Abstract
We have previously shown that IκB kinase-β (IKKβ) interacts with the epithelial Na+ channel (ENaC) β-subunit and enhances ENaC activity by increasing its surface expression in Xenopus oocytes. Here, we show that the IKKβ-ENaC interaction is physiologically relevant in mouse polarized kidney cortical collecting duct (mpkCCDc14) cells, as RNA interference-mediated knockdown of endogenous IKKβ in these cells by ∼50% resulted in a similar reduction in transepithelial ENaC-dependent equivalent short circuit current. Although IKKβ binds to ENaC, there was no detectable phosphorylation of ENaC subunits by IKKβ in vitro. Because IKKβ stimulation of ENaC activity occurs through enhanced channel surface expression and the ubiquitin-protein ligase Nedd4-2 has emerged as a central locus for ENaC regulation at the plasma membrane, we tested the role of Nedd4-2 in this regulation. IKKβ-dependent phosphorylation of Xenopus Nedd4-2 expressed in HEK-293 cells occurred both in vitro and in vivo, suggesting a potential mechanism for regulation of Nedd4-2 and thus ENaC activity. 32P labeling studies utilizing wild-type or mutant forms of Xenopus Nedd4-2 demonstrated that Ser-444, a key SGK1 and protein kinase A-phosphorylated residue, is also an important IKKβ phosphorylation target. ENaC stimulation by IKKβ was preserved in oocytes expressing wild-type Nedd4-2 but blocked in oocytes expressing either a dominant-negative (C938S) or phospho-deficient (S444A) Nedd4-2 mutant, suggesting that Nedd4-2 function and phosphorylation by IKKβ are required for IKKβ regulation of ENaC. In summary, these results suggest a novel mode of ENaC regulation that occurs through IKKβ-dependent Nedd4-2 phosphorylation at a recognized SGK1 and protein kinase A target site.
The epithelial Na+ channel (ENaC)2 is the rate-limiting step in electrogenic Na+ reabsorption in a variety of epithelia that develop steep transepithelial Na+ concentration gradients, including the aldosterone-sensitive distal nephron, colon, and alveoli (1). ENaC is therefore critical to the regulation of extracellular and airway fluid volume (1, 2). Given its importance to normal homeostasis, channel activity is highly regulated by a number of hormonal influences, including aldosterone and vasopressin, as well as numerous intra- and extracellular stimuli such as flow rate, Na+ concentration, and proteolytic activation (1).
Several kinases have been shown to be important in mediating these regulatory influences, including SGK1, protein kinase A (PKA), protein kinase C, ERK, the G-protein-coupled receptor kinase (Grk2), and AMP-activated protein kinase (AMPK) (1, 3–8). In some cases, as with ERK and Grk2, these kinases phosphorylate ENaC directly (5, 6); but in many cases, kinase activity alters the activity of key intracellular regulatory proteins that control the apical membrane insertion or retrieval of ENaC (1, 9). We have previously described the interaction of IκB kinase (IKK)-β with the β-subunit of ENaC and have provided evidence that IKKβ increases ENaC activity in an oocyte expression system, primarily by increasing cell surface expression of the channel (10). Moreover, expression of a kinase-dead IKKβ had the opposite effect of decreasing ENaC activity, implying that tonic activation of IKKβ may be required for a significant proportion of basal ENaC activity (10).
IKKβ is a part of the IKK complex formed by IKKα, IKKβ, and IKKγ (or NEMO, the nuclear factor-κB (NF-κB) essential modulator), which mediates the activation of the NF-κB pathway by proinflammatory cytokines, bacterial products such as lipopolysaccharide (LPS), and other stimuli such as heat shock or oxidative stress (11, 12). Interaction between this canonical proinflammatory and stress signaling pathway and ENaC function has been suggested by a number of observations and appears to be biphasic. Acute exposure to the cytokine tumor necrosis factor-α has been shown to increase amiloride-sensitive Na+ transport in alveolar epithelium (13) and distal tubule cells from diabetic rats (14), whereas LPS acutely increases Na+ transport in mouse cortical collecting duct cells (15). Amiloride-sensitive alveolar fluid clearance, a function of ENaC activity, increases in a rat model of pneumonia by a tumor necrosis factor-α-dependent mechanism (16). In contrast, prolonged (12–24 h) exposure of alveolar epithelial cells to tumor necrosis factor-α (17) or of mouse collecting duct cells to LPS (18) results in down-regulation of ENaC activity and expression. We have found that aldosterone, the major hormonal regulator of ENaC, increases IKKβ levels in renal epithelia and activates NF-κB and have suggested that long term stimulation of NF-κB might represent a negative feedback loop modulating long term ENaC activation in conditions of oxidative or metabolic stress (10).
Despite evidence of cross-talk between the IKK complex and ENaC-mediated Na+ transport, the significance of the interaction between IKKβ and ENaC remains unclear, as does the potential mechanism of IKKβ-dependent up-regulation of ENaC activity. The current studies were performed to examine whether ENaC is a substrate for IKKβ phosphorylation, the significance of the IKKβ-ENaC interaction in natively expressing cells, and the mechanism by which IKKβ regulates ENaC expression. We have found that IKK-dependent regulation of ENaC is physiologically relevant in mouse kidney collecting duct cells and that the regulatory mechanism involves phosphorylation of the E3 ubiquitin-protein ligase Nedd4-2 by IKKβ at a previously identified SGK1 and PKA phosphorylation site.
EXPERIMENTAL PROCEDURES
Reagents, Chemicals, and Cell Culture—All reagents and chemicals used were purchased from Sigma unless otherwise noted. Plasmids expressing NH2-terminal hemagglutinin-tagged and COOH-terminal V5-tagged ENaC subunits were kindly provided by Dr. Thomas Kleyman. HEK-293 and mpkCCDc14 cells were cultured in high glucose, Dulbecco's modified Eagle's medium and defined medium supplemented with hormones and nutrients, respectively, as described previously (7).
