | This study |
| YHC234 | As Y816 chk1-L506R, 3AQ::URA3 | This study |
| YJP1124 | As Y816 rad9Δ::HIS3 | This study |
| YJP1159 | As Y816 chk1-L506R::URA3 rad9Δ::HIS3 | This study |
| YJP1160 | As Y816 chk1-L506R, 3AQ::URA3 rad9Δ::HIS3 | This study |
| PJ69-4A | MATa trpl-901 leu2-3,112 ura3-52 his3-200 ga14Δ ga18Δ LYSZ::GALl-HIS3 GAL2-ADE2 metZ::GAL7-lacZ | Ref. 17 |
Growth Conditions—Yeast cells were grown on yeast extract/peptone/dextrose-rich medium or synthetic complete medium as indicated. To examine sensitivity to HU on plates, serial dilutions of cells were spotted on to yeast extract/peptone/dextrose solid medium containing HU ranging in concentration from 0 to 80 mm, incubated at 30 °C for 3 days, and then photographed. The micro-colony assays were conducted by growing cells in yeast extract/peptone/dextrose at 22 °C overnight and then diluted and plated on prewarmed plates and incubated at 30 °C. After 12–14 h, cells were examined for formation of microcolonies, and the number of cells in each of 50–100 microcolonies was counted for each strain. For Western analysis of the Chk1 shift caused by DNA damage, cells were grown in synthetic complete–Leu medium at 30 °C to log phase, and MMS was added to a final concentration of 0.1%. Cells were then incubated at 30 °C for 2 h before harvest.
Western Analysis—Protein extracts from cells expressing HA-CHK1 and 3XHA-PDS1 were prepared as previously described (25), separated on 10% acrylamide, 0.066% bisacrylamide gels, and transferred to nitrocellulose membranes. HA-Chk1 and HA-Pds1 were detected by Western analysis using anti-HA antibody (16B12, Covance, Madison, WI).
Two-hybrid Assay—The two-hybrid vectors and host strain of James et al. (17) were used. The CHK1 coding region and its mutant forms were inserted into pGBD-C1 to produce Chk1-Gal4 DNA binding domain (BD) fusion proteins, whereas the RAD9 coding region was inserted into pGAD-C1 to yield a Rad9-Gal4 activation domain (AD) fusion protein. Chk1-BD and Rad9-AD plasmids were co-transformed into strain PJ69-4A, which carries a GAL2-ADE2 reporter gene. Transformants were picked and suspended in media with a starting cell concentration of 2 × 107/ml. A series of 10-fold dilutions were made, and five μl of each dilution was plated on complete minimal medium and on minimal medium lacking adenine. The plates were incubated at 30 °C and photographed after 3–5 days.
Kinase Assay—Pulldown and kinase assays were carried out as previously described (5), except that the complexes were washed 5 times for 10 min at 4 °C with NETN buffer and once with kinase buffer before carrying out the kinase reaction using 20 μm cold ATP.
RESULTS
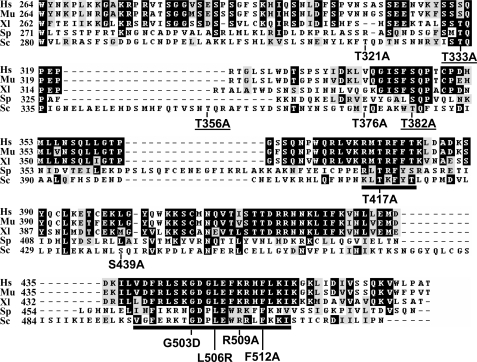
The ATRMec1/ATMTel1 (S/TQ) Sites of Chk1 Are Required for Its Function in Response to DNA Damage and Replication Blocks—The Chk1 protein consists of two primary domains, the well conserved N-terminal kinase domain and the less conserved non-catalytic C-terminal domain. The current model suggests that in response to DNA damage or replication stress, Chk1 is activated through phosphorylation by ATR/ATM on SQ or TQ motifs on the C-terminal domain. The yeast Chk1 C terminus contains six SQ/TQ sites that could be the phosphorylation targets for the yeast ATR/ATM orthologues, Mec1/Tel1 (Fig. 1). Two of the S/TQ sites, Thr-333 and Thr-382, correspond respectively to the sites, Ser-317 and Ser-345 on mammalian Chk1 known to be phosphorylated in response to DNA damage and replication blocks. Other S/TQ sites in the yeast Chk1 do not seem to be conserved in the mammalian Chk1 proteins.
FIGURE 1.
The C termini of Chk1 orthologs. The amino acid sequences of Chk1 C termini from human (Hs), mouse (Mu), frog (Xl), fission yeast (Sp) and budding yeast (Sc) are lined up with conserved residues highlighted in dark boxes. The two conserved TKF domain (amino acids (aa) 416–421) and GD domain (aa 496–515) in yeast Chk1 are underlined. ScChk1 point mutants made in the conserved regions, and the putative ATRMec1 phosphorylation sites are shown in arrows denoting the wild-type and the substituting amino acids. Three point mutants at ATR phosphorylation sites important for Chk1 function underlined.
