Abstract
The enzyme 5-lipoxygenase (5-LO) initiates the biosynthesis of leukotrienes, inflammatory mediators involved in immune diseases and defense. The subcellular localization of 5-LO is regulated, with nuclear import commonly leading to increased leukotriene production. We report here that 5-LO is constitutively phosphorylated on Ser-271 in transfected NIH 3T3 cells. This residue is nested in a classical nuclear export sequence, and phosphorylated Ser-271 5-LO was exclusively found in the nucleus by immunofluorescence and by fractionation techniques. Mutation of Ser-271 to Ala allowed nuclear export of 5-LO that was blocked by the specific nuclear export inhibitor leptomycin b, suggesting that phosphorylation of Ser-271 serves to interfere with exportin-1-mediated nuclear export. Consistent with previous reports that purified 5-LO can be phosphorylated on Ser-271 in vitro by MAPK-activated protein kinase 2, the nuclear export of 5-LO was increased by either treatment with the p38 inhibitor SB 203,580 or co-expression of a kinase-deficient p38 MAPK. Nuclear export of 5-LO can also be induced by KN-93, an inhibitor of Ca2+/calmodulin-dependent kinase II, and the effects of SB 203,580 plus KN-93 are additive. Finally, HeLa cells, which lack nuclear 5-LO, also lack constitutive phosphorylation of Ser-271. Taken together, these results indicate that the phosphorylation of Ser-271 serves to inhibit the nuclear export of 5-LO. This action works in concert with nuclear import, which is regulated by phosphorylation on Ser-523, to determine the subcellular distribution of 5-LO, which in turn regulates leukotriene biosynthesis.
Leukotrienes (LTs)2 are intercellular messengers that play critical roles in inflammatory and allergic diseases (for review, see Ref. 1). In particular, LTB4 promotes the recruitment and activation of leukocytes, stimulates phagocytosis and killing of pathogens, and promotes the synthesis of proteins involved in the inflammatory process. Through these effects and others, the overproduction of LTB4 helps drive the chronic inflammation that characterizes asthma (2–4), bronchitis (5–7), and rhinitis (8). On the other hand, the underproduction of LTB4 represents a form of immune suppression, resulting in impaired host defense against infectious pathogens. Thus, LTB4 promotes immune defense against pathogenic bacteria (9–11), fungi (12), viruses (13), and parasites (14–16). Significantly, reduced LT biosynthesis correlates with increased susceptibility to infectious disease in numerous conditions, including cigarette smoking (17, 18), malnutrition (19–22), vitamin D deficiency (23), human immunodeficiency virus infection (24, 25), and type II diabetes (26).
The enzyme 5-lipoxygenase (5-LO) initiates the biosynthesis of LTs from the polyunsaturated fatty acid arachidonic acid and represents a key point of regulation. In its resting state 5-LO is a soluble enzyme that can reside in either the cytoplasm or the nucleus (27–29). The 5-LO protein has three nuclear import sequences that can be regulated and function independently from one another (30, 31). These sequences determine the degree of nuclear localization of 5-LO, as they act additively rather than redundantly (32). This is important because the amount of LTB4 produced upon 5-LO activation increases with the amount of 5-LO present within the nucleus (33, 34). Furthermore, phosphorylation of Ser-523 on 5-LO via the cAMP/protein kinase A pathway serves to inhibit nuclear import (35) and inhibit LTB4 biosynthesis (36). Exaggerated nuclear import of 5-LO could, therefore, play an important role in the overproduction of LTB4 associated with chronic inflammatory diseases.
Much less is known about the regulation of nuclear export of 5-LO. It appears that 5-LO can be exported by an exportin-1-like mechanism, as treatment of CHO cells overexpressing a fusion protein involving 5-LO and green fluorescent protein (GFP) with the exportin-1 inhibitor leptomycin b (lmb) reduced cytoplasmic 5-LO (37). Exportin-1 binds a nuclear export sequence (NES) on target proteins. By forming a heterotrimer with a target protein and ranGTP, exportin-1 mediates one form of nuclear export (for review, see Ref. 38). Exportin-1 binds leucine-rich NESs with the consensus pattern of LXXLXL or LXXXLXL, where X is any intervening residue. Other hydrophobic residues and, in particular, isoleucine, may replace the leucines. Some NESs have been recognized to have a specific secondary structure, with a portion of the NES residing on an α-helix and a portion extending onto an adjacent random coil (39, 40). This structure is found in NESs from p53, IκBα, MAPK-activated protein kinase (MK)-2 and 14–3-3. To our knowledge, no region of 5-LO that matches these features has been identified to date.
The nuclear export of proteins can be regulated by a variety of mechanisms, including phosphorylation. For example, phosphorylation of the retinoic acid receptor RXRα by Jun kinase activates its export from the nucleus (41), and phosphorylation of ERK5 by MEK5 inhibits its nuclear export (42). Notably, the purpose of phosphorylation in these examples is not to alter intrinsic activity; indeed, RXRα is not an enzyme. In vitro experiments have shown that recombinant 5-LO can be phosphorylated on Ser-271, by MAPK-activated protein kinase-2 (MK-2) and possibly by MK-3 (43), by Ca2+/calmodulin-dependent kinase II (CaMKII) and by protein kinase A (44). Although phosphorylation of Ser-271 has been demonstrated in vitro, it has yet to be demonstrated in cells or tissues. Importantly, assays of 5-LO activity indicated that phosphorylation of Ser-271 in vitro had no appreciable effect on enzyme activity (43, 44). This suggests that phosphorylation of Ser-271 serves some purpose other than to alter the intrinsic activity of 5-LO.
In this study we sought to determine whether phosphorylation of Ser-271 affected nuclear export of 5-LO. Specifically, we asked if Ser-271 might already be phosphorylated in cells with nuclear 5-LO and, hypothesizing that phosphorylation might inhibit export, would dephosphorylation or mutation of Ser-271 allow exportin-1-dependent export of 5-LO. This would be particularly relevant to inflammatory signaling given that the subcellular localization of 5-LO strongly affects LTB4 biosynthesis.