RNA Interference Knockdown of IKKβ in mpkCCDc14 Cells—Approximately 2 × 106 cells at 80–90% confluency were trypsinized and then resuspended in Nucleofector T solution containing 100 pmol of either SMARTpool® siRNA IKKβ (mixtures of sequences 5′-GGAAGUACCUGAACCAGUU-3′, 5′-CCAAUAAUCUUAACAGUGU-3′, 5′-GGAUUCAGCUUCUCCUAAA-3′, and 5′-GUGGUGAGCUUAAUGAAUG-3′) or siGENOME® non-targeting siRNA (mixtures of sequences 5′-UAGCGACUAAACACAUCAA-3′, 5′-UAAGGCUAUGAAGAGAUAC-3′, 5′-AUGUAUUGGCCUGUAUUAG-3′, and 5′-AUGAACGUGAAUUGCUCAA-3′) (Thermo Fisher/Dharmacon). Following the manufacturer's instructions, these were mixed in the cuvette and directly placed in the Nucleofector® electroporation device (Amaxa Biosystems). The siRNA oligonucleotides were electroporated into the cells using Amaxa Program K-29. An equal volume of warm culture medium was added, and the cell suspension was then plated on a plastic 12-well plate containing 2 ml of culture medium. The following day, the cells were trypsinized and plated onto 12-mm filter inserts (Costar Transwells) at superconfluency. Two to four days later, the cells were used for electrophysiological and biochemical studies.
Equivalent Short-circuit Current Measurements—A portable epithelial volt ohmmeter was used to measure Isc across polarized mpkCCDc14 cell monolayers as described (19). Amiloride-sensitive equivalent Isc was calculated as the difference in equivalent Isc measured before versus after addition of 10 μm amiloride to the apical medium.
In Vitro Phosphorylation Assays—For in vitro phosphorylation assays of ENaC, plasmids expressing NH2-terminal hemagglutinin-tagged and COOH-terminal V5-tagged ENaC subunits were translated in vitro using the TnT coupled reticulocyte lysate system (Promega) in the presence or absence of [35S]Met/Cys following the manufacturer's instructions. ENaC subunits were immobilized using 1 μl of anti-V5 monoclonal antibody (Invitrogen) per reaction coupled to protein A/G beads (15-μl bead volume; Pierce). In vitro phosphorylation was performed by adding 1 μl of purified active IKKβ enzyme (Upstate) versus control buffer (50 mm Tris-HCl, pH 7.5, 150 mm NaCl, 0.1 mm benzamidine, 0.2 mm phenylmethylsulfonyl fluoride, and 0.1% 2-mercaptoethanol) with [γ-32P]ATP (20 μCi; MP Biomedicals, Irvine, CA) at room temperature for 1 h. As a positive control for ENaC phosphorylation, parallel in vitro phosphorylation of β-ENaC was performed using 1 μl of purified casein kinase-2 (Promega) following the manufacturer's recommendations. As a positive control for IKKβ activity, another parallel in vitro phosphorylation was performed using glutathione-Sepharose beads (Pierce) to pull down purified glutathione S-transferase (GST)-tagged IκBα (Upstate) for use as a reaction substrate. After SDS-PAGE and transfer to nitrocellulose membranes, phosphorylated bands on the membrane were imaged by exposure to a phospho-screen (Molecular Dynamics), and the bands were quantitated using a Bio-Rad phosphorimaging device.
Assays of Nedd4-2 in vitro phosphorylation were performed as described previously (20). HEK-293T cells transiently transfected with 3 μg of plasmid DNA/60-mm dish using Lipofectamine 2000 (Invitrogen) to express wild-type or mutant FLAG-xNedd4-2 were lysed 1 day after transfection. Nedd4-2 was immunoprecipitated from cell lysates using 1 μl of anti-FLAG monoclonal antibody M2 coupled to protein A/G beads (20-μl bead volume). In vitro phosphorylation reactions were performed as above using either purified active IKKβ enzyme or control buffer. As a positive control for FLAG-xNedd4-2 phosphorylation, parallel in vitro phosphorylations were performed using 1 μl of purified SGK1 (Upstate). As a positive control to check IKKβ enzymatic activity, GST-tagged IκBα was again used as a reaction substrate. After SDS-PAGE and transfer to nitrocellulose membranes, immunoblotting for expression of FLAG-xNedd4-2 was first performed (anti-Nedd4 WW2 domain antibody (Upstate) at 1:10,000 and secondary horseradish peroxidase-conjugated goat anti-rabbit antibody (Amersham Biosciences) at 1:5000) and quantitated using a Versa-Doc imager with Quantity One software (BioRad). After the chemiluminescent signal had decayed, phosphorylated bands on the membrane were imaged by exposure to a phospho-screen, and the bands were quantitated using a Bio-Rad phosphorimaging device. The intensity of each band was corrected by subtracting out the local background in the same lane.
In Vivo Phosphorylation Assays—In vivo phosphorylation assays were performed based on our previously described protocol (20). Either Mam-X (stably transfected with a short hairpin RNA that does not target any known mammalian gene), HEK-293 cells, or HEK-293T cells were transiently co-transfected using Lipofectamine 2000 with the pMO-FLAG-xNedd4-2 plasmid and either SMARTpool® IKKβ siRNA or siGENOME® non-targeting siRNA according to the manufacturer's instructions 2 days prior to experimentation. For labeling, cells were washed twice in phosphate-free efflux buffer containing 140 mm NaCl, 3 mm KCl, 1 mm MgSO4, 1 mm CaCl2, 10 mm glucose, and 10 mm HEPES, pH 7.4 (with 1 m Tris base). Each dish of cells was then incubated at 37 °C for 3 h in 1.5 ml of this buffer containing 0.3 mCi of [32P]orthophosphate (MP Biomedicals). After incubation, cells were washed once in ice-cold phosphate-free buffer before lysis in ice-cold radioimmune precipitation assay buffer containing 50 mm Tris-HCl, pH 7.5, 100 mm NaCl, 50 mm NaF, 1 mm EDTA, 1 mm EGTA, 1 mm phenylmethylsulfonyl fluoride, 1 mm sodium orthovanadate, 0.1 mg/ml aprotinin, 1× complete protease inhibitor mixture (Roche Applied Science), 0.1% SDS, 1% sodium deoxycholate, and 1% Triton X-100 (phosphatase and protease inhibitors added just prior to use). After clearing the lysates by high speed centrifugation, FLAG-xNedd4-2 was immunoprecipitated using the anti-FLAG monoclonal antibody M2 coupled to protein A/G beads, washed, eluted in Laemmli sample buffer, subjected to SDS-PAGE, and then transferred to a nitrocellulose membrane. Nedd4-2 Western blotting was performed first, followed by a phosphorimaging device analysis of the same membrane. The ratio of the phosphorylation signal to the immunoblot signal in each lane was compared across conditions to derive relative phosphorylation levels.