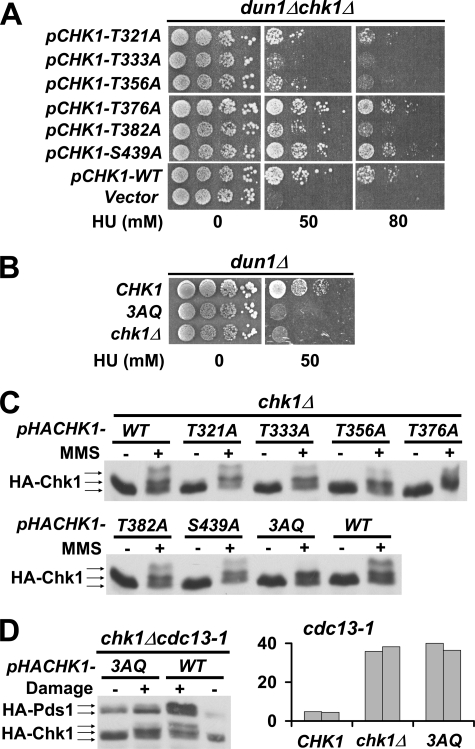
To examine the functional importance of the S/TQ sites of the yeast Chk1, we mutated the CHK1 gene on a plasmid to produce six different chk1 alleles, each conferring alanine substitution of the serine or threonine residue in one of the six S/TQ sites. Although Chk1 is not required for the response to replication blocks by HU in wild-type cells, we have shown that Chk1 is essential in cells that lack Dun1 when grown on HU (7). We, therefore, introduced plasmids carrying wild-type CHK1, mutated chk1, or empty vector into chk1Δ dun1Δ cells to determine whether the mutated Chk1 would rescue the lethality of the chk1Δ dun1Δ cells on HU. chk1Δ dun1Δ cells with an empty vector failed to grow on plates containing 50 mm HU, whereas introduction of a plasmid encoding wild-type CHK1 rescued the lethality (Fig. 2A). The chk1Δ dun1Δ cells harboring plasmids encoding the mutated Chk1T333A failed to grow on HU, whereas cells expressing Chk1T321A, Chk1T356A, and Chk1T382A showed an intermediate phenotype on 50 mm HU and failed to grow on plates containing 80 mm HU. Cells expressing Chk1T376A and Chk1S439A grew as well on HU as cells expressing wild-type Chk1. These results indicated that the three TQ sites at Thr-333, Thr-356, and Thr-382 that gave severe to intermediate phenotypes were important for Chk1 function in response to replication blocks by HU. We, therefore, generated a dun1Δ strain in which the chromosomal CHK1 was mutated to confer alanine substitutions on all the three TQ sites at 333, 356, and 382 (3AQ). As expected, the dun1Δ chk1–3AQ cells, like dun1Δchk1Δ cells, failed to grow on plates containing 50 mm HU (Fig. 2B).
FIGURE 2.
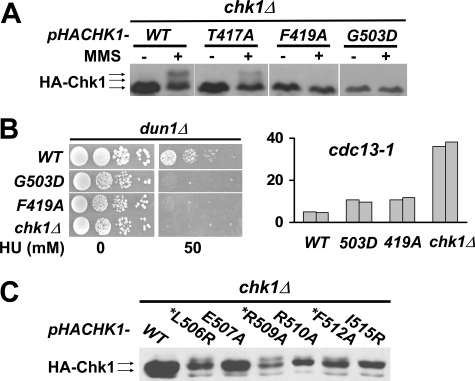
The conserved ATRMec1 phosphorylation sites on Chk1 are essential for the checkpoint response in vivo. A, plasmids carrying Chk1 mutants with alanine substitution of threonine/serine at one of the six putative ATR Mec1 phosphorylation sites were transformed into dun1Δ chk1Δ cells. Transformants were spotted on plates containing 0 or 50 or 80 mm HU. B, the 3AQ mutant combining three point mutations (T333A, T356A, and T382A) was integrated to the CHK1 locus in dun1Δ cells and tested for HU resistance under the same condition. C, chk1Δ cells harboring plasmids carrying HA-tagged Chk1 phosphorylation sites mutants were treated with 0.1% MMS, and protein extracts were analyzed by Western blots using anti-HA antibody. D, left, plasmids carrying HA-tagged 3AQ mutant were introduced into cdc13-1 chk1Δ cells, and DNA damage was elicited by incubating the cells at the nonpermissive temperature. The mobility shifts of Chk1 and accumulation of Pds1 were monitored by Western blots. Right, the 3AQ mutant was integrated into cdc13-1 cells, and checkpoint function of Chk1 in the resulting strains was evaluated using micro-colony assay. The bars represent the average number of cells per colony from 50 colonies. The experiment was carried out in duplicate.
To determine whether the phosphorylation of the mutated Chk1 proteins was compromised in response to DNA damage, chk1Δ cells harboring plasmid-borne HA-tagged Chk1 were treated with MMS, and the Chk1 proteins from these cells were analyzed by Western blotting. The wild-type Chk1 from MMS-treated cells exhibited slower migrating forms, indicating modification of the protein in response to DNA damage induced by MMS. Surprisingly, despite the growth phenotypes observed for some of the SQ/TQ mutants on HU, all single AQ mutant proteins exhibited mobility patterns similar to that of wild-type Chk1, although we observed a reduction in the amount of the slowest migrating form in these mutant proteins. However, the triple mutant, Chk13AQ, did not exhibit the slowest migrating forms that were apparent for the wild-type or single mutant proteins (Fig. 2C). These results suggest that phosphorylation or modification of Chk1 took place at multiple sites in addition to the 3TQ sites.