EXPERIMENTAL PROCEDURES
Sequence and Structural Analysis—Amino acid sequences were obtained from the National Center for Biotechnology Information. Primary accession numbers for proteins are: for 5-LOs, human P09917, mouse P48999, rat P12527, hamster P51399, dog XP_534950, opossum XP_001364255, cow XP_613515; for rabbit 15-LO, P12630. Alignment of protein sequences was performed using ClustalW (45).
Plasmids, Mutagenesis, and DNA Construction—Plasmids containing the wild type (WT) 5-LO and the S523A and the S271A mutants of 5-LO alone in pcDNA or fused to green fluorescent protein (GFP) in pEGFP have been described previously(34, 36, 46). Substitutions of Leu-270 and Leu-272 with Ala in 5-LO were performed using the QuikChange site-directed mutagenesis kit (Stratagene, La Jolla, CA) following the manufacturer's instructions. Mutations and protein frame reading were verified by DNA sequence analysis (DNA Sequencing Core, University of Michigan). All oligonucleotides were synthesized by Integrated DNA Technologies Inc. (Coralville, IA). Plasmid and oligonucleotide sequences are available upon request.
Cell Culture, Transfection, and Immunoblotting—NIH 3T3, HeLa, and COS-1 cells obtained from ATCC (Manassas, VA) were grown under 5% CO2 in Dulbecco's modified Eagle's medium (Invitrogen) with 10% calf serum and 100 units/ml each of penicillin and streptomycin (complete medium). IMR-90 cells stably transfected with pcDNA3 empty vector, pcDNA3-p38-kinase-deficient, or pcDNA3-MAPK kinase 3, described previously (47), were maintained in similar medium with 200 μg/ml Geneticin before performing experiments. Cells were plated at 70% confluency and transfected with plasmids using Polyfect transfection reagent (Qiagen) following the manufacturer's instructions. 16–20 h post-transfection cells were treated with complete medium containing various compounds including 1–30 μm SB 203,580, 0.1–10 μm KN-93, or 5 ng/ml lmb for 0–6 h. After treatment cells were washed with PBS, harvested by a rubber policeman, and lysed with 50 mm Tris-HCl (pH 7.5), 150 mm NaCl, 0.1% Nonidet P-40 (Sigma) with complete protease inhibitor and phosphatase inhibitor cocktails (Roche Applied Science). Protein samples were separated by 10% SDS-PAGE and transferred to Immobilon-P polyvinylidene difluoride membranes. After blocking, the membranes were probed with either a polyclonal antibody raised against a phosphorylated Ser-271 peptide kindly provided by Dr. Qingyuan Ge (Cell Signaling Technologies), or LO-32 or H-5, two antisera against human 5-LO and FLAP, respectively (a generous gift of Dr. J. F. Evans, Merck Research Laboratories) followed by peroxidase-conjugated secondary antibody and enhanced chemiluminescence detection (Amersham Pharmacia Biosciences).
Cell Fractionation—NIH 3T3 cells overexpressing WT 5-LO and FLAP were harvested, washed with cold (4 °C) PBS, then fractionated as described previously (48). Briefly, cells were disrupted in cold (4 °C) lysis buffer (0.1% Nonidet P-40, 10 mm Tris-HCl (pH 7), 10 mm NaCl, 3 mm MgCl2, 1 mm EDTA, 10 μg/ml of both aprotinin and leupeptin, and complete proteases and phosphatases inhibitor tablets (Roche Applied Science)), vortexed for 20 s, placed on ice for 5 min, then centrifuged (500 × g, 10 min, 4 °C). Laemmli sample buffer (62.5 mm Tris (pH 6.8), 2% (w/v) SDS, 10% (v/v) glycerol, 5% β-mercaptoethanol, 0.01% (w/v) bromphenol blue) was then added to the resulting supernatants (non-nuclear fraction), pellets (nuclear fraction), or whole cell lysates. Samples were boiled for 10 min then analyzed by SDS-PAGE and immunoblot as described in the previous section.
Indirect Immunofluorescence Microscopy—NIH 3T3 cells grown on chamber slides (four chambers per slide) were transfected with 0.5 μg of plasmid DNA overnight, then washed and treated as described. For immunostaining, cells were washed with PBS, fixed in 4% paraformaldehyde in PBS for 25 min at room temperature, and permeabilized with 0.3% Triton X-100 in PBS containing 0.1% bovine serum albumin. The cells were then blocked with 1% bovine serum albumin in PBS, 0.3% Triton X-100 and incubated with primary antibody for 1 h at room temperature. Cells were washed in permeabilization buffer and incubated with fluorescein isothiocyanate-conjugated goat anti-rabbit secondary antibody (titer 1:250) in blocking buffer for 1 h. Mounting was done in mounting medium containing 4′,6-diamidino-2-phenylindole (Vector Laboratories, Inc., Burlingame, CA). Cells were visualized and imaged using a Nikon E600 microscope equipped for epifluorescence and digital image capture using a SPOT RT camera or using an Olympus FluoView 500 laser scanning confocal microscope.
Quantitation of Subcellular Distribution—As described previously (30), 100 positively transfected NIH 3T3 cells were scored by blinded evaluators as to whether nuclear fluorescence was greater than, equal to, or less than cytosolic fluorescence. Care was taken to avoid damaged, dead, or autofluorescent cells. Results from at least three independent transfections were used for statistical analysis.
Statistical Analysis—Statistical significance was evaluated by one-way analysis of variance using p < 0.05 as indicative of statistical significance. Pairs of group means were analyzed using the Tukey-Kramer post test.