Oocyte Two-electrode Voltage Clamp Measurements—Xenopus laevis oocytes were harvested, collagenase-treated, and maintained as described previously (7). Oocytes were injected with cRNAs encoding mouse α-, β-, and γ-ENaC (7), NH2-terminal FLAG-xNedd4-2 (20), and/or IKKβ (10) cRNAs in the combinations and amounts indicated in the legend to Fig. 7. Two-electrode voltage clamp measurements of amiloride-sensitive ENaC currents in oocytes were performed 1–2 days after cRNA injection as described previously (7).
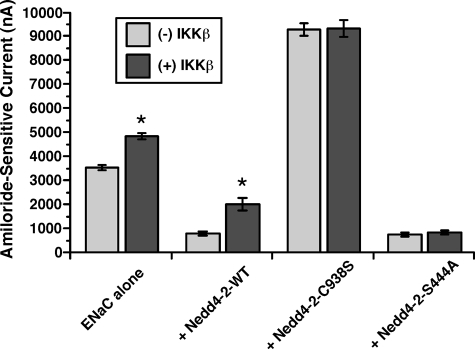
FIGURE 7.
IKKβ-dependent regulation of ENaC requires functional Nedd4-2 and phosphorylation at Ser-444. The mean ± S.E. of amiloride-sensitive ENaC currents measured by two-electrode voltage clamp at –100 mV in oocytes that were co-injected with 2 ng each of mouse α-, β-, and γ-ENaC cRNAs ± 5 ng of IKKβ ± 0.7 ng of WT or S444A Nedd4-2 or 5 ng of C938S Nedd4-2, as indicated, is shown. For each condition, ENaC currents were measured and compared with or without IKKβ co-expression (*, p < 0.001 compared with corresponding control in the absence of IKKβ co-expression; two-tailed unpaired t tests; n = 18–68 oocytes, three batches for each of the Nedd4-2 co-expression conditions, and nine batches for ENaC alone condition).
Statistics—Statistical analyses were performed using either StatView (SAS) or SigmaPlot software (Jandel Scientific). Analysis of variance was used to compare data obtained from different batches of oocytes for two-electrode voltage clamp experiments. For other biochemical experiments, statistics were performed using Student's t tests, as described in each figure legend. In all cases, p values <0.05 were considered significant.
RESULTS
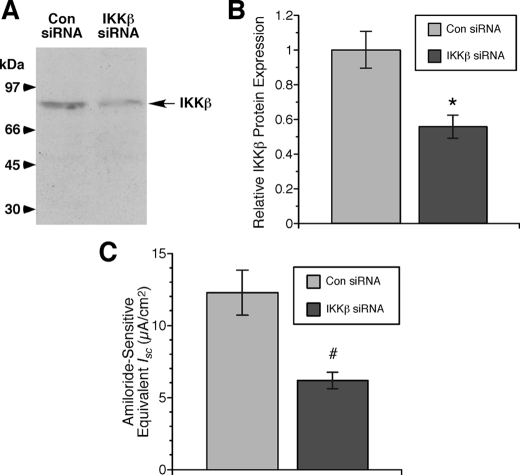
Endogenous Regulation of ENaC by IKKβ in mpkCCDc14 Cells—We previously discovered that IKKβ interacts with the COOH-terminal cytoplasmic tail of β-ENaC through a yeast two-hybrid screen, and this interaction was confirmed biochemically through co-immunoprecipitation studies. Functionally, IKKβ co-expression was found to enhance ENaC activity by increasing its surface expression in Xenopus oocytes (10). To test whether the regulation of ENaC by IKKβ is physiologically relevant in an endogenously expressing cell system, we used polarized mpkCCDc14 cells and knocked down IKKβ expression through RNA interference (Fig. 1). Transfection of siRNAs directed specifically against IKKβ knocked down its protein expression by a mean of ∼40–50% relative to control siRNA-transfected cells (Fig. 1, A and B). Concomitant with this knockdown of IKKβ expression, amiloride-sensitive, ENaC-dependent equivalent Isc was similarly reduced by ∼50% (Fig. 1C). These findings suggest that IKKβ expression in these cells is required to sustain ENaC currents under basal conditions; when IKKβ levels are reduced, ENaC activity is also inhibited.
FIGURE 1.
Knockdown of IKKβ causes parallel inhibition of ENaC currents in mpkCCDc14 cells. A, typical immunoblot demonstrating significant knockdown of IKKβ protein expression in cells transfected with IKKβ siRNA relative to that of cells transfected with control siRNA (Con). B, summary of relative IKKβ protein expression in cells transfected with IKKβ versus control siRNA (*, p = 0.01; two-tailed unpaired t test relative to control siRNA; n = five samples for each group from two separate experiments). C, summary of mean (±S.E.) amiloride-sensitive equivalent Isc measurements by epithelial volt-ohmmeter in IKKβ siRNA- and control siRNA-transfected cells (#, p = 0.002; two-tailed unpaired t test relative to control siRNA; n = 15 samples for each group).