DNA damage sensed outside the context of a replication fork can be mimicked in cells by inactivation of Cdc13, a protein that participates in telomere capping (4). Chk1 plays a role in the M-A delay after Cdc13 inactivation; thus, we then investigated the role of the 3TQ sites in the DNA damage checkpoint activated by a lesion in late S/G2 induced by a temperature-sensitive allele of CDC13. Inactivation of Cdc13 in cdc13-1 cells at the nonpermissive temperature causes DNA damage that triggers the M-A checkpoint response, which includes stabilization of the securin Pds1 mediated by Chk1 (4, 5, 18, 19). A plasmid encoding an HA-tagged Chk13AQ was introduced into a temperature-sensitive strain, cdc13-1 chk1Δ, whose chromosomal PDS1 was also tagged with HA. cdc13-1 cells expressing the Chk13AQ-mutated protein failed to accumulate Pds1 when DNA damage was induced, indicating the Chk13AQ protein was not functional in the checkpoint response (Fig. 2D). Checkpoint activation in cdc13-1 strains results in cell cycle delay at the M-A transition which leads to micro-colonies of 2–4 cells (4, 5). To determine the contribution of the 3 TQ sites to cell cycle arrest, we generated a cdc13-1 strain in which chk1–3AQ was integrated into its chromosomal locus. We then counted the cells in micro-colonies formed by cdc13-1 cells expressing wild-type CHK1, chk1–3AQv or chk1Δ alleles after triggering the checkpoint via inactivation of Cdc13 at the nonpermissive temperature. As expected, cells expressing wild-type Chk1 formed micro-colonies with only a few cells when grown at the nonpermissive temperature for cdc13-1. In contrast, chk1–3AQ cells had many cells per micro-colony, essentially the same as chk1Δ cells (Fig. 2D). Theses results indicate that the ATRMec1 phosphorylation sites, as defined in the 3AQ mutant, which include Thr-333 and Thr-382 that are conserved in mammalian Chk1, are required for Chk1 function in response to replication blocks and DNA damage sensed in late S/G2.
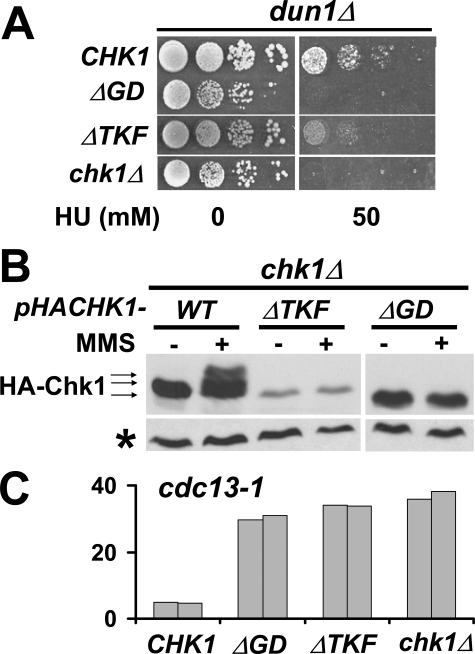
The GD and TKF Domains of Chk1 Mediate Interaction with Rad9 and Are Required for Both the M-A and HU Checkpoint Responses—In addition to the ATRMec1 phosphorylation sites, the C termini of Chk1 proteins have two conserved clusters of amino acids called TRF (TKF in yeast Chk1) and GD domains (11) (Fig. 1). To determine the importance of the two domains in Chk1 function, we made two CHK1 deletion mutants, which lacked amino acids (aa) 416–421 (ΔTKF) and aa 496–517 (ΔGD), respectively. dun1Δ and cdc13-1 cells expressing the mutants integrated into the CHK1 loci were examined for their ability to grow on 50 mm HU or to halt the cell cycle in response to DNA damage. The dunΔ chk1-ΔGD cells were unable to grow on 50 mm HU, whereas dun1Δ chk1-ΔTKF cells showed some micro-colonies at higher plating densities after 5 days on HU (Fig. 3A). Both cdc13-1chk1-ΔGD and cdc13-1chk1-ΔTKF failed to elicit the M-A DNA damage checkpoint as shown in the micro-colony assay (Fig. 3C). In addition, both ΔTKF and ΔGD proteins did not generate slower migrating forms when cells were treated with MMS (Fig. 3B), suggesting that the growth and checkpoint phenotypes observed were likely due to absence of Chk1 activation by phosphorylation even though the ATRMec1 sites remained intact in both deletion mutants. We also observed that the Chk1 ΔTKF protein seemed to be unstable as the protein levels of this mutated protein were much lower than that of wild-type protein (Fig. 3B). It is unknown whether the lower protein level contributed to the phenotypes observed for chk1-ΔTKF cells.
FIGURE 3.
The conserved GD and TRF domains in the C terminus of Chk1 are essential for the checkpoint response in vivo. A, the chk1 gene lacking the GD domain (ΔGD) or TKF domain (ΔTKF) was integrated into dun1Δ cells. The resulting strains were evaluated for resistance to replication blocks on 50 mm HU. B, plasmids encoding HA-tagged deletion mutants were introduced into chk1Δ cells. The mobility shifts of Chk1 proteins after MMS treatment were monitored by Western blots. *, cross-reaction signal indicative of the relative amounts of protein loaded. C, the deletion mutants were also integrated into cdc13-1 cells, and their ability to halt the cell cycle was evaluated by micro-colony assay. Numbers for duplicate experiments are shown.