RESULTS
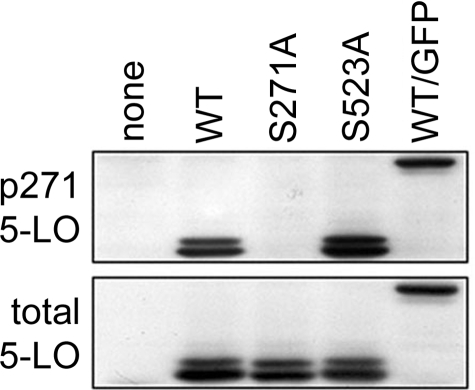
Ser-271 Is Phosphorylated on 5-LO in Untreated NIH 3T3 Cells—The finding that 5-LO could be phosphorylated on Ser-271 by MAPK-activated protein kinase-2 in vitro (43) was the first unequivocal report of direct phosphorylation of the 5-LO protein. However, an absence of evidence that this phosphorylation could occur in living cells (e.g. when p38 MAPK was activated in cells in the presence of radiolabeled phosphate) raised many questions. We hypothesized that induced phosphorylation of Ser-271 would be difficult to demonstrate if that residue was already phosphorylated. This was tested using an antibody raised against a peptide containing phosphorylated Ser-271. Cellular proteins from NIH 3T3 cells overexpressing WT 5-LO or GFP/5-LO, when assayed for 5-LO with phosphorylated Ser-271 (p271 5-LO) by immunoblot, produced bands at ∼78 kDa for WT 5-LO and ∼105 kDa for GFP/5-LO, and these corresponded to bands detected with an antibody for total 5-LO (Fig. 1). The p271 5-LO antibody did not detect 5-LO when Ser-271 was mutated to Ala, and it did not detect a band in untransfected NIH 3T3 cells. The antibody successfully detected S523A 5-LO; stripping and reprobing of the blot for total unphosphorylated 5-LO indicated similar levels of 5-LO expression in the different sets of transfected cells. 5-LO as well as p271 5-LO appeared as a doublet when expressed in NIH 3T3 cells, as has been observed previously (36). These results indicate that at least a portion of the total cellular 5-LO in transfected, but otherwise untreated, NIH 3T3 cells is phosphorylated on Ser-271.
FIGURE 1.
Antibody against p271 5-LO indicates that this residue is phosphorylated on 5-LO in NIH 3T3 cells. Cells were transfected with empty plasmid (none) or plasmids encoding WT 5-LO, S271A 5-LO, S523A 5-LO, or WT 5-LO/GFP as described under “Experimental Procedures.” After 20 h cells were harvested and processed, and total proteins were analyzed by immunoblot for p271 5-LO, then stripped and reprobed for total 5-LO as described under “Experimental Procedures.” Results are from one experiment representative of three independent experiments.
Structural Analysis of the Ser-271 Region—To potentially gain some insight into the purpose of phosphorylation on Ser-271, we evaluated its structural context. In primary sequences from the known or predicted 5-LOs of different species, Ser-271 is conserved as either a Ser or Thr (Fig. 2). In fact, the only variations near Ser-271 are conservative substitutions at Gln-269 and Gln-274. The predicted sequences for dog, opossum, and cow indicate that Ser-271 may be replaced with Thr, which could still be targeted by a Ser/Thr kinase. Importantly, the Leu residues surrounding Ser-271 (Leu-266, Leu-270, Leu-272) are conserved in all 5-LO species. The remarkable similarity of primary sequence of the Ser-271 region across the 5-LOs from different species contrasts with its dissimilarity with the parallel region from rabbit reticulocyte 15-LO (Fig. 2). The Ser-271 region rests atop the catalytic domain of 5-LO, distant from the regulatory PLAT domain, according to published putative models of 5-LO tertiary structure (49, 50). The Ser-271 region is held in place by two β-strands of a four β-strand complex that appears to be found in all LOs, plant, and animal. In the different 5-LOs there are consistently 33 residues between the conserved β-strands, whereas there are 29 residues in the 15-LOs, which accounts for 4 missing residues in any homology alignment (e.g. Fig. 2). Computer modeling (50) indicates that the Ser-271 region is a short α-helix, with Ser-271 at the amino terminus. In addition, the hydrophobic residue Leu-270 sits just outside the helix and projects outward, away from the catalytic domain. Thus, the Ser-271 region is highly conserved among and unique to the 5-LOs, structurally resembles an NES (at least according to computer modeling), and is projected externally.
FIGURE 2.
Alignment of primary sequences of known and predicted mammalian 5-LO species near Ser-271 (arrowhead) compared with rabbit reticulocyte 15-LO. Asterisks indicate identical residues; colons indicate similar residues.
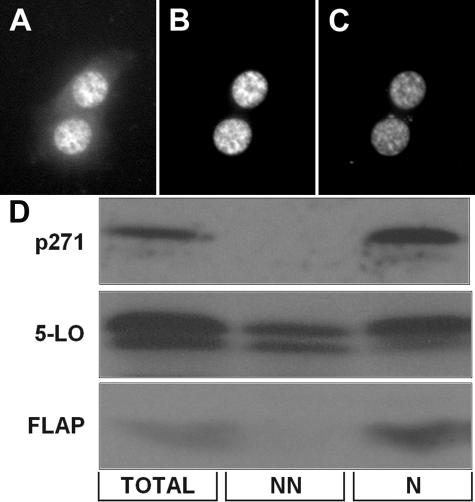
Subcellular Localization of p271 5-LO—The finding that Ser-271 is embedded in a potential NES suggested that the subcellular localization of p271 5-LO as well as total 5-LO should be determined. When NIH 3T3 cells were transfected with 5-LO/GFP and stained for p271 using the phospho-specific antibody, 5-LO/GFP was found in both the nucleus and the cytoplasm (Fig. 3A), whereas p271 5-LO was strictly nuclear (Fig. 3B), as indicated by DNA staining (Fig. 3C). The phosphorylated 5-LO was indeed intranuclear, as analysis utilized confocal microscopy. Similar results were obtained by analyzing cells transfected to express WT 5-LO and FLAP by cell fractionation combined with immunoblotting (Fig. 3D). That is, p271 5-LO was predominantly found in nuclear fractions, whereas total 5-LO was evident in both non-nuclear and nuclear fractions. The 5-LO activating protein, FLAP, known to be a nuclear membrane-associated protein (51), was used as a nuclear marker.
FIGURE 3.
Subcellular localization of 5-LO with and without phosphorylation on Ser-271. NIH 3T3 cells overexpressing 5-LO/GFP were fixed and stained for p271 and for DNA; confocal images indicate total 5-LO, from GFP (A), p271 5-LO (B), and DNA (C). D, immunoblot detection of p271 5-LO, total 5-LO, and FLAP in total cell proteins, non-nuclear (NN) proteins, and nuclear (N) proteins from NIH 3T3 cells overexpressing 5-LO.