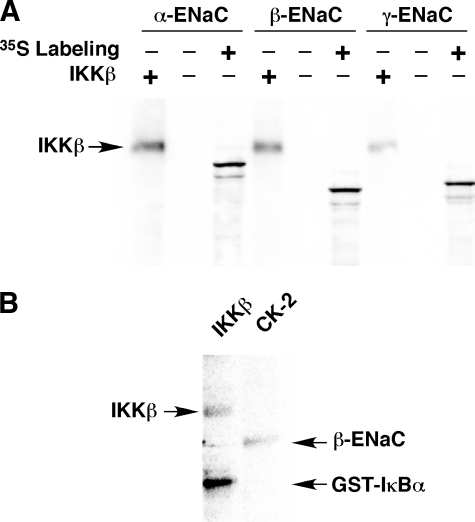
IKKβ Does Not Directly Phosphorylate ENaC in Vitro—As IKKβ is a kinase that binds to β-ENaC, we next tested whether any of the ENaC subunits may serve as direct substrates for IKKβ phosphorylation in vitro. In vitro transcribed and translated epitope-tagged mouse α-, β-, or γ-ENaC subunits were immunoprecipitated and then incubated with [γ-32P]ATP in the presence or absence of purified IKKβ as described under “Experimental Procedures.” The samples were then subjected to SDS-PAGE and transferred to nitrocellulose membranes (Fig. 2). [35S]Met/Cys labeling of ENaC was performed for some samples so that the ENaC subunit mobilities could be assessed on the membrane. No appreciable 32P incorporation into ENaC was apparent in the samples exposed to IKKβ, yet IKKβ autophosphorylation bands were observed at ∼90 kDa, as indicated in Fig. 2A. The presence of co-precipitated IKKβ confirms that this kinase binds to both α- and β-ENaC and, to a much lesser extent, γ-ENaC, findings consistent with our earlier work (10). GST-tagged IκBα was used as an additional positive control to demonstrate IKKβ activity, and casein kinase-2 was used as a positive control to demonstrate phosphorylation of ENaC by another kinase (Fig. 2B). Similar negative ENaC in vitro phosphorylation data were derived from experiments where ENaC subunits were transiently expressed in HEK-293 cells and then immunoprecipitated from cell lysates prior to performing in vitro phosphorylation assays (data not shown). The failure of IKKβ to phosphorylate ENaC in vitro suggests that its regulation of ENaC is indirect, not occurring through direct phosphorylation of the channel.
FIGURE 2.
IKKβ fails to phosphorylate ENaC subunits in vitro. A, epitope-tagged α-, β-, or γ-ENaC subunits, synthesized by coupled in vitro transcription/translation with (+) or without (–)[35S]Met/Cys labeling, were subjected to in vitro [γ-32P]ATP labeling in the presence (+) or absence (–) of purified IKKβ enzyme, as indicated. Autophosphorylation of IKKβ is apparent in the lanes where IKKβ is added, but no phosphorylation bands at the mobilities of the ENaC subunits are apparent in these lanes (compare with 35S-labeled ENaC subunits in nearby lanes). B, in vitro phosphorylation of GST-IκBα by IKKβ (left lane) and that of β-ENaC by casein kinase-2 (CK-2; right lane) are shown as positive controls. The data shown are representative of three similar experiments.
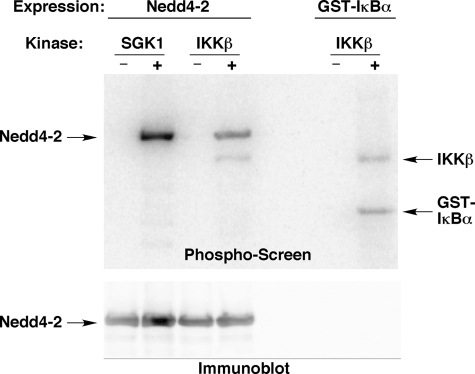
IKKβ-dependent Phosphorylation of Nedd4-2 in Vitro and in Vivo—We have previously reported that IKKβ stimulation of ENaC activity occurs through enhanced channel plasma membrane expression in Xenopus oocytes (10). As the E3 ubiquitin-protein ligase Nedd4-2 has emerged as a central locus for the regulation of ENaC expression at the apical plasma membrane (21), we considered that ENaC regulation by IKKβ may be mediated indirectly through Nedd4-2. Of note, Nedd4-2 has been recently reported to be a substrate for various kinases, including SGK1, PKA, AMPK, and Grk2, which may modulate Nedd4-2 function (3, 4, 20, 22). To test whether IKKβ phosphorylates Nedd4-2 in vitro, FLAG-xNedd4-2 was expressed in and immunoprecipitated from HEK-293 cell lysates, and in vitro phosphorylation assays were performed (Fig. 3). As a positive control for Nedd4-2 phosphorylation, we confirmed that purified SGK1 robustly phosphorylated xNedd4-2 (Fig. 3, upper panel, left). IKKβ similarly phosphorylated xNedd4-2 (Fig. 3, upper panel, middle) and its positive control, GST-tagged IκBα (upper panel, right). As observed in Fig. 2, autophosphorylation of IKKβ was also detected in these assays.
FIGURE 3.
In vitro phosphorylation of Nedd4-2 by IKKβ. Phosphorylation of Nedd4-2 (left) or GST-IκBα (right) in the absence (–) or presence (+) of purified SGK1 or IKKβ, as indicated, is shown. Phospho-screen (upper panel) and Nedd4-2 immunoblot (lower panel) images from the same nitrocellulose membrane are shown. Autophosphorylation of IKKβ is also indicated on the phospho-screen. Images shown are representative of those obtained in three replicate experiments.
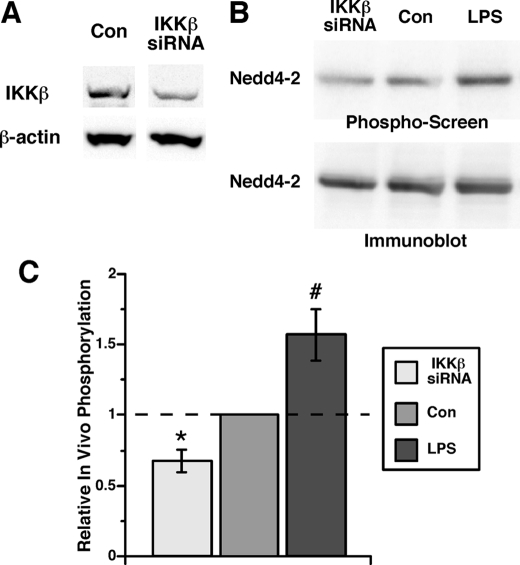
To determine whether IKKβ-dependent phosphorylation of Nedd4-2 occurs in intact cells, in vivo labeling with [32P]orthophosphate of HEK-293 cells expressing xNedd4-2 was performed under control conditions, after IKKβ protein expression was knocked down by RNA interference (Fig. 4A), or after overnight stimulation by 100 ng/ml LPS, an upstream activator of the IKK/NF-κB pathway. After immunoprecipitation of FLAG-xNedd4-2 from cell lysates, immunoblotting was performed first, followed by a phosphorimaging device analysis of the same membrane (Fig. 4B). The ratio of the phosphorylation signal to the immunoblot signal in each lane was compared across conditions to derive relative phosphorylation levels (Fig. 4C). Knockdown of IKKβ reduced in vivo [32P]orthophosphate incorporation into xNedd4-2 by 33 ± 8% relative to control siRNA-transfected cells (Fig. 4C, left). Conversely, LPS stimulation of these cells enhanced in vivo [32P]orthophosphate labeling of xNedd4-2 by 57 ± 19% (Fig. 4C, right). Taken together, these data indicate that IKKβ-dependent phosphorylation of Nedd4-2 occurs both in vitro and in vivo, suggesting a potential mechanism for regulation of Nedd4-2 and thus ENaC activity by IKKβ.
