Abstract
The function of retinoblastoma protein (pRb) in the regulation of small intestine epithelial cell homeostasis has been challenged by several groups using various promoter-based Cre transgenic mouse lines. Interestingly, different pRb deletion systems yield dramatically disparate small intestinal phenotypes. These findings confound the function of pRb in this dynamic tissue. In this study, Villin-Cre transgenic mice were crossed with Rb (flox/flox) mice to conditionally delete pRb protein in small intestine enterocytes. We discovered a novel hyperplasia phenotype as well as ectopic cell cycle reentry within villus enterocytes in the small intestine. This phenotype was not seen in other pRb family member (p107 or p130) null mice. Using a newly developed crypt/villus isolation method, we uncovered that expression of pRb was undetectable, whereas proliferating cell nuclear antigen, p107, cyclin E, cyclin D3, Cdk2, and Cdc2 were dramatically increased in pRb-deficient villus cells. Cyclin A, cyclin D1, cyclin D2, and Cdk4/6 expression was not affected by absent pRb expression. pRb-deficient villus cells appeared capable of progressing to mitosis but with higher rates of apoptosis. However, the cycling villus enterocytes were not completely differentiated as gauged by significant reduction of intestinal fatty acid-binding protein expression. In summary, pRb, but not p107 or p130, is required for maintaining the postmitotic villus cell in quiescence, governing the expression of cell cycle regulatory proteins, and completing of absorptive enterocyte differentiation in the small intestine.
The small intestine is a very dynamic tissue with proliferative epithelial cells invaginating into the mesenchyme to form flask-shaped crypts, whereas differentiated cells project into the lumen, covering finger-like villi. Fresh cells are generated from the stems cells that are either seated at the traditional +4 position from the bottom of the crypts or scattered between Paneth cells (so-called crypt base columnar cells) (1–3). Newly produced cells either migrate upward onto the villus and differentiate into absorptive enterocytes, goblet cells, or enteroendocrine cells or migrate down to the crypt bottom and differentiate into Paneth cells. After the cells pass the cryptvillus junction, they have already exited the cell cycle and are terminally differentiated into functional absorptive or secretory cells. These functionally differentiated cells finally shed into the lumen roughly 3–5 days after original generation (4).
Retinoblastoma protein (pRb)2 belongs to a pocket protein family, which also includes p107 and p130 (5). The major function of the pocket protein family members is to regulate the G1/S checkpoint and cell cycle progression, mainly through interaction with the E2F family of transcription factors that, in turn, regulate many genes necessary for both the G1/S and G2/M transition (6). Because germ line Rb–/– is lethal, pRb function has recently been studied in a conditional tissue-specific ablation fashion (7, 8). In the small intestine, three different groups have reported knocking out pRb protein specifically in the intestine, each with disparate findings. One group crossed I-FABP Cre transgenic mice with Rb (flox/flox) mice (9). The generated pRb intestine conditional nulls still expressed pRb, although at a reduced level. These mice did not demonstrate an abnormal small intestinal phenotype, but they did show ectopic cell cycle in colon enterocytes. Another group crossed collagen 1A1 Cre mice with Rb (flox/flox) mice to delete pRb in the small intestine (10). They identified hyper-proliferation in the putative crypts region and in the villi. The mice died shortly after birth. This phenotype, however, could not be conclusively tied to conditionally deleting pRb in enterocytes, as the author acknowledged that the collagen 1A1 promoter is not enterocyte-specific. Finally, another group used Villin Cre transgenic mice that were crossed with Rb (flox/flox) mice to delete pRb in the intestine (11). These investigators found aggressive tumors developed outside of the intestine after the mice reached 12–17 months of age. The detailed small intestinal effects from this pRb-deficient mouse strain, however, were not described.
The purpose of this study was to delineate the effects of enterocyte-specific deletion of pRb expression on the phenotype of the small intestine. To accomplish this, we bred Villin Cre transgenic mice with Rb (flox/flox) mice to delete pRb. Furthermore, we developed a new method to effectively separate villi from crypts, which enabled us to study pRb function in proliferating and postmitotic enterocytes, respectively. We found a unique and not previously described phenotype in the pRb ablation mice in which pRb expression was undetectable, and we discovered that pRb deficiency alone was sufficient to perturb the cell cycle and differentiation in villus cells.