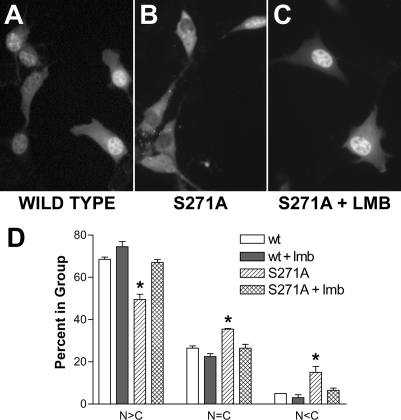
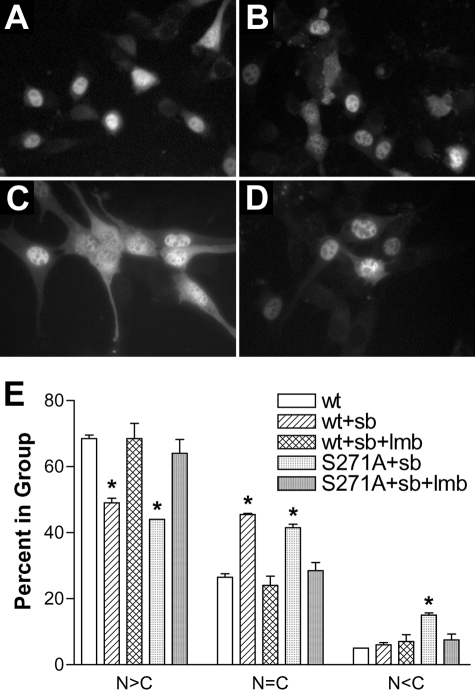
Site-directed Mutagenesis of Ser-271—The similarity of the Ser-271 region to a classical NES suggested that this region might interact with the classical exportin, exportin-1. Furthermore, knowing that Ser-271 is phosphorylated (Fig. 1) and that p271 5-LO is predominantly nuclear in NIH 3T3 cells (Fig. 3), we hypothesized that phosphorylation of Ser-271 blocks the export of 5-LO from the nucleus toward the cytosol. To assess this, we mutated Ser-271 to Ala and examined the effect on 5-LO subcellular distribution. Replacing only Ser-271 with Ala produced a significant change in the subcellular distribution of 5-LO; significantly more cells had either balanced nuclear and cytoplasmic 5-LO or predominantly cytoplasmic 5-LO (Fig. 4B). Importantly, NIH 3T3 cells overexpressing S271A 5-LO produce significantly less than half as much LTB4 when stimulated with calcium ionophore (10 μm) with added arachidonic acid (10 μm) versus cells with WT 5-LO (1912 + 232 versus 4570 + 807 pg/ml; p < 0.01) (34). Treatment of NIH 3T3 cells expressing S271A 5-LO with the nuclear export inhibitor lmb (5 ng/ml, 2 h) resulted in the redistribution of 5-LO to the nucleus (Fig. 4C). These changes were evaluated by scoring more than 100 cells per condition for whether nuclear fluorescence was greater than, equal to, or less than cytosolic fluorescence and analyzing values from three independent experiments. Quantitative analysis indicated that for the S271A mutation, statistically more cells had a balanced (n = C) or cytoplasmic (N < C) fluorescence distribution than did WT 5-LO (Fig. 4D). In addition, there was no statistical difference between the subcellular distribution of fluorescence in cells expressing S271A treated with lmb and that of cells expressing WT 5-LO with or without lmb (Fig. 4D). Similar results were obtained when fluorescence was from GFP using GFP/5-LO fusion proteins or from immunofluorescence of 5-LO in constructs lacking GFP (not shown). Taken together, these results indicated that site-directed mutagenesis of Ser-271 increased the rate of lmb-sensitive nuclear export of 5-LO.
FIGURE 4.
Site-directed mutation of Ser-271 allows lmb-sensitive nuclear export of 5-LO. NIH 3T3 cells were transfected with plasmids encoding WT 5-LO/GFP or S271A 5-LO/GFP. After 20 h cells were left untreated or treated with lmb (5 ng/ml, 2 h, 37 °C), then fixed and imaged as described under “Experimental Procedures.” A, WT 5-LO/GFP, untreated. B, S271A 5-LO/GFP, untreated. C, S271A 5-LO/GFP, lmb-treated. D, the subcellular distribution of fluorescence in more than 100 cells per condition was scored as predominantly nuclear (N>C), balanced between nucleus and cytoplasm (n=C), or cytoplasmic (N<C). Results show the means ± S.E. of triplicate determinations; *, p < 0.05 versus WT 5-LO/GFP. Results are from one experiment and are representative of three independent experiments.
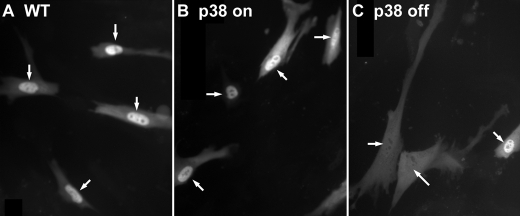
Role of p38 MAPK on 5-LO Distribution—As a first approach to assess the role of p38 MAPK in phosphorylating Ser-271 on 5-LO, we used IMR-90 cells (normal fetal lung fibroblasts) stably transfected with MAPK kinase 3, which activates p38, or a kinase-deficient p38. These cells have been characterized previously (47). IMR-90 cells stably expressing MAPK kinase 3, and thus having constitutively active p38 MAPK, and transfected with GFP/5-LO have predominantly nuclear 5-LO (Fig. 5B), like WT IMR-90 cells transfected with GFP/5-LO (Fig. 5A). However, cells stably expressing a kinase-deficient p38 MAPK have more cells with 5-LO balanced in distribution between the nucleus and cytoplasm (Fig. 5C). Quantitative analysis supported that the distribution of 5-LO in the latter cells was statistically different from that in either the WT cells or the MAPK kinase 3 cells (data not shown).
FIGURE 5.
Functional p38 MAPK activity is required for nuclear accumulation of 5-LO in IMR90 cells. IMR90 cells stably transfected with control plasmid (WT), MAPK kinase 3 (p38 on), or a kinase-deficient p38 MAPK construct (p38 off) were transfected with 5-LO/GFP and imaged after 20 h. Arrows point to nuclei. Results are from one experiment and are representative of three independent experiments.