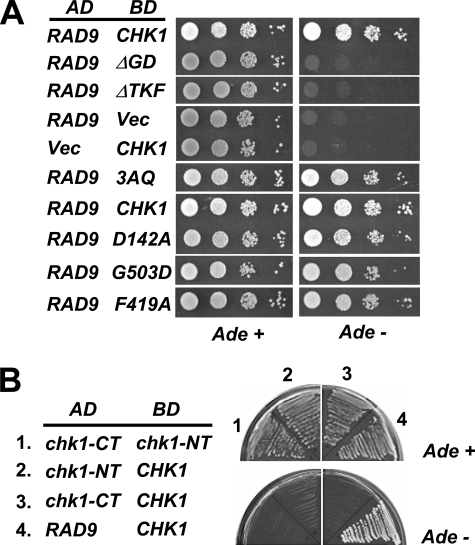
The phosphorylation of Chk1 by ATRMec1 is mediated by an adapter/mediator protein, Rad9, which has been shown to physically interact with Chk1 (5). It was formally possible that the ΔTKF and ΔGD Chk1 proteins failed to interact with Rad9 and was, thus, incapable of being activated. To test this, we used a two-hybrid system to examine the interaction between Rad9 and wild-type and mutated Chk1 proteins. Rad9 protein fused to the AD of Gal4 and wild-type or mutated Chk1 proteins fused to the DNA binding domain (BD) of Gal4 were co-expressed in yeast cells carrying an ADE2 reporter. Cells expressing AD-Rad9 and BD-Chk1 grew well on plates lacking adenine, whereas cells expressing AD-Rad9 with an empty BD vector or BD-Chk1 with an empty AD vector did not grow (Fig. 4A), confirming the interaction between Chk1 and Rad9. No growth was observed in cells expressing AD-Rad9 with BD-Chk1 proteins lacking the GD or TKF domains (Fig. 4A), indicating that the GD and TKF domains are important determinants for Chk1-Rad9 interaction. However, single amino acid substitutions of invariant residues in the GD (G503) and TKF (F419) domains did not lead to a loss of interaction with Rad9 (Fig. 4A and see “Discussion”). It should be mentioned that the levels of fused BD-Chk1 deletion proteins were comparable to that of the wild-type BD-Chk1 in the cell (data not shown); hence, the lack of interaction between Rad9 and Chk1 deletion mutants was not due to lower levels of Chk1 mutated proteins. Interestingly, although cells expressing Chk1–3AQ fail to grow on HU and fail in the DNA damage checkpoint (Fig. 2), mutation of the 3TQs had no effect on Chk1 interaction with Rad9 (Fig. 4A). Likewise, the catalytically inactive mutant, Chk1-D142A (5), also defective in HU response and checkpoint function (data not shown), exhibited normal interaction with Rad9 (Fig. 4A). These results indicate that residues within the GD and TKF domains of Chk1 but not the ATRMec1 phosphorylation sites nor its kinase activity are necessary for the interaction with Rad9. Studies with Chk1 proteins from other organisms and our studies presented here suggest that the Chk1 C terminus acts to inhibit the catalytic activity of the N terminus. Despite this, we did not observe an interaction between the kinase domain and C-terminal domains using this two-hybrid system (Fig. 4B).
FIGURE 4.
The GD and TRF domains but not the ATRMec1 phosphorylation sites on Chk1 are required for interaction with Rad9p. A, two-hybrid reporter cells were co-transformed with a plasmid expressing Rad9 fused to the GAL4 AD and a plasmid carrying wild-type or mutant Chk1 fused to the GAL4 DNA BD. Transformants were spotted in a series of dilutions on the media indicated. Interaction between AD and BD fusion proteins allowed growth on the media lacking adenine. D142A, catalytically inactive mutant. B, the Chk1 N terminus (chk1-NT, 1–304) and the C terminus (chk1-CT, 281–527) were fused to the GAL4 activation and DNA binding domains to test for interactions between these two domains and between these domains with the full-length protein (CHK1). The transformants were streaked out on media with or without adenine.
Point Mutations in the GD Domain, but Not in the TKF Domain, Activate Chk1 in the Absence of DNA Damage Signal—To further delineate the role of the TKF and GD domains in response to DNA damage and replication blocks, we generated Chk1 proteins with single amino acid substitutions of the conserved residues in both domains (Fig. 1). Cells expressing two point mutants, F419A and G503D, were defective in responding to HU in dun1Δ cells (Fig. 5B), which was similar to what was observed with cells expressing Chk1-mutated proteins lacking the TKF and GD domains. However, unlike the deletion mutants, cells expressing both point mutants showed only a mild checkpoint defect in cdc13-1 cells (Fig. 5B) despite the observation that both Chk1F419A and Chk1G503D proteins had lost all of the MMS-induced mobility shift observed for the wild-type protein (Fig. 5A). Thus, the Chk1F419A and Chk1G503D point mutations may define separation of function alleles of CHK1.
FIGURE 5.
Single amino acid substitutions in the TKF and GD domains of Chk1 separate the function of Chk1 in the recovery from replication blocks and DNA damage outside of S phase. A, plasmids encoding HA-tagged point mutants of CHK1 in TKF domain (T417A or F419A) or GD domain (G503D) were transformed into chk1Δ cells. The mobility shift of Chk1 proteins in response to MMS treatment was analyzed by Western blotting. B, growth on HU of dun1Δ cells expressing CHK1 alleles (left) and checkpoint proficiency of cdc13-1 cells expressing (right) TKF or GD point mutants were evaluated as described above. C, Chk1 mutants in the second half of GD domain were transformed into chk1Δ cells and analyzed by Western analysis in the absence of exogenous DNA damage signal. *, amino acids that map to the same face of a predicted α-helix.
Single amino acid substitutions in the second half of the GD domain yielded results different from those in the first half of the GD domain and in the TKF domain. Several amino acid substitutions in the second half of the GD domain, L506R, R509A, and F512A, gave rise to slower migrating forms of Chk1 in the absence of exogenous DNA damage (Fig. 5C). The second half of the GD domain is predicted to form an α-helix structure, and interestingly, all the three residues, Leu-506, Arg-509, and Phe-512, are located on the same face of the predicted helix (Fig. 5C).
To test whether the GD mutants that exhibited slower migrating forms without DNA damage represented activated alleles of CHK1, we cloned two of them CHK1L506R and CHK1R509A into a low copy vector and introduced them into cdc13-1 chk1Δ cells. Chk1 regulates Pds1 abundance via phosphorylation (5). We reasoned that if the mutated Chk1 proteins were activated, that their expression would lead to accumulation of Pds1. Expression of the catalytically inactive Chk1D142A protein caused cells to fail to accumulate Pds1 in response to a DNA damage signal (supplemental Fig. S1). Expression of Chk1L506R or Chk1R509A led to higher levels of Pds1 in the absence of DNA damage than cells expressing wild-type Chk1 from the same low copy vector (Fig. 6A and supplemental S1), suggesting that these GD mutations cause activation of Chk1 in the absence of DNA damage. However, expression of Chk1L506R from the same vector did not result in dramatic changes in cell cycle distribution of otherwise wild-type undamaged cells (Fig. supplemental S1).