EXPERIMENTAL PROCEDURES
Enterocyte Isolation—Sections of distal jejunum (5–6 cm) were removed, flushed with ice-cold phosphate-buffered saline, cut open longitudinally, and immersed in fresh ice-cold phosphate-buffered saline with protease inhibitors and phosphatase inhibitors (0.2 mm phenylmethylsulfonyl fluoride, 5 μg/ml aprotinin, 1 mm benzamidine, 1 mm sodium orthovanadate, 2 μm cantharidin). The bowel was then cut into three equal pieces and put into 5 ml of ice-cold BSS buffer (1.5 mm KCl, 96 mm NaCl, 27 mm sodium citrate, 8 mm KH2PO4, 5.6 mm Na2HPO4, 15 mm EDTA, and 1 mm dithiothreitol) with inhibitors as above, vortexed at 4 °C at maximum speed for 5 min. This process was repeated two additional times for 3 and 7 min, respectively, using fresh BSS solution. After a total of 15 min of vortexing, almost all epithelial cells were recovered in the BSS solution. The BSS solution was examined under light microscopy after each round of vortexing. The first sample consisted of debris and a few villi. The second sample had the majority of the villi. The third sample had the majority of the crypts with the remaining muscle. The muscle was collected and washed with BSS buffer to get rid of contamination by epithelial cells. The BSS solution (with crypts and villi) was then passed through a 70-μm cell strainer (BD Biosciences) to isolate crypts from villi. The smaller size crypts passed through the cell strainer and were collected in the effluent. The larger size villi were captured in the cell strainer. The crypt and villus fractions were then spun down at 1000 × g for 10 min at 4 °C and washed once with Tris buffer (150 mm NaCl, 1 mm EDTA, 1 mm EGTA, 50 mm Tris-HCl, pH 8.0) with inhibitors as above. The resulting pellets were saved at –80 °C for future RNA or protein experiments.
Mice—Mice containing Rb alleles in which exon 19 is flanked by loxP sites (Rb(flox/flox)) (12, 13), p107-null mice and p130-null mice were the generous gift of Dr. Eric Knudsen (University of Cincinnati, Cincinnati, OH). Villin Cre transgenic mice were obtained from the Mouse Repository of the Mouse Model of Human Cancers Consortium (Frederick, MD) and have been previously characterized (14). Villin Cre mice were intercrossed with Rb(flox/flox) mice to generate small intestine conditional pRb knock-out mice that were homozygous for the floxed Rb and contained Villin Cre (we will thereafter call this mouse strain VC+ Rb (f/f)). The animals were housed at the animal facilities at the Washington University School of Medicine, St. Louis.
BrdUrd Labeling and Immunostaining—Ninety minutes prior to tissue harvest, the mice received a subcutaneous injection with 1 ml/100 g body weight of 5-bromodeoxyuridine (BrdUrd) labeling agent (3 mg of BrdUrd/ml; Zymed Laboratories Inc.). The sections from the distal jejunum were then fixed in 10% neutral buffered formalin, embedded in paraffin, and cut into 5-μm sections. Immunostaining was performed according to the instructions associated with the specific antibodies. The following antibodies were used in this study: anti-BrdUrd (Zymed Laboratories Inc.), anti-p-histone 3, anti-PCNA, anti-cleaved caspase-3 (all from Cell Signaling; Danvers, MA), Alexa Fluor 488-conjugated goat anti-mouse IgG, and Texas Red-conjugated goat anti-rabbit IgG (all from Invitrogen). An index of proliferation rate was determined by counting the number of BrdUrd-labeled cells and total crypt cells in 20 full well oriented crypts. Apoptosis rate was determined by counting the number of cleaved caspase 3-positive cells within 100 villi.