The role of p38 MAPK in 5-LO distribution was also assessed using the p38 inhibitor SB 203,580. Initial experiments indicated that SB 203,580 produced time- and dose-dependent changes in the subcellular distribution of 5-LO (not shown). At 1–10 μm, SB 203,580 caused a slow shift of GFP/5-LO from predominantly nuclear to a more balanced (nuclear versus cytoplasm) distribution, as seen after 6 h of treatment in Fig. 6, A and C. This change was completely blocked by the addition of lmb simultaneously with SB 203,580 (Fig. 6D). Quantitative analysis, as described above, indicated that the effects of SB 203,580 were statistically significant (Fig. 6E). The distribution of S271A GFP/5-LO treated with SB 203,580 was similar to that of identically treated WT GFP/5-LO, and the addition of lmb again returned the distribution to that of vehicle-treated cells (Fig. 6E). Thus, SB 203,580 does not produce effects that are additive to mutation of Ser-271, and both effects are blocked by lmb, indicating that redistribution is through nuclear export.
FIGURE 6.
Treatment with the p38 inhibitor SB 203,580 allows lmb-sensitive nuclear export of WT 5-LO but not S271A 5-LO. NIH 3T3 cells were transfected overnight with WT 5-LO, then treated with vehicle (DMSO) (A), lmb (5 ng/ml) (B), SB 203,580 (3 μm)(C), or SB 203,580 (D) plus lmb for 6 h then fixed and imaged. E, quantitation of these cells plus cells transfected with S271A 5-LO and treated identically in parallel was performed as described under “Experimental Procedures.” Results show the means ± S.E. of triplicate determinations; *, p < 0.05 versus WT 5-LO/GFP given vehicle. Results are from one experiment and are representative of three independent experiments.
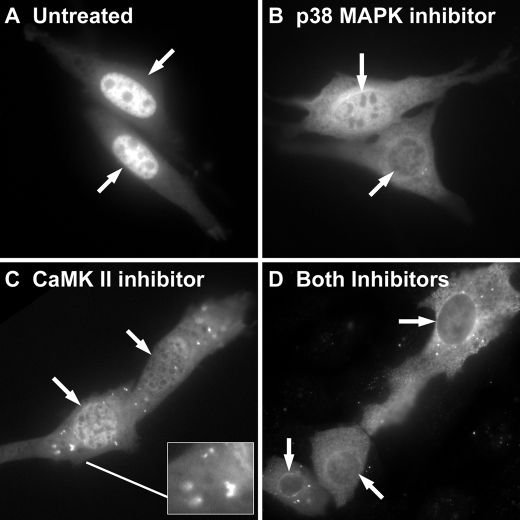
Role of CaMKII on 5-LO Distribution—Because 5-LO can be phosphorylated on Ser-271 in vitro by CaMKII (44), we asked if a selective inhibitor of CaMKII could emulate or add to the effect of SB 203,580 on 5-LO distribution. The addition of KN-93 (3 μm, 6 h) consistently produced a shift in 5-LO distribution that was similar in timing and appearance to that produced by SB 203,580 (Fig. 7). Treatment with KN-93 also repeatedly produced punctate cytoplasmic fluorescence of unknown significance, as shown in the detail to Fig. 7C. The combination of SB 203,580 plus KN-93 for 6 h appeared to have an additive effect on 5-LO localization, producing cells with predominantly cytoplasmic fluorescence (Fig. 7D). This suggested that, in NIH 3T3 cells, both p38 MAPK and CaMKII may act to retain 5-LO within the nucleus.
FIGURE 7.
Treatment with the CaMKII inhibitor KN-93 allows export of 5-LO that is additive in effect with SB 203,580. NIH 3T3 cells were left untreated (A) or treated with SB 203,580 (3 μm)(B), KN-93 (3 μm)(C), or SB 203,580 plus KN-93 (D) for 6 h, then fixed and imaged. Arrows indicate nuclei. Results are from one experiment and are representative of three independent experiments.
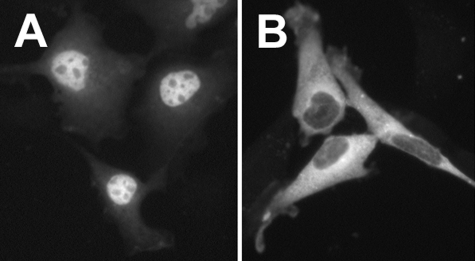
Mutational Evaluation of Leu-270 and Leu-272—If either Leu-270 or Leu-272 is necessary for binding exportin-1 to mediate export of 5-LO, then site-directed replacement of either residue should result in nuclear localization of 5-LO. This result was obtained for L270A 5-LO (Fig. 8A) but not for L272A 5-LO (Fig. 8B). The cytoplasmic localization of L272A-5-LO may have resulted from improper folding of the protein, which has been shown to prevent nuclear import of 5-LO (29). Importantly, computer modeling indicates that Leu-272, but not Leu-270, is involved in the formation of an α-helix (50), consistent with this explanation.
FIGURE 8.
Localization of 5-LO after site-directed mutagenesis of leucines adjacent to Ser-271. NIH 3T3 cells transfected with plasmids encoding 5-LO with L270A (A) or L272A (B) substitutions were fixed, and 5-LO was localized by indirect immunofluorescent staining as described under “Experimental Procedures.”
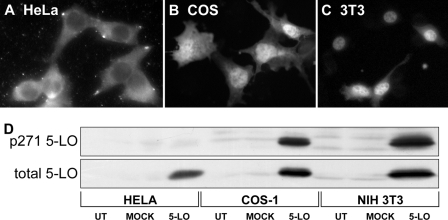
Other Cell Lines—The subcellular localization of 5-LO varies in different cell types (52). In HeLa cells transfected to overexpress 5-LO, 5-LO is mainly cytoplasmic (Fig. 9A), whereas transfected COS-1 cells have slightly more nuclear than cytoplasmic 5-LO (Fig. 9B) and, in transfected NIH 3T3 cells many cells have almost exclusively nuclear 5-LO (Fig. 9C). Examination of whole cell lysates from the transfected cell lines indicates that HeLa cells lack phosphorylation on Ser-271, whereas both COS-1 and 3T3 cells have phosphorylation on Ser-271 of 5-LO.