FIGURE 6.
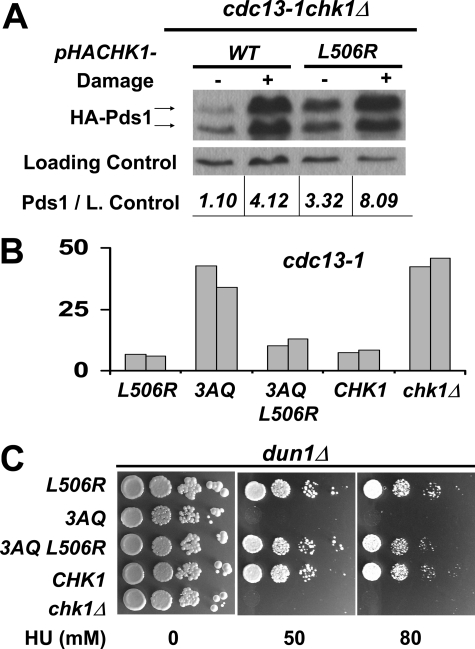
A single amino acid substitution in the GD domain rescued the lethality of cells expressing Chk1 protein lacking the ATRMec1 phosphorylation sites. A, constructs encoding the indicated HA-tagged CHK1 alleles were transformed into a cdc13-1 chk1Δ HA-PDS1 yeast strain, and transformants were grown at the permissive temperature (no DNA damage) and restrictive temperature (DNA damage) for cdc13-1. Chk1 and Pds1 were visualized by Western analysis using anti-HA antibodies. B, Chk1 mutants 3AQ, L506R, and the combined mutants 3AQ L506R were integrated into cdc13-1 cells. The resulting strains were evaluated for checkpoint function by micro-colony assay. The bars represent the average number of cells per micro-colony from 100 micro-colonies. C, the same chk1 alleles were also integrated into dun1Δcells and evaluated for growth on HU.
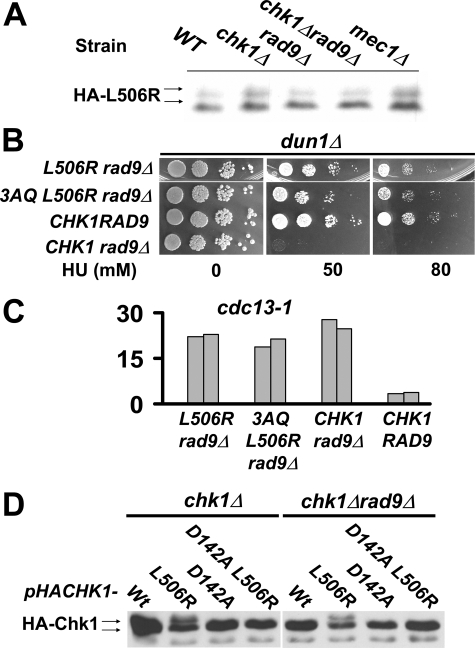
Chk1 Activation by GD Mutations Does Not Require ATRMec1 Phosphorylation Sites—The activation of Chk1 is thought to occur through phosphorylation by ATRMec1. Thus, we set out to examine whether the activation of Chk1 by GD mutation required the ATRMec1 sites corresponding to Ser-317 and Ser-345 in vertebrate Chk1 by combining the 3AQ and L506R mutations. When integrated into cdc13-1 cells (Fig. 6B) or dun1Δ cells (Fig. 6C), the single L506R mutation showed no checkpoint defect, whereas the 3AQ mutant, as shown previously, had lost checkpoint response in both assays. The double CHK1 3AQ L506R mutant behaved essentially like wild-type CHK1 in both cdc13-1 and dun1Δ cells, suggesting that the L506R substitution completely rescued the defect(s) due to the 3AQ mutations (Fig. 6, B and C). These results suggested that the L506R mutation resulted in activation of Chk1 and that activation was via a mechanism that did not require the ATRMec1 phosphorylation sites. Consistent with this, Chk1L506R proteins prepared from cells lacking Rad9 or ATRMec1 retained the slower migrating forms that were not different from Chk1L506R proteins from wild-type cells (Figs. 7, A and D), whereas wild-type Chk1 was not modified in the same strain backgrounds (Fig. 7D and data not shown).
FIGURE 7.
Chk1 activation in the absence of the upstream mediator Rad9. A, plasmids encoding HA-tagged Chk1L506R were transformed into different yeast strains as indicated above. The electrophoretic mobility shift of Chk1L506R in these cells in the absence of exogenous DNA damage was analyzed by Western blotting. B, growth on HU of chk1dun1Δ cells with or without Rad9. C, checkpoint proficiency by micro-colony assay of integrated CHK1L506R alleles in cdc13-1 cells in the absence of Rad9. The bars represent the average number of cells per micro-colony from 100 micro-colonies. D, plasmids encoding HA-tagged wild-type and Chk1 mutants combining CHK1L506R with mutation at the catalytic domain (D142A, kinase dead) were transformed into chk1Δ and chk1Δ rad9 Δ cells. The Chk1 proteins in the absence of DNA damage were analyzed by Western blotting.