Western Blotting—Isolated enterocyte pellets stored at –80 °C were reconstituted with Tris buffer and sonicated at 4 °C. The samples were then lysed with 1× SDS loading buffer and heated at 100 °C for 5 min. The protein concentration was determined using an RC DC kit from Bio-Rad. Equal amounts of protein were subjected to Western blotting assay. The primary antibodies against the following proteins were used in this study: Cdk2 and pRb (Pharmingen); cyclin A, cyclin E, and tubulin (Millipore; Billerica, MA); p107, p130, intestinal fatty acid-binding protein (I-FABP), MCM6 (minichromosome maintenance-deficient 6), PTK6 (protein-tyrosine kinase 6), and cell division cycle 2 (Cdc2) (Santa Cruz Biotechnology, Santa Cruz, CA); p-pRb (T821) and cyclin D2 (Invitrogen). The remaining antibodies against the following proteins were from Cell Signaling: platelet-derived growth factor receptor α, PCNA, p-pRb (Ser-807/811), p-pRb (Ser-780), cyclin D1, cyclin D3, Cdk4, Cdk6, and p-histone 3.
RESULTS
VC+ Rb(f/f) Mice Have Small Intestine Mucosal Hyperplasia—Mice that contained Villin Cre and were homozygous for Rb (flox/flox) were born at an expected ratio with normal body weight. These mice have survived for 8 months so far with no obvious health problems. Strikingly, hyperplasia was clearly identified in the small intestine mucosal layer, which has not been previously reported. The crypts were significantly deeper and villi taller when compared with the wild type littermates by using hematoxylin and eosin staining (Fig. 1).
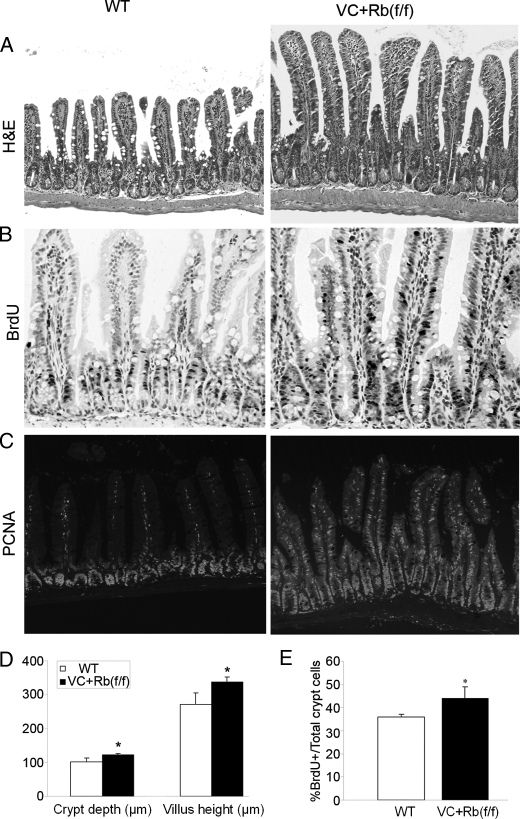
FIGURE 1.
Small intestine morphology in VC+Rb(f/f) and WT littermate mice (6–8 weeks of age). A, hematoxylin and eosin (H&E) staining revealed hyperplastic crypts and villi in VC+Rb(f/f) when compared with the WT mice. B, BrdUrd (BrdU) was administered to mice 90 min prior to sacrifice. Immunohistochemistry was then performed to detect BrdUrd-labeled cells (cells in S phase). Note the expansion of BrdUrd-labeled cells onto the villi. C, immunofluorescence was performed to detect the cell proliferation marker PCNA. In WT mice, PCNA was restricted to the proliferation zone, whereas in VC+Rb(f/f) mice, it spread onto the villi. D, crypt depth and villus height measurements in VC+Rb(f/f) or WT small intestine. * denotes p < 0.05; Student's t test. Results shown are from eight WT and four VC+Rb(f/f) mice. E, measurement of BrdUrd-labeled cells in crypts from VC+Rb(f/f) or WT mice. Results shown are from eight wild type and four VC+Rb(f/f) mice (*, p < 0.05; Student's t test).