FIGURE 9.
Comparison of 5-LO subcellular distribution and phosphorylation of Ser-271 in different cell types. The subcellular distribution of 5-LO was determined by immunofluorescence in HeLa (A), COS-1 (B), and NIH 3T3 cells (C). In D whole cell lysates obtained from parallel cultures that had been untreated (UT) or transfected with empty vector (MOCK) or pcDNA 5-LO for 20 h were analyzed by immunoblot for p271 5-LO, then stripped and reprobed for total 5-LO as described under “Experimental Procedures.” Results are from one experiment representative of three independent experiments.
DISCUSSION
Previous studies demonstrated that purified 5-LO could be phosphorylated on Ser-271 when combined in vitro with specific kinases, including MAPK-activated protein kinase-2, CaMKII, and protein kinase A. Because phosphorylation of this site could not be achieved in cells, the biological significance of this posttranslational modification of 5-LO has remained elusive. In this study we demonstrate that 5-LO can be constitutively phosphorylated on Ser-271 in cultured cells. Furthermore, we show that Ser-271 is nested in an NES motif, that p271 5-LO is intranuclear in untreated cells, and that site-directed mutagenesis (S271A) allows lmb-sensitive nuclear export of 5-LO. This suggests that phosphorylation of this site prevents exportin-1 binding and the subsequent nuclear export of 5-LO, which alters eventual maximal capacity for LT synthesis.
As noted above, the positioning of resting 5-LO within the nucleus can lead to a substantial increase in LTB4 biosynthesis after cell stimulation. This has been demonstrated in primary cells, such as neutrophils (33) and macrophages (53), as well as in molecular model systems, such as transfected NIH 3T3 cells (34). In addition, we have previously demonstrated that impairment of nuclear import of 5-LO, achieved by selectively inactivating a nuclear import sequence, reduces LTB4 synthesis by more than 60% (34). In the same study we found that mutation of Ser-271 to Ala, preventing phosphorylation at that site, also led to both reduced nuclear 5-LO and a 60% reduction in LTB4 production after cell stimulation. Importantly, the S271A mutation of 5-LO did not alter the intrinsic, cell-free activity of 5-LO (34), much like the phosphorylation of Ser-271 in vitro had no effect on activity (43, 44). Taken together, these data indicate that the nuclear export of 5-LO is a regulated process and that phosphorylation of Ser-271 serves to increase LTB4 biosynthesis by inhibiting the export of 5-LO.
The timing of events related to the phosphorylation of 5-LO, relative to any changes in enzymatic activity, appears to be important. Generally speaking, 5-LO exists in leukocytes as a resting enzyme, and leukotriene secretion is negligible. Phosphorylation of Ser-271 on 5-LO does not appear to lead to activation of the enzyme; there is no increase in intrinsic, cell-free enzymatic activity (34). Also, the membrane association of 5-LO, a hallmark of 5-LO activation, does not appear to differ between WT 5-LO, S271A 5-LO, or WT 5-LO cells treated with kinase inhibitors. Similarly, we find that S271A 5-LO is comparable with WT 5-LO in terms of membrane association after cell stimulation (not shown), indicating that phosphorylation of Ser-271 is not necessary for membrane association. When leukocytes are stimulated in a way that activates 5-LO and triggers LT biosynthesis, 5-LO moves to membranes within seconds (54), newly biosynthesized LTs are detectable outside the cell within seconds, and LT production ends after several minutes (55). Contrasting with this are the rates of import and export of 5-LO. The addition of lmb to S271A 5-LO, blocking export and allowing nuclear accumulation of 5-LO, requires 30–120 min for completion of nuclear import of 5-LO. The addition of kinase inhibitors to allow nuclear export of 5-LO requires 3–6 h to see appreciable redistribution of 5-LO to the cytoplasm. These points suggest that, as the direct effects of phosphorylation of Ser-271 on 5-LO activity or membrane association are minimal, the immediate effects on LT biosynthesis will be small. Instead, by inhibiting the export of 5-LO, phosphorylation will allow accumulation of the protein in the nucleus (assuming ongoing import), which indirectly alters LT biosynthesis.
In this study we provide evidence that the inhibition of both p38 MAPK and CaMKII alters 5-LO localization in NIH 3T3 cells. The p38 MAPK is one of a family of kinases that are activated by mitogens and is known to be activated in proliferating cells (like those used in this study). Interestingly, we find that the addition of serum can increase nuclear accumulation of 5-LO in many types of leukocytes and cell lines and that depletion or omission of serum as well as contact inhibition of proliferation can lead to nuclear export of 5-LO (not shown). The relevance of the presence of serum, growth factors, or cell-cell contact to 5-LO subcellular distribution and LT biosynthesis in vivo has not been elucidated. In leukocytes, adhesion activates both p38 MAPK (55) and CaMKII (56), and we have shown that adhesion promotes nuclear accumulation of 5-LO in neutrophils (33) and eosinophils (57). However, it is not clear at this point whether phosphorylation of Ser-271 plays a role in the nuclear accumulation of 5-LO after adhesion or during proliferation.
It is notable that mutation of Ser-271, which must completely prevent phosphorylation at that residue, only alters 5-LO localization in a portion of NIH 3T3 cells. As we have demonstrated previously (30–32, 36), 5-LO contains three nuclear import sequences that are additive in action and regulated independently from one another. Given that the subcellular distribution of a protein will be determined by both its rate of import and its rate of export, the results of this study can be explained solely by three nuclear import sequences opposing a single NES. That is, if export is relatively slow, then activation of two or three nuclear import sequences may be sufficient to result in the accumulation of nuclear 5-LO. On the other hand, it remains entirely possible that 5-LO contains additional NES, either classical or non-classical.