FIGURE 4.
IKKβ-dependent in vivo phosphorylation of Nedd4-2. A, representative immunoblot of lysates from siRNA-transfected Mam-X HEK-293 cells demonstrating knockdown of IKKβ protein expression relative to β-actin expression levels. Typical IKKβ knockdown from three replicate experiments was 50–60% in the IKKβ siRNA-transfected cells relative to control siRNA-transfected cells. B, representative image showing differential [32P]orthophosphate labeling of Nedd4-2 relative to total Nedd4-2 immunoprecipitated from Mam-X HEK-293 cell lysates under baseline conditions (Con), following knockdown of IKKβ (IKKβ siRNA) or following overnight treatment with 100 ng/ml Escherichia Coli-derived LPS. Phospho-screen (upper panel) and Nedd4-2 immunoblot (lower panel) images from the same nitrocellulose membrane are shown. C, summary of the mean (±S.E.) Nedd4-2 in vivo phosphorylation signals normalized to the control condition from three replicate experiments (*, p = 0.015; #, p = 0.006; two-tailed unpaired t tests relative to the control).
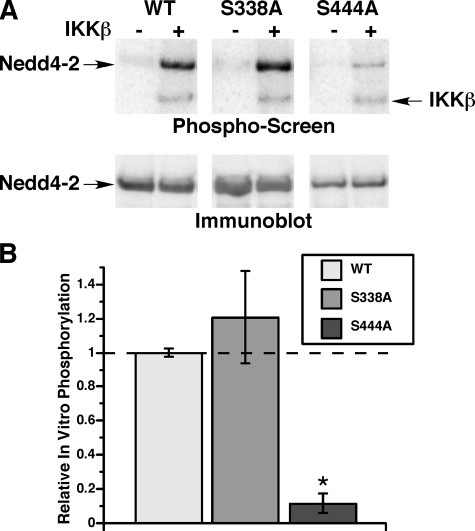
Identification of Ser-444 in xNedd4-2 as the Target for IKKβ Phosphorylation in Vitro and in Vivo—Although several IKKβ target phosphorylation sites have been identified within various components of the NF-κB·IκB protein complex (11, 23–25), no single consensus IKKβ target peptide sequence motif has emerged that would allow prediction of IKKβ phosphorylation sites in new protein substrates. It has been shown previously that both SGK1 and PKA stimulate ENaC activity via phosphorylation of Nedd4-2 at two key residues, Ser-338 and Ser-444 (Xenopus numbering), which has an inhibitory effect on Nedd4-2 function (3, 4). As IKKβ also stimulates ENaC activity, we tested whether IKKβ phosphorylates xNedd4-2 at the same SGK1 and PKA phosphorylation sites. In vitro phosphorylation assays were performed in the presence or absence of purified IKKβ using immunoprecipitated WT Nedd4-2 or Nedd4-2 with either Ser-338 or Ser-444 mutated to Ala (S338A or S444A) (Fig. 5). Nedd4-2 phosphorylation signal normalized to Nedd4-2 protein expression was quantitated and compared in the two mutant Nedd4-2 constructs relative to that of WT Nedd4-2 (Fig. 5B). Although the relative in vitro phosphorylation of the S338A mutant was not different from WT Nedd4-2, the 32P incorporation of the S444A mutant was dramatically reduced by 80–90% in vitro.
FIGURE 5.
In vitro phosphorylation of wild-type versus mutant Nedd4-2 by IKKβ. A, phosphorylation of WT, S338A, or S444A Nedd4-2 in the absence (–) or presence (+) of purified IKKβ, as indicated. Representative phosphoscreen (upper panel) and Nedd4-2 immunoblot (lower panel) images from the same nitrocellulose membrane are shown. Autophosphorylation IKKβ band is also indicated on the phospho-screen. B, summary of IKKβ-dependent Nedd4-2 in vitro phosphorylation corrected for protein expression reported relative to WT Nedd4-2 within each experiment. Results shown are the mean ± S.E. from four replicate experiments (*, p < 0.001 relative to WT; two-tailed unpaired t test).
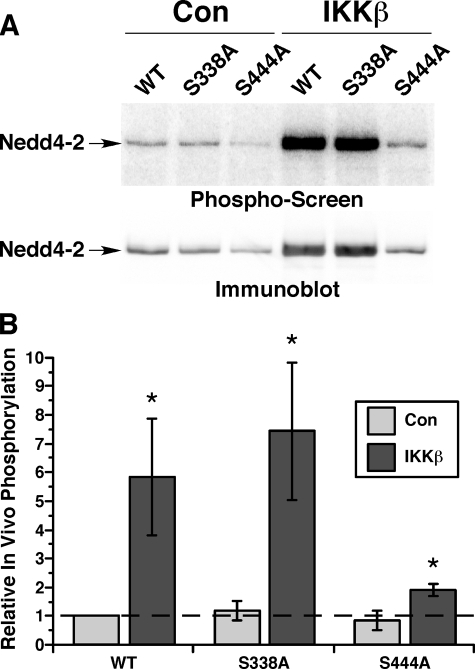
To test whether differential IKKβ-dependent phosphorylation at Ser-444 also occurs in vivo, we performed in vivo [32P]orthophosphate labeling studies using the same three Nedd4-2 constructs in control green fluorescent protein-transfected cells and in cells transfected to overexpress IKKβ (Fig. 6). Of note, IKKβ overexpression appeared to enhance the steady-state expression of transfected Nedd4-2 in these cells, whereas expression of the S444A mutant was decreased compared with the other Nedd4-2 constructs under both control and IKKβ co-expression conditions (Fig. 6A). Nevertheless, when normalized for Nedd4-2 protein expression, overexpression of IKKβ dramatically increased in vivo phosphate labeling of WT and S338A Nedd4-2 by ∼5–8-fold. However, there was only a smaller, albeit significant, ∼2-fold increase in phosphate labeling with the S444A Nedd4-2 mutant (Fig. 6B). These results suggest that Ser-444 is also the dominant target for IKKβ-dependent phosphorylation in vivo, although additional IKKβ-dependent Nedd4-2 phosphorylation site(s) could exist.