As a gauge of enterocyte proliferation, the ratio of BrdUrd-positive cells to total crypt cells increased about 20% in VC+Rb(f/f) small intestine when compared with the wild type littermates. Moreover, in the absence of pRb, BrdUrd-labeled cells were no longer restricted to the crypt compartment, as BrdUrd-labeled cells clearly extended into the villus (Fig. 1B). This suggests that pRb is required for postmitotic villus cells to exit the cell cycle. To exclude the possibility of faster migration from crypts, villus cells were further checked for the expression of the cell proliferation marker PCNA. PCNA staining was confined to the crypt area in wild type small intestine. In contrast, PCNA staining in the VC+Rb(f/f) mice expanded into the villi (Fig. 1C), thus confirming that the villus cells were indeed proliferating. Because PCNA is a well known pRb/E2F targeted protein, these results are consistent with the loss of functional pRb in the villi.
pRb Is Undetectable in VC+Rb(f/f) Mice—Our VC+Rb(f/f) mice yielded a distinctive small intestinal phenotype compared with previous I-FABP Cre;Rb(flox/flox) mice (9). We therefore wondered if the phenotype could be due to different deletion efficiency because I-FABP promoter activity is known to be mosaic (9), whereas the Villin promoter is more evenly distributed across the small intestine (14). To investigate the efficiency of pRb deletion in VC+Rb(f/f) mice and the distinct function of pRb in crypt versus villus enterocytes, separation of these distinct cellular regions was necessary. Although there are several methods to isolate crypts from villi in the literature, each is associated with pitfalls (see under “Discussion”). We therefore developed a new isolation method as detailed under “Experimental Procedures.” Briefly, by using vigorous shaking (vortexing) and a cell strainer, we successfully separated crypts, villi, and underlying mesenchyme/muscle from the small intestine at a low temperature (4 °C) that is critical for keeping the recovered protein and RNA intact.
To validate the purity and integrity of isolated crypts, villi, and mesenchyme/muscle fractions, we first evaluated morphology by light microscopy. As demonstrated in Fig. 2A, crypt fractions revealed a U-shaped structure with dark granules on the bottom representing the Paneth cell region. In contrast, the villus fraction showed typical cylindrical shapes with the crypt-villus junction areas still intact. The mesenchyme/muscle fraction showed intact lamina propria connecting to the muscle layer. Purity was further established with distinct molecule markers for each cellular compartment by Western blotting. The expression of platelet-derived growth factor receptor α is known to be present only in mesenchyme (15). PTK6 is a marker for villus enterocytes (16), whereas pRb phosphorylation at Ser-807/811 was shown to be present only in crypt enterocytes (17). The localization of these cell-specific proteins was validated by Western blotting. Phosphorylation of pRb at Ser-807/811 was revealed in the mesenchyme/muscle fraction, which might reflect low proliferative activity in this compartment.
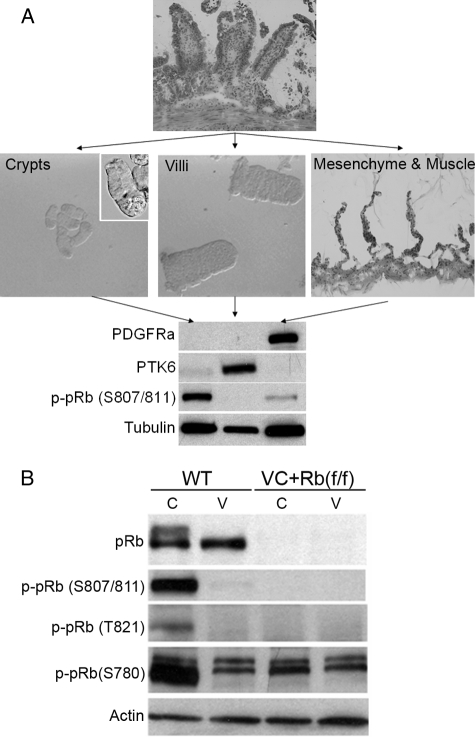
FIGURE 2.