The activity of phosphatases may be relevant to the phosphorylation of Ser-271 in primary leukocytes in vivo. In many transformed cell lines, like the NIH 3T3 cells used in this study, phosphatase activity is suppressed. In preliminary studies we do not find constitutive phosphorylation of Ser-271 in a variety of primary leukocytes (not shown). It seems reasonable that the phosphorylation status of Ser-271 may reflect a balance of kinase and phosphatase activities that change in a dynamic fashion.
In summary, we find that Ser-271 is constitutively phosphorylated in NIH 3T3 and COS-1 cells and that, in NIH 3T3 cells, phosphorylation is associated with nuclear accumulation of 5-LO. As Ser-271 is nested in a site whose primary and secondary structure resemble an NES and mutation of Ser-271 allows lmb-sensitive export, we suggest that phosphorylation of Ser-271 inhibits expotin-1-mediated export of 5-LO. In this way phosphorylation affects the subcellular distribution of resting 5-LO, which in turn affects LT biosynthesis upon cell stimulation and 5-LO activation.
Acknowledgments
We thank Dr. Victor Thannickal for supplying the IMR-90 cells used in these studies. We thank Dr. Qingyuan Ge from Cell Signaling Technologies for kindly providing the p271 antibody.
This work was supported by National Institutes of Health Grants RO1 AI43955, HL50496, and HL078727. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: LT, leukotriene; CaMK, calcium/calmodulin kinase; FLAP, 5-LO-activating protein; GFP, green fluorescent protein; lmb, leptomycin b; LO, lipoxygenase; MAPK, mitogen-activated protein kinase; NES, nuclear export sequence; p271, phospho-Ser-271 5-LO; WT, wild type; PBS, phosphate-buffered saline.
References
- 1.Flamand, N., Mancuso, P., Serezani, C. H., and Brock, T. G. (2007) Cell. Mol. Life Sci. 64 2657-2670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Drazen, J. M., Lilly, C. M., Sperling, R., Rubin, P., and Israel, E. (1994) Adv. Prostaglandin Thromboxane Leukotriene Res. 22 251-262 [PubMed] [Google Scholar]
- 3.Turner, C. R., Breslow, R., Conklyn, M. J., Andresen, C. J., Patterson, D. K., Lopez-Anaya, A., Owens, B., Lee, P., Watson, J. W., and Showell, H. J. (1996) J. Clin. Investig. 97 381-387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shindo, K., Fukumura, M., and Miyakawa, K. (1995) Eur. Respir. J. 8 605-610 [PubMed] [Google Scholar]
- 5.Biernacki, W. A., Kharitonov, S. A., and Barnes, P. J. (2003) Thorax 58 294-298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crooks, S. W., Bayley, D. L., Hill, S. L., and Stockley, R. A. (2000) Eur. Respir. J. 15 274-280 [DOI] [PubMed] [Google Scholar]
- 7.Beeh, K. M., Kornmann, O., Buhl, R., Culpitt, S. V., Giembycz, M. A., and Barnes, P. J. (2003) Chest 123 1240-1247 [DOI] [PubMed] [Google Scholar]
- 8.Cáp, P., Pehal, F., Chládek, J., and Malý, M. (2005) Allergy 60 171-176 [DOI] [PubMed] [Google Scholar]
- 9.Demitsu, T., Katayama, H., Saito-Taki, T., Yaoita, H., and Nakano, M. (1989) Int. J. Immunopharmacol. 11 801-808 [DOI] [PubMed] [Google Scholar]
- 10.Mancuso, P., Standiford, T., Marshall, T., and Peters-Golden, M. (1998) Infect. Immun. 66 5140-5146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coffey, M. J., Phare, S. M., and Peters-Golden, M. (2004) Prostaglandins Leukot. Essent. Fatty Acids 71 185-190 [DOI] [PubMed] [Google Scholar]
- 12.Medeiros, A. I., Sa-Nunes, A., Soares, E. G., Peres, C. M., Silva, C. L., and Faccioli, L. H. (2004) Infect. Immun. 72 1637-1644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gosselin J, B. P. F. L. (2005) J. Immunol. 174 1587-1593 [DOI] [PubMed] [Google Scholar]
- 14.Wirth, J. J., and Kierszenbaum, F. (1985) J. Immunol. 134 1989-1993 [PubMed] [Google Scholar]
- 15.Serezani, C. H., Perrela, J. H., Russo, M., Peters-Golden, M., and Jancar, S. (2006) J. Immunol. 177 3201-3208 [DOI] [PubMed] [Google Scholar]
- 16.Moqbel, R., Sass-Kuhn, S., Goetzl, E., and Kay, A. (1983) Clin. Exp. Immunol. 52 519-527 [PMC free article] [PubMed] [Google Scholar]
- 17.Laviolette, M., Coulombe, R., Picard, S., Braquet, P., and Borgeat, P. (1986) J. Clin. Investig. 77 54-60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Balter, M. S., Toews, G. B., and Peters-Golden, M. (1989) J. Lab. Clin. Med. 114 662-673 [PubMed] [Google Scholar]
- 19.Skerrett, S. J., Henderson, W. R., and Martin, T. R. (1990) J. Immunol. 144 1052-1061 [PubMed] [Google Scholar]
- 20.Anstead, G. M., Chandrasekar, B., Zhang, Q., and Melby, P. C. (2003) J. Leukocyte Biol. 74 982-992 [DOI] [PubMed] [Google Scholar]
- 21.Mancuso, P., Huffnagle, G. B., Olszewski, M. A., Phipps, J., and Peters-Golden, M. (2006) Am. J. Respir. Crit. Care Med. 173 212-218 [DOI] [PubMed] [Google Scholar]
- 22.Cederholm, T., Lindgren, J., and Palmblad, J. (2000) J. Intern. Med. 247 715-722 [DOI] [PubMed] [Google Scholar]