FIGURE 6.
IKKβ-dependent in vivo phosphorylation of wild-type versus mutant Nedd4-2. A, HEK-293 cells were co-transfected to express WT or mutant Nedd4-2 constructs along with either green fluorescent protein (Con) or IKKβ prior to in vivo [32P]orthophosphate labeling assays. Phospho-screen (upper panel) and Nedd4-2 immunoblot (lower panel) images from the same nitrocellulose membrane are shown. B, a summary of in vivo phosphorylation levels of the indicated Nedd4-2 constructs corrected for protein expression relative to WT Nedd4-2 within each experiment is shown. Results shown are the mean ± S.E. from four replicate experiments (*, p < 0.05 relative to corresponding control condition; one-tailed unpaired t tests).
IKKβ-dependent Regulation of ENaC Requires Functional Nedd4-2 and Phosphorylation at Ser-444—To explore the potential functional role of Nedd4-2 in the regulation of ENaC by IKKβ, we measured amiloride-sensitive whole cell currents in oocytes co-expressing mouse ENaC, either WT or mutant xNedd4-2 constructs, and/or IKKβ (Fig. 7). Confirming our previous findings (10), co-expression of IKKβ with ENaC increased ENaC current by ∼30% over that of oocytes expressing ENaC alone. Wild-type xNedd4-2 co-expression dramatically reduced ENaC currents under control conditions, but this reduction was substantially reversed by IKKβ co-expression. Indeed, ENaC current was increased ∼150% by IKKβ co-expression relative to ENaC alone in the presence of Nedd4-2, consistent with the idea that the presence of additional Nedd4-2 magnified the regulatory effect of IKKβ on ENaC. Co-expression of a ubiquitin ligase-deficient, dominant-negative xNedd4-2 mutant (C938S) (26) greatly increased ENaC currents, presumably by preventing Nedd4-2-dependent ENaC ubiquitination and degradation. Under this condition, IKKβ failed to activate ENaC, suggesting that inhibition of Nedd4-2-mediated ENaC ubiquitination is involved in the IKKβ-dependent activation of ENaC. Finally, co-expression of the IKKβ phosphorylation-deficient S444A xNedd4-2 mutant substantially inhibited ENaC current. Again, IKKβ failed to activate ENaC current with co-expression of this mutant, suggesting that IKKβ-mediated phosphorylation of Ser-444 in xNedd4-2 is required for the IKKβ-dependent regulation of ENaC.
DISCUSSION
In this study, we have further characterized the mechanism and relevance of ENaC regulation by IKKβ, a novel interaction partner and activator of ENaC that we recently identified (10). Knockdown of endogenous IKKβ in polarized mpkCCDc14 cells inhibited ENaC-dependent transepithelial currents, suggesting that under baseline conditions in these cultured cells, without exogenously induced stimulation of the IKK/NF-κB pathway, there is tonic IKKβ activity supporting basal ENaC activity. This result is consistent with our previous finding that overexpression of a dominant-negative mutant of IKKβ down-regulated ENaC currents expressed in oocytes under basal conditions (10). Although IKKβ binds to ENaC, we have found that Nedd4-2, rather than ENaC, is the phosphorylation target for this kinase. We have further shown that phosphorylation of Ser-444 in xNedd4-2, a dominant SGK1/PKA target site, is the relevant target of IKKβ in vitro and in vivo. Nedd4-2 mediates the IKKβ-dependent regulation of ENaC, and phosphorylation at Ser-444 appears to be required for this regulation to occur. Although our data demonstrate that IKKβ phosphorylates xNedd4-2, we feel it is likely that IKKβ will also phosphorylate mammalian Nedd4-2 based on the highly conserved sequence near Ser-444 across species (3), but this prediction remains to be tested.
Additional molecular details regarding the mechanism for IKKβ-dependent regulation of ENaC are not yet clear, but a few intriguing possibilities are suggested by our findings. It has been shown previously that SGK1-dependent phosphorylation of Ser-444 in xNedd4-2 enhances the binding of Nedd4-2 to 14-3-3 scaffolding proteins, which sequesters Nedd4-2 and thereby prevents it from interacting with ENaC (27–29). Therefore, as the effect of IKKβ on ENaC, like that of SGK1 and PKA, is stimulatory and requires phosphorylation at Ser-444, it is reasonable to propose that these three kinases share this common mechanism for ENaC regulation. However, the SGK1/PKA regulation of ENaC appears to require the additional phosphorylation at one of two other minor SGK1/PKA phosphorylation sites, Ser-338 or Thr-363. Such phosphorylation may enable the binding of another 14-3-3 molecule within a dimeric 14-3-3 complex to the same Nedd4-2 molecule, and this additional interaction appears to be necessary for the efficient sequestration of Nedd4-2 (3, 4). However, Ser-338 was not found to be a site for IKKβ phosphorylation of Nedd4-2. Moreover, at least based on our in vitro analysis of the IKKβ-dependent phosphorylation of Nedd4-2 (cf. Fig. 5), there are no other apparent significant IKKβ target phosphorylation sites in Nedd4-2 besides Ser-444. Therefore, an interesting question for future study is to determine whether SGK1 or PKA phosphorylation at another site is required to confer the IKKβ-mediated regulation of ENaC.
Normally, IKKα and IKKβ exist together in a complex, along with IKKγ/NEMO and other adaptor proteins such as Hsp90 and Cdc37 (12). Of note, as observed for IKKβ, we have also found that siRNA-mediated knockdown of IKKα produces a similar inhibition of ENaC-dependent current in mpkCCDc14 cells (data not shown). This finding indicates that disruption of the IKK complex in vivo may be sufficient to modulate ENaC currents. Furthermore, based on the finding that IKKβ co-precipitates when added to immunoprecipitates of either ENaC subunits (especially α and β) (Fig. 2) or Nedd4-2 (Figs. 3 and 5) in vitro, it is likely that at least the IKK complex, ENaC, and Nedd4-2 can exist together as a larger signaling complex in cells. We propose that this complex may afford the efficient coupling and integration of signals from various potential upstream pathways that impinge on IKK to regulate ENaC activity via its phosphorylation and functional regulation of Nedd4-2. Moreover, as Nedd4-2 appears to be an important regulator of a growing list of other ion transport proteins in cells (e.g. voltage-gated Na+ and K+ channels, KCNQ channels, ClC Cl– channels, the SGLT1 Na+/glucose co-transporter, and the NaPi IIb Na+/phosphate co-transporter) (30–35), IKKβ-dependent regulation of Nedd4-2 function may serve as a general transduction mechanism that couples changes in inflammation and potentially other signaling pathway inputs to transport protein regulation.