A, separation of small intestine into crypts, villi, and mesenchyme and muscle fractions and confirmation of purity by Western blot analysis of putative markers for each fraction. B, Western blot demonstrated efficient deletion of pRb from crypts and villi in VC+Rb(f/f) mice. The pRb protein from isolated crypts revealed hyperphosphorylation. Actin was used as a loading control. C, crypts; V, villi.
This method was then used to investigate pRb deletion efficiency in crypt and villus enterocytes in the VC+Rb(f/f) mice. As shown in Fig. 2B, we saw a near-complete deletion of pRb in both crypts and villi. At the same time, we noticed that pRb displayed multiple bands in the crypts, which might reflect hyperphosphorylation of this protein in the aggressively proliferating compartment. Indeed, when site-specific antibodies for pRb phosphorylation were used for Western blotting, we found that pRb was phosphorylated only in crypts on sites Ser-780, Thr-821, and Ser-807/811. The p-pRb (Ser-780) antibody also picked up two slower migrating bands that is likely nonspecific, because they appeared in the small intestine taken from pRb-null mice. These results endorse the notion that pRb phosphorylation is likely to be an important regulatory mechanism for enterocyte proliferation and differentiation under normal conditions.
VC+Rb(f/f) Mice Small Intestine Show Compensatory Expression of p107 and p130, but Failed to Rescue pRb Function—The expression and function of pRb may be compensated by the other pocket protein family members p107 and p130 (18, 19). We first looked at p107 and p130 protein distribution in crypts and villi by Western blotting. As shown in Fig. 3, p107 protein was strongly present in the highly proliferating crypts, which agrees with the reported immunostaining pattern for this protein (9). The expression of p130 was evenly spread between the crypts and villi in normal mice and was absent in p130-null mice, as expected. Similar to pRb, p107 expression in crypts also displayed multiple bands, which may reflect possible differential phosphorylation of this protein within crypt enterocytes. For p130, its migration pattern in SDS-PAGE appeared to be no different between crypts and villi.
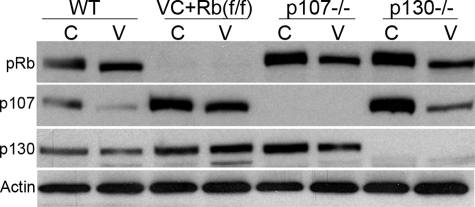
FIGURE 3.
Rb family proteins compensate for each other in small intestine enterocytes. Western blot analysis of protein indicated in wild type littermates (WT), pRb-null (VC+Rb(f/f)), p107-null (p107–/–), and p130-null (p130–/–) crypts and villi. The result was representative of at least three experiments. C, crypts; V, villi. Actin was used as a control for protein loading.
We then looked at the compensatory expression of these pocket proteins in the absence of one another. As shown in Fig. 3, we found that all the pocket proteins were capable of compensatory elevated expression in the absence of the others in both crypts and villi. The single exception was in the villi of either p107- or p130-null animals, in which the expression of pRb was not enhanced. Despite these compensatory expression changes, villus and crypt morphology and rates of proliferation were not affected by loss of either p107 or p130 (data not shown). These results suggest that high levels of either p107 or p130 protein in the pRb-null villi cannot suppress villus cell cycle reentry. In summary, our isolation method permitted demonstration of a different distribution and phosphorylation pattern of all three pocket proteins within different cellular compartments of the small intestine. Even though pRb expression was compensated by p107 and p130 in crypts and villi, its function was not, indicating a distinct and non-overlapping role for pRb in the regulation of normal small intestine homeostasis.
Deficient pRb Perturbs Expression of Multiple Cell Cycle-associated Proteins in Enterocytes—pRb has been well known to regulate cell cycle-associated proteins via E2F transcription factor family proteins. We therefore sought to investigate the expression of well characterized pRb/E2F-targeted proteins as well as atypical cell cycle-associated proteins in the pRb-null small intestine (Fig. 4). Because pRb phosphorylation status is different in crypts and villi, we decided to look at both compartments. By immunostaining, PCNA has been identified in the villi in pRb-null small intestine. Now the immunoblotting results clearly showed PCNA was indeed spread into villi only in the absence of pRb but not p107 or p130.