- 23.Coffey, M. J., Wilcoxen, S. E., Phare, S. M., Simpson, R. U., Gyetko, M. R., and Peters-Golden, M. (1994) Prostaglandins 48 313-329 [DOI] [PubMed] [Google Scholar]
- 24.Coffey, M., Phare, S. M., Kazanjian, P. H., and Peters-Golden, M. (1996) J. Immunol. 157 393-399 [PubMed] [Google Scholar]
- 25.Mayatepek, E., Flock, B., Zelezny, R., Kreutzer, K., and von Giesen, H. J. (1999) J. Infect. Dis. 179 714-716 [DOI] [PubMed] [Google Scholar]
- 26.Jubiz, W., Draper, R. E., Gale, J., and Nolan, G. (1984) Prostaglandins Leukotrienes Med. 14 305-311 [DOI] [PubMed] [Google Scholar]
- 27.Brock, T. G., Paine, R. I., and Peters-Golden, M. (1994) J. Biol. Chem. 269 22059-22066 [PubMed] [Google Scholar]
- 28.Brock, T. G., McNish, R. W., and Peters-Golden, M. (1995) J. Biol. Chem. 270 21652-21658 [DOI] [PubMed] [Google Scholar]
- 29.Chen, X.-S., Naumann, T. A., Kurre, U., Jenkins, N. A., Copeland, N. G., and Funk, C. D. (1995) J. Biol. Chem. 270 17993-17999 [DOI] [PubMed] [Google Scholar]
- 30.Jones, S. M., Luo, M., Healy, A. M., Peters-Golden, M., and Brock, T. G. (2002) J. Biol. Chem. 277 38550-38556 [DOI] [PubMed] [Google Scholar]
- 31.Jones, S. M., Luo, M., Peters-Golden, M., and Brock, T. G. (2003) J. Biol. Chem. 278 10257-10263 [DOI] [PubMed] [Google Scholar]
- 32.Luo, M., Pang, C. W. M., Gerken, A. E., and Brock, T. G. (2004) Traffic 5 847-854 [DOI] [PubMed] [Google Scholar]
- 33.Brock, T. G., McNish, R. W., Bailie, M. B., and Peters-Golden, M. (1997) J. Biol. Chem. 272 8276-8280 [DOI] [PubMed] [Google Scholar]
- 34.Luo, M., Jones, S. M., Peters-Golden, M., and Brock, T. G. (2003) Proc. Natl. Acad. Sci. U. S. A 100 12165-12170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Luo, M., Jones, S. M., Flamand, N., Aronoff, D. M., Peters-Golden, M., and Brock, T. G. (2005) J. Biol. Chem. 280 40609-40616 [DOI] [PubMed] [Google Scholar]
- 36.Luo, M., Jones, S. M., Coffey, M. J., Peters-Golden, M., and Brock, T. G. (2004) J. Biol. Chem. 279 41512-41520 [DOI] [PubMed] [Google Scholar]
- 37.Hanaka, H., Shimizu, T., and Izumi, T. (2002) Biochem. J. 361 505-514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pemberton, L. F., and Paschal, B. M. (2005) Traffic 6 187-198 [DOI] [PubMed] [Google Scholar]
- 39.Rittinger, K., Budman, J., Xu, J., Volinia, S., Cantley, L. C., Smerdon, S. J., Gamblin, S. J., and Yaffe, M. B. (1999) Mol. Cell 4 153-166 [DOI] [PubMed] [Google Scholar]
- 40.Meng, W., Swenson, L. L., Fitzgibbon, M. J., Hayakawa, K., Ter Haar, E., Behrens, A. E., Fulghum, J. R., and Lippke, J. A. (2002) J. Biol. Chem. 277 37401-37405 [DOI] [PubMed] [Google Scholar]
- 41.Zimmerman, T. L., Thevananther, S., Ghose, R., Burns, A. R., and Karpen, S. J. (2006) J. Biol. Chem. 281 15434-15440 [DOI] [PubMed] [Google Scholar]
- 42.Kondoh, K., Terasawa, K., Morimoto, H., and Nishida, E. (2006) Mol. Cell. Biol. 26 1679-1690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Werz, O., Klemm, J., Samuelsson, B., and Rådmark, O. (2000) Proc. Natl. Acad. Sci. U. S. A 97 5261-5266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Werz, O., Szellas, D., Steinhilber, D., and Rådmark, O. (2002) J. Biol. Chem. 277 14793-14800 [DOI] [PubMed] [Google Scholar]
- 45.Thompson, J. D., Higgins, D. G., and Gibson, T. J. (1994) Nucleic Acids Res. 22 4673-4680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Healy, A. M., Peters-Golden, M., Yao, J. P., and Brock, T. G. (1999) J. Biol. Chem. 274 29812-29818 [DOI] [PubMed] [Google Scholar]
- 47.Horowitz, J. C., Lee, D. Y., Waghray, M., Keshamouni, V. G., Thomas, P. E., Zhang, H., Cui, Z., and Thannickal, V. J. (2004) J. Biol. Chem. 279 1359-1367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Flamand, N., Lefebvre, J., Surette, M. E., Picard, S., and Borgeat, P. (2006) J. Biol. Chem. 281 129-136 [DOI] [PubMed] [Google Scholar]
- 49.Hemak, J., Gale, D., and Brock, T. G. (2002) J. Mol. Model 8 75-83 [DOI] [PubMed] [Google Scholar]
- 50.Lütteke, T., Krieg, P., Fürstenberger, G., and von der Lieth, C. W. (2003) Bioinformatics 19 2482-2483 [DOI] [PubMed] [Google Scholar]
- 51.Soberman, R. J., and Christmas, P. (2003) J. Clin. Investig. 111 1107-1113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brock, T. G. (2005) J. Cell. Biochem. 96 1203-1211 [DOI] [PubMed] [Google Scholar]
- 53.Covin, R. B., Brock, T. G., Bailie, M. B., and Peters-Golden, M. (1998) Am. J. Physiol. Lung Cell. Mol. Physiol. 275 303-310 [DOI] [PubMed] [Google Scholar]
- 54.Brock, T. G., McNish, R. W., and Peters-Golden, M. (1998) Biochem. J. 329 519-525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vliagoftis, H. (2002) J. Immunol. 169 4551-4558 [DOI] [PubMed] [Google Scholar]
- 56.Rosengart, M. R., Arbabi, S., Garcia, I., and Maier, R. V. (2000) Shock 13 183-189 [DOI] [PubMed] [Google Scholar]
- 57.Brock, T. G., Anderson, J. A., Fries, F. P., Peters-Golden, M., and Sporn, P. H. S. (1999) J. Immunol. 162 1669-1676 [PubMed] [Google Scholar]