The above results also suggested that Chk1L506R could bypass the requirement of upstream factors for checkpoint function. To test this, we integrated CHK1L506R into dun1Δ cells that also lacked Rad9. Rad9 functions as an adaptor protein for ATRMec1 phosphorylation of Chk1 (5) and just like chk1Δ dun1Δ cells, dun1Δ rad9Δ cells die on low concentrations of HU (7). dun1Δ rad9Δ cells expressing wild-type Chk1 failed to grown on 50 mm HU; however, dun1Δ rad9Δ cells expressing Chk1L506R or Chk13AQL506R also lacking the ATRMec1 phosphorylation sites grew as well as the wild-type cells on HU (Fig. 7B). These data indicate that Chk1L506R can bypass the requirement for Rad9 and the ATRMec1 phosphorylation sites for the role of Chk1 in the response to replication blocks by HU.
The CHK1L506R mutation was also integrated into cdc13-1 rad9Δ cells to evaluate whether the mutant could bypass Rad9 for the M-A checkpoint; however, the results were different from those obtained in dun1Δ rad9Δ cells. The CHK1-L506R cdc13-1 rad9Δ cells had very similar checkpoint defects as cdc13-1 rad9Δ cells (Fig. 7C). This could be explained by the fact that both Chk1 and Rad53 contribute to the DNA damage checkpoint downstream of Rad9, and Rad53 also requires Rad9 for signal amplification (20, 21). Additionally, activated Chk1 may not be able to compensate for the loss of other Rad9 functions such as blocking processing of telomeres in cdc13-1 cells (22).
The Mobility Shift of Chk1L506R Requires Chk1 Catalytic Activity—Introducing the substitution D142A, which results in a catalytically inactive protein, abolished the electrophoretic mobility shift of Chk1L506R (Fig. 7D) in wild-type and rad9Δ cells. These results suggest that mutations in the GD domain cause Chk1 autophosphorylation.
To determine whether the autophosphorylation could take place via intra- or intermolecular mechanisms, we introduced into chk1Δ cells two plasmids expressing different versions of Chk1. One plasmid encoded an untagged Chk1L506R, the other, a HA-tagged wild-type, Chk1 kinase dead Chk1D142A, or kinase dead Chk1D142AL506R. No electrophoretic shift was detected for any of the HA-tagged Chk1 proteins that were co-transformed with untagged Chk1L506R (supplemental Fig. S2), indicating that the mobility shift observed in Chk1L506R is not likely to occur via an intermolecular mechanism.
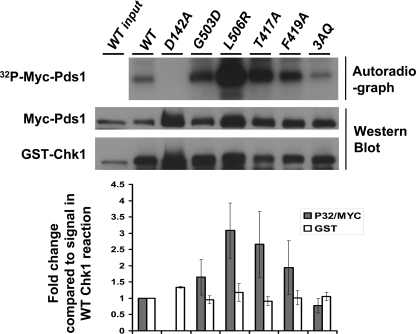
The Impact of Mutations of the ATRMec1 Phosphorylation Sites and GD and TK Domains of Chk1 on Checkpoint Function in Vivo Does Not Correlate with Loss of Kinase Activity of Mutated Proteins in Vitro—The kinase activity of Chk1 is clearly required for function, as a mutation (D142A) in the catalytic domain of Chk1 rendered dun1Δ cells unviable on HU and cdc13-1 cells checkpoint-defective (data not shown). Given the different phenotypes observed in vivo in cells expressing Chk1 proteins that lacked the ATRMec1 phosphorylation sites or GD or TK domains, we asked how the kinase activity of the different mutants would correlate with the phenotype of yeast cells expressing the mutated proteins. Measuring kinase activity from Chk1 proteins isolated from budding yeast has been challenging. This is probably due to fact that the known substrate for Chk1, securin (Pds1), is labile, and its levels fluctuate during the cell cycle. However, we had previously shown that Chk1 can form a complex and phosphorylate securin when co-expressed in insect cells from baculoviruses (5); thus, we used this approach to measure whether the yeast Chk1 proteins could form a complex and phosphorylate Pds1 in vitro. We did not obtain baculoviruses expressing the two deletion mutants (ΔGD and ΔTKF) despite multiple attempts, possibly due to instability of the mutated proteins. To measure the activity of Chk1, glutathione S-transferase (GST)-Chk1 and the GST-Chk1 mutated proteins, expressed at the same levels, were pulled down in a complex with Myc-Pds1, the amount of Pds1 that was bound to Chk1 was determined by Western analyses, and the amount of Pds1 phosphorylated by Chk1 was determined by autoradiography, measuring the incorporation of 32P from [γ-32P] ATP. As previously shown, the catalytically inactive mutant, Chk1D142A, showed no activity. The Chk1L506R mutation resulted in a Chk1 protein that was hyperactive (Fig. 8). Surprisingly, the other point mutants including 3AQ, despite their apparent defects observed in vivo (Figs. 2B and 5B), showed higher or the same activity compared with wild-type Chk1. Also the ability of Chk1 to bind its substrate was not affected by the mutations since all mutants showed higher levels of Pds1 in a complex with Chk1 compared with the wild-type Chk1 (Fig. 9). We have identified other residues in Chk1 that decrease the interaction with Pds1 (data not shown), which gives us confidence that we can measure the impact of mutations on the interaction between Chk1 and Pds1 using this approach. These results suggest that the defects of Chk1 mutants seen in yeast cells could be due to factors other than Chk1 capacity to bind or phosphorylate Pds1.
FIGURE 8.