There are differing reports in the literature as to the functional consequences of IKK/NF-κB pathway stimulation on ENaC activity. Certain studies suggest that stimulation of the IKK/NF-κB pathway by either tumor necrosis factor-α or LPS inhibits ENaC activity, at least over relatively long time intervals (hours to days) (17, 18). This inhibition may occur via down-regulation of SGK1 in renal collecting duct epithelial cells and is earliest seen at 6 h (18). However, shorter term, acute effects may occur within 30 min and appear to be stimulatory (13–15), indicating a probable biphasic response of ENaC to this pathway. Our findings in this study show a stimulatory effect, at least under baseline conditions. There is good evidence that interstitial inflammation enhances renal Na+ reabsorption in association with proteinuric glomerular damage (36). We speculate that inflammation-associated activation of IKK/NF-κB and ENaC may contribute to this response. Acute up-regulation of renal salt absorption via this mechanism could also help the body defend against hypotension that occurs with systemic vasodilatation in the setting of sepsis and generalized inflammation.
As there is apparent tonic activity of IKK with respect to ENaC function in our system in the absence of induced inflammatory stimuli, it seems likely that other noninflammatory signaling pathways regulate the IKK complex under the conditions of our experiments. However, it is not yet clear what the potential upstream regulators of the IKK pathway are in this case. Interestingly, the metabolic sensor AMPK has been reported to be an upstream inhibitor of IKK in several cell systems (37–39), and we have shown that AMPK inhibits ENaC, also via effects on Nedd4-2 in an AMPK phosphorylation-dependent manner (7, 20). Further studies to test for the potential involvement of AMPK and other cellular signaling pathways as upstream regulators of the IKKβ-dependent regulation of ENaC are thus warranted.
In summary, we have identified a previously characterized node of Nedd4-2 regulation by SGK1 and PKA as the target for IKKβ-dependent regulation of ENaC. Via effects on Nedd4-2 function, the IKK/NF-κB pathway may play an important role in integrating the response of ENaC and other epithelial ion transport proteins and thus transepithelial salt transport to various pathways (e.g. inflammation, hormonal (aldosterone versus vasopressin), and metabolic).
Acknowledgments
We thank Lauren Kester for excellent technical assistance.
This work was supported, in whole or in part, by National Institutes of Health Grants K08 DK067143 (to J. L.), R01 DK057718 and DK047874 (to J. P. J.), and R01 DK075048 (to K. R. H.). This work was also supported by American Heart Association Postdoctoral Fellowship Award AHA 0825540D (to R. A.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: ENaC, epithelial Na+ channel; IKK, IκB kinase; ERK, extracellular signal-regulated kinase; siRNA, short interfering RNA; Isc, short-circuit current; xNedd4-2, Xenopus Nedd4-2; WT, wild-type; PKA, protein kinase A; AMPK, AMP-activated protein kinase; NF-κB, nuclear factor-κB; LPS, lipopolysaccharide; GST, glutathione S-transferase; SGK1, serum and glucocorticoid-regulated kinase 1; Grk2, G-protein coupled receptor kinase 2.
References
- 1.Bhalla, V., and Hallows, K. R. (2008) J. Am. Soc. Nephrol. 19 1845–1854 [DOI] [PubMed] [Google Scholar]
- 2.Mall, M., Grubb, B. R., Harkema, J. R., O'Neal, W. K., and Boucher, R. C. (2004) Nat. Med. 10 487–493 [DOI] [PubMed] [Google Scholar]
- 3.Debonneville, C., Flores, S. Y., Kamynina, E., Plant, P. J., Tauxe, C., Thomas, M. A., Munster, C., Chraibi, A., Pratt, J. H., Horisberger, J. D., Pearce, D., Loffing, J., and Staub, O. (2001) EMBO J. 20 7052–7059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Snyder, P. M., Olson, D. R., Kabra, R., Zhou, R., and Steines, J. C. (2004) J. Biol. Chem. 279 45753–45758 [DOI] [PubMed] [Google Scholar]
- 5.Shi, H., Asher, C., Chigaev, A., Yung, Y., Reuveny, E., Seger, R., and Garty, H. (2002) J. Biol. Chem. 277 13539–13547 [DOI] [PubMed] [Google Scholar]
- 6.Dinudom, A., Fotia, A. B., Lefkowitz, R. J., Young, J. A., Kumar, S., and Cook, D. I. (2004) Proc. Natl. Acad. Sci. U. S. A. 101 11886–11890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carattino, M. D., Edinger, R. S., Grieser, H. J., Wise, R., Neumann, D., Schlattner, U., Johnson, J. P., Kleyman, T. R., and Hallows, K. R. (2005) J. Biol. Chem. 280 17608–17616 [DOI] [PubMed] [Google Scholar]
- 8.Booth, R. E., and Stockand, J. D. (2003) Am. J. Physiol. 284 F938–F947 [DOI] [PubMed] [Google Scholar]
- 9.Butterworth, M. B., Edinger, R. S., Frizzell, R. A., and Johnson, J. P. (2008) Am. J. Physiol., in press