In vitro catalytic activity of Chk1 and Chk1 mutants toward Pds1. Complexes containing glutathione S-transferase (GST)-tagged Chk1 proteins co-expressed with myc-tagged Pds1 in insect cells were pulled down using glutathione-Sepharose, washed 4 times and incubated in a kinase reaction with [γ-32P]ATP. γ-32P incorporation was visualized by autoradiography. The amount Chk1 added to the reaction and the amount of Pds1 bound to Chk1 were determined by Western analyses using anti-glutathione S-transferase and anti-Myc antibodies, respectively. The quantification of the signal from each lane in the autoradiograph and Western blots are represented graphically below each lane. The bar graph represents 32P signals incorporation into Myc-Pds1 normalized to the amount of myc-Pds1 in each lane and relative to the phosphorylation of Pds1 by wild-type Chk1. The bars represent averages from three independents assays. The lanes in the bar graph are in same order as figure above.
DISCUSSION
Although many studies have suggested models regarding the structural requirements to set up the checkpoint, these models are for the most part based on data from in vitro assays, and to date many of these models have not been tested in vivo. In addition, several studies suggest that ATR regulates Chk1 kinase activity, and thus, many laboratories have employed assays that measure Chk1 kinase activity as a means to measure checkpoint activation in cells after DNA damage. Here we describe a model system that allowed us to explore in vivo the role of Chk1 in the recovery from replication blocks and in the response to DNA damage generated outside of S phase. Our study examines the possible mechanisms of Chk1 regulation by phosphorylation in vivo and combines genetic and biochemical approaches to examine the impact of ATRMec1 phosphorylation sites and the conserved C-terminal domains in the activation of Chk1.
We show that the conserved ATRMec1 phosphorylation sites were essential for the checkpoint response in vivo; that is, that mutation of the sites caused lethality when DNA replication was stalled by HU. However, a single amino acid substitution at an invariant leucine in a conserved domain of the C terminus restored viability to cells expressing the phosphorylation defective-mutated protein, suggesting that an allosteric change in the protein relieved the requirement of the conserved ATR phosphorylation sites on Chk1 for checkpoint function.
The Conserved GD Domain in the C Terminus of Chk1 Regulates Chk1 Function—We show that the GD domain contains two regions that are important for Chk1 regulation. Deletion of the GD domain resulted in a protein defective for the interaction with Rad9 and for the checkpoint response. In contrast, amino acid substitutions at invariant resides in the second half of the GD domain of the Chk1 C terminus gave rise to slower migrating forms of Chk1, suggesting activation of Chk1 without exogenous DNA damage, and mutations in this region of the GD domain, which are predicted to form an α-helix, rescued the lethality on HU of cells expressing the Chk1 proteins lacking the ATRMec1 phosphorylation sites.
Recently Palermo et al. (10) have reported studies on the mutations of the conserved aspartic acid (Asp-469) in the first half of the GD domain of Schizosaccharomyces pombe Chk1 (SpChk1). The region containing Asp-469 in SpChk1 resembles the Chk1 phosphorylation consensus motif, except that Asp-469 is in place of the serine/threonine phosphoacceptor residue in a true Chk1 site. The authors hypothesized that this region may constitute a pseudosubstrate of Chk1 that functions to inhibit Chk1 activity and that mutations of Asp-469 would relieve the inhibition resulting in constitutive activation of Chk1. However, these authors did not observe elevated activity of SpChk1 Asp-469 mutants; instead, Asp-469 mutations compromised the function of SpChk1 in response to DNA damage. The sequence for the pseudo-substrate of SpChk1 is not present in Chk1 from other organisms, and we did not observe mobility shifts of ScChk1 with mutations made in the first half of the GD domain including D504A, G503D, and F498A (Fig. 5, data not shown).
Our data suggests that instead of the first half of the GD domain, the second half of the GD domain could interact and inhibit the kinase domain of Chk1. Despite this, we did not observe an interaction between the kinase domain and C-terminal domains in the two-hybrid assay (Fig. 4B).
We show that the GD and TKF domains are critical for the interaction between Chk1 and the signal amplifier Rad9. In addition, we identified two single amino acid substitutions in the conserved GD and TKF domains of Chk1 that maintained interaction with Rad9 (Fig. 4A) but were defective in the DNA damage-induced mobility shift (Fig. 5A). These alleles of CHK1, G503D and F419A, acted as separation of function mutations. dun1Δ cells expressing these proteins were unable to grow on HU, but cdc13-1 cells expressing these proteins exhibited little defect in the M-A checkpoint elicited by DNA damage at telomeres (Fig. 5B). Because Rad9 is required for dun1Δ cells to survive on HU, these findings suggest that different levels of Chk1 function are required for the different roles of Chk1 (23) or that Chk1 has different/additional substrates in its role to block mitosis in response to damage and in the recovery from replication blocks.
Correlation of Checkpoint Function with Kinase Activity of Chk1—Several single amino acid substitutions that rendered Chk1 ineffective in the response to DNA damage and/or replication blocks had little effect on the ability of Chk1 to interact with and phosphorylate its known substrate securin in vitro, indicating that ATRMec1 activation of Chk1 by phosphorylation is through a mechanism other than regulation of Chk1 kinase activity. In this line it has been reported that alanine substitution of the threonine in the TRF domain (T377) of Xenopus Chk1 resulted in hyperphosphorylation of the protein without DNA damage (24). The yeast Chk1T417A-mutated protein, which corresponds to Xenopus Chk1T377, did not exhibit hyperphosphorylation in vivo as no shifts were observed in the absence of DNA damage despite the fact that we observed an increase in activity of the Chk1T417A protein in vitro. Our studies indicate that Chk1 activity measured in vitro does not correlate with checkpoint activation in vivo and is probably not a good indicator of the status of the pathway in vivo.
Our work in vivo and in vitro supports the model in which Chk1 GD and TKF domains target Chk1 to Rad9 for phosphorylation by ATRMec1, events required for the response to DNA damage from replication forks. Phosphorylation of Chk1 by ATRMec1 could act to relieve the inhibition of Chk1 by the second half of the GD domain, as changes caused by an amino acid substitution in the second half of the GD domain restored checkpoint function to Chk1 in vivo in the absence of Rad9 or ATRMec1 phosphorylation. Together these findings provide in vivo evidence that activation of Chk1 by phosphorylation is through a mechanism other than, or in addition to, regulation of Chk1 kinase activity.
Supplementary Material
Acknowledgments
We are grateful to David Robbins, Mark R. Spaller, Matthew D. Wood, and other members of the Sanchez laboratory for helpful comments and discussions. We thank Stephen Elledge for strains and Alice L. Givan for help with flow cytometry.
This work was supported, in whole or in part, by National Institutes of Health Grants P30 ES06096 and U01 ES11038 (NIEHS) and RO1 CA84463 (NCI) (to Y. S.). This work was also supported by Pew Scholars Program in the Biomedical Sciences (to Y. S.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1 and S2.
Footnotes
The abbreviations used are: HU, hydroxyurea; HA, hemagglutinin; BD, binding domain; AD, activation domain; SpChk1, S. pombe Chk1; MMS, methyl methanesulfonate; WT, wild type; 3AQ, A333Q, A356Q, and A382Q.
References
- 1.Nyberg, K. A., Michelson, R. J., Putnam, C. W., and Weinert, T. A. (2002) Annu. Rev. Genet. 36 617–656 [DOI] [PubMed] [Google Scholar]
- 2.Branzei, D., and Foiani, M. (2005) Curr. Opin. Cell Biol. 17 568–575 [DOI] [PubMed] [Google Scholar]
- 3.Gardner, R., Putnam, C. W., and Weinert, T. (1999) EMBO J. 18 3173–3185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weinert, T. A., Kiser, G. L., and Hartwell, L. H. (1994) Genes Dev. 8 652–665 [DOI] [PubMed] [Google Scholar]
- 5.Sanchez, Y., Bachant, J., Wang, H., Hu, F., Liu, D., Tetzlaff, M., and Elledge, S. J. (1999) Science 286 1166–1171 [DOI] [PubMed] [Google Scholar]
- 6.Searle, J. S., Schollaert, K. L., Wilkins, B. J., and Sanchez, Y. (2004) Nat. Cell Biol. 6 138–145 [DOI] [PubMed] [Google Scholar]
- 7.Schollaert, K., Poisson, J. M., Searle, J. S., Scwanekamp, J. A., Tomlinson, C. R., and Sanchez, Y. (2004) Mol. Biol. Cell 15 4051–4063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caldwell, J. M., Chen, Y., Schollaert, K., Theis, J. F., Babcock, G., Newlon, C. S., and Sanchez, Y. (2008) J. Cell Biol. 180 1073–1086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu, Q., Guntuku, S., Cui, X. S., Matsuoka, S., Cortez, D., Tamai, K., Luo, G., Carattini-Rivera, S., DeMayo, F., Bradley, A., Donehower, L. A., and Elledge, S. J. (2000) Genes Dev. 14 1448–1459 [PMC free article] [PubMed] [Google Scholar]
- 10.Palermo, C., Hope, J. C., Freyer, G. A., Rao, H., and Walworth, N. C. (2008) PLoS ONE 3 e1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sanchez, Y., Wong, C., Thoma, R. S., Richman, R., Wu, Z., Piwnica-Worms, H., and Elledge, S. J. (1997) Science 277 1497–1501 [DOI] [PubMed] [Google Scholar]
- 12.Katsuragi, Y., and Sagata, N. (2004) Mol. Biol. Cell 15 1680–1689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheetham, G. M. (2004) Curr. Opin. Struct. Biol. 14 700–705 [DOI] [PubMed] [Google Scholar]
- 14.Pellicena, P., and Kuriyan, J. (2006) Curr. Opin. Struct. Biol. 16 702–709 [DOI] [PubMed] [Google Scholar]
- 15.Das, R., Esposito, V., Abu-Abed, M., Anand, G. S., Taylor, S. S., and Melacini, G. (2007) Proc. Natl. Acad. Sci. U. S. A. 104 93–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sikorski, R. S., and Hieter, P. (1989) Genetics 122 19–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.James, P., Halladay, J., and Craig, E. A. (1996) Genetics 144 1425–1436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weinert, T. A., and Hartwell, L. H. (1993) Genetics 134 63–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cohen-Fix, O., and Koshland, D. (1997) Proc. Natl. Acad. Sci. U. S. A. 94 14361–14366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sun, Z., Hsiao, J., Fay, D. S., and Stern, D. F. (1998) Science 281 272–274 [DOI] [PubMed] [Google Scholar]
- 21.Navas, T. A., Sanchez, Y., and Elledge, S. J. (1996) Genes Dev. 10 2632–2643 [DOI] [PubMed] [Google Scholar]
- 22.Lydall, D., and Weinert, T. (1995) Science 270 1488–1491 [DOI] [PubMed] [Google Scholar]
- 23.Purdy, A., Uyetake, L., Cordeiro, M. G., and Su, T. T. (2005) J. Cell Sci. 118 3305–3315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang, S. X., and Dunphy, W. G. (2000) FEBS Lett. 487 277–281 [DOI] [PubMed] [Google Scholar]
- 25.Foiani, M., Marini, F., Gamba, D., Lucchini, G., and Plevani, P. (1994) Mol. Cell. Biol. 14 923–933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Allen, J. B., Zhou, Z., Siede, W., Friedberg, E. C., and Elledge, S. J. (1994) Genes Dev. 8 2401–2415 [DOI] [PubMed] [Google Scholar]
- 27.Desany, B. A., Alcasabas, A. A., Bachant, J. B., and Elledge, S. J. (1998) Genes Dev. 12 2956–2970 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.