- 10.Lebowitz, J., Edinger, R. S., An, B., Perry, C. J., Onate, S., Kleyman, T. R., and Johnson, J. P. (2004) J. Biol. Chem. 279 41985–41990 [DOI] [PubMed] [Google Scholar]
- 11.Zandi, E., Rothwarf, D. M., Delhase, M., Hayakawa, M., and Karin, M. (1997) Cell 91 243–252 [DOI] [PubMed] [Google Scholar]
- 12.Hacker, H., and Karin, M. (2006) Sci. STKE 2006, RE13. [DOI] [PubMed]
- 13.Fukuda, N., Jayr, C., Lazrak, A., Wang, Y., Lucas, R., Matalon, S., and Matthay, M. A. (2001) Am. J. Physiol. 280 L1258–L1265 [DOI] [PubMed] [Google Scholar]
- 14.DiPetrillo, K., Coutermarsh, B., Soucy, N., Hwa, J., and Gesek, F. (2004) Kidney Int. 65 1676–1683 [DOI] [PubMed] [Google Scholar]
- 15.Vinciguerra, M., Hasler, U., Mordasini, D., Roussel, M., Capovilla, M., Ogier-Denis, E., Vandewalle, A., Martin, P.-Y., and Feraille, E. (2005) J. Am. Soc. Nephrol. 16 2576–2585 [DOI] [PubMed] [Google Scholar]
- 16.Rezaiguia, S., Garat, C., Delclaux, C., Meignan, M., Fleury, J., Legrand, P., Matthay, M. A., and Jayr, C. (1997) J. Clin. Investig. 99 325–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dagenais, A., Frechette, R., Yamagata, Y., Yamagata, T., Carmel, J. F., Clermont, M. E., Brochiero, E., Masse, C., and Berthiaume, Y. (2004) Am. J. Physiol. 286 L301–L311 [DOI] [PubMed] [Google Scholar]
- 18.de Seigneux, S., Leroy, V., Ghzili, H., Rousselot, M., Nielsen, S., Rossier, B. C., Martin, P.-Y., and Feraille, E. (2008) J. Biol. Chem. 283 25671–25681 [DOI] [PubMed] [Google Scholar]
- 19.Mohan, S., Bruns, J. R., Weixel, K. M., Edinger, R. S., Bruns, J. B., Kleyman, T. R., Johnson, J. P., and Weisz, O. A. (2004) J. Biol. Chem. 279 32071–32078 [DOI] [PubMed] [Google Scholar]
- 20.Bhalla, V., Oyster, N. M., Fitch, A. C., Wijngaarden, M. A., Neumann, D., Schlattner, U., Pearce, D., and Hallows, K. R. (2006) J. Biol. Chem. 281 26159–26169 [DOI] [PubMed] [Google Scholar]
- 21.Snyder, P. M. (2005) Endocrinology 146 5079–5085 [DOI] [PubMed] [Google Scholar]
- 22.Sanchez-Perez, A., Kumar, S., and Cook, D. I. (2007) Biochem. Biophys. Res. Commun. 359 611–615 [DOI] [PubMed] [Google Scholar]
- 23.Sakurai, H., Chiba, H., Miyoshi, H., Sugita, T., and Toriumi, W. (1999) J. Biol. Chem. 274 30353–30356 [DOI] [PubMed] [Google Scholar]
- 24.Lang, V., Janzen, J., Fischer, G. Z., Soneji, Y., Beinke, S., Salmeron, A., Allen, H., Hay, R. T., Ben-Neriah, Y., and Ley, S. C. (2003) Mol. Cell. Biol. 23 402–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu, C., and Ghosh, S. (2003) J. Biol. Chem. 278 31980–31987 [DOI] [PubMed] [Google Scholar]
- 26.Abriel, H., Loffing, J., Rebhun, J. F., Pratt, J. H., Schild, L., Horisberger, J. D., Rotin, D., and Staub, O. (1999) J. Clin. Investig. 103 667–673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ichimura, T., Yamamura, H., Sasamoto, K., Tominaga, Y., Taoka, M., Kakiuchi, K., Shinkawa, T., Takahashi, N., Shimada, S., and Isobe, T. (2005) J. Biol. Chem. 280 13187–13194 [DOI] [PubMed] [Google Scholar]
- 28.Bhalla, V., Daidie, D., Li, H., Pao, A. C., LaGrange, L. P., Wang, J., Vandewalle, A., Stockand, J. D., Staub, O., and Pearce, D. (2005) Mol. Endocrinol. 19 3073–3084 [DOI] [PubMed] [Google Scholar]
- 29.Liang, X., Peters, K. W., Butterworth, M. B., and Frizzell, R. A. (2006) J. Biol. Chem. 281 16323–16332 [DOI] [PubMed] [Google Scholar]
- 30.Fotia, A. B., Ekberg, J., Adams, D. J., Cook, D. I., Poronnik, P., and Kumar, S. (2004) J. Biol. Chem. 279 28930–28935 [DOI] [PubMed] [Google Scholar]
- 31.Henke, G., Maier, G., Wallisch, S., Boehmer, C., and Lang, F. (2004) J. Cell. Physiol. 199 194–199 [DOI] [PubMed] [Google Scholar]
- 32.Jespersen, T., Membrez, M., Nicolas, C. S., Pitard, B., Staub, O., Olesen, S. P., Baro, I., and Abriel, H. (2007) Cardiovasc. Res. 74 64–74 [DOI] [PubMed] [Google Scholar]
- 33.Palmada, M., Dieter, M., Boehmer, C., Waldegger, S., and Lang, F. (2004) Biochem. Biophys. Res. Commun. 321 1001–1006 [DOI] [PubMed] [Google Scholar]
- 34.Dieter, M., Palmada, M., Rajamanickam, J., Aydin, A., Busjahn, A., Boehmer, C., Luft, F. C., and Lang, F. (2004) Obes. Res. 12 862–870 [DOI] [PubMed] [Google Scholar]
- 35.Palmada, M., Dieter, M., Speil, A., Bohmer, C., Mack, A. F., Wagner, H. J., Klingel, K., Kandolf, R., Murer, H., Biber, J., Closs, E. I., and Lang, F. (2004) Am. J. Physiol. 287 G143–G150 [DOI] [PubMed] [Google Scholar]
- 36.Rodriguez-Iturbe, B., Herrera-Acosta, J., and Johnson, R. J. (2002) Kidney Int. 62 1379–1384 [DOI] [PubMed] [Google Scholar]
- 37.Giri, S., Nath, N., Smith, B., Viollet, B., Singh, A. K., and Singh, I. (2004) J. Neurosci. 24 479–487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu, X., Mahadev, K., Fuchsel, L., Ouedraogo, R., Xu, S. Q., and Goldstein, B. J. (2007) Am. J. Physiol. 293 E1836–E1844 [DOI] [PubMed] [Google Scholar]
- 39.Huang, N. L., Chiang, S. H., Hsueh, C. H., Liang, Y. J., Chen, Y. J., and Lai, L. P. (2008) Int. J. Cardiol., in press [DOI] [PubMed]