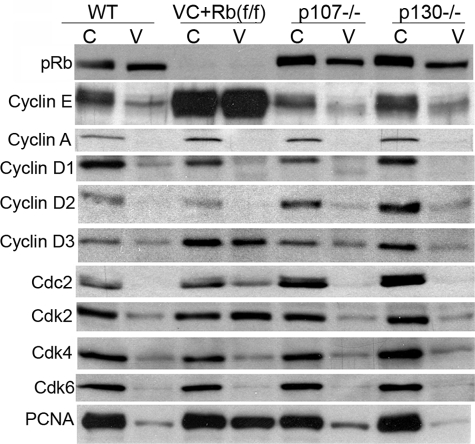
FIGURE 4.
Cell cycle regulatory protein expression is perturbed after pocket protein deletion. Western blot analysis of proteins indicated in wild type littermates (WT), pRb-null (VC+Rb(f/f)), p107-null (p107–/–), and p130-null (p130–/–) crypts and villi. The results shown were representative of at least three experiments. C, crypts; V, villi.
Cyclin E expression was mostly in crypts in normal mice. Deletion of pRb increased its expression no less than 5-fold in crypts with a more dramatic increase in villi. In contrast, p107 or p130 deficiency had no effect on cyclin E expression in the small intestine. The fact that cyclin E expression was largely repressed by pRb in the small intestine, even in highly proliferating crypts, indicates that cyclin E expression is mainly regulated by pRb itself and probably only partially regulated by pRb phosphorylation. This notion is also supported by the fact that deletion of hypophosphorylated pRb in villi released cyclin E expression. Cyclin A was exclusively expressed in crypts in normal mice. Its expression has been shown to be regulated by pRb (13, 20, 21). However, in small intestine, its expression seemed unaffected by any pocket protein studied. Three cyclin D proteins (D1, D2, and D3) were expressed in the small intestine with the highest levels in the crypts of normal mice. Cyclin D1 expression was less affected by the different pocket protein deletions, although the expression of cyclins D2 and D3 was regulated by different pocket proteins. Cyclin D2 expression was increased in crypts in the absence of p107 or p130. On the other hand, cyclin D3 expression was increased in both crypts and villi upon pRb depletion. Interestingly, β-catenin, which could regulate cyclin D1 expression, was found to be evenly spread across crypts and villi, and its expression and distribution were not affected by the absent expression of any pocket family protein (supplemental Fig. S1).
The expression of the cyclin-dependent kinases (Cdks) was also affected by pocket protein deletion. The majority of Cdk2/4/6 and Cdc2 (Cdk1) expression was located in the proliferating crypts in normal mice. pRb deletion elicited Cdk2 and Cdc2 expression in villi. Furthermore, Cdc2 expression was increased in p107- or p130-null crypts. Cdk4/6 expression and location were not affected by Rb family proteins.
To confirm that pRb was deleted only in enterocytes in VC+Rb(f/f) small intestine, Western blotting for Rb family and cell cycle regulatory proteins were performed on the isolated mesenchyme and muscle fraction (stromal tissue and lacking enterocytes). The expression of pRb as well as other cell cycle-related proteins was indeed intact in the stromal tissue (supplemental Fig. S2). As expected, p107 and p130 expression was absent in the stromal tissue of p107-null and p130-null mice, respectively. There was a compensatory increase in the expression of pRb in response to the loss of p107 or p130 in the stromal tissue. The expression of all the other cell cycle proteins, except for cyclin E and Cdc2, was not affected by the absence of p107 or p130 (supplemental Fig. S2).
In summary, our results revealed that cell cycle-associated protein expression was highest in crypts, dramatically decreased as cells migrated into villi, and was differentially regulated by Rb family proteins in the small intestine. These data also imply that pRb regulates cell cycle exit in the villi via active repression of cell cycle protein expression such as cyclin E, cyclin D3, Cdk2, and Cdc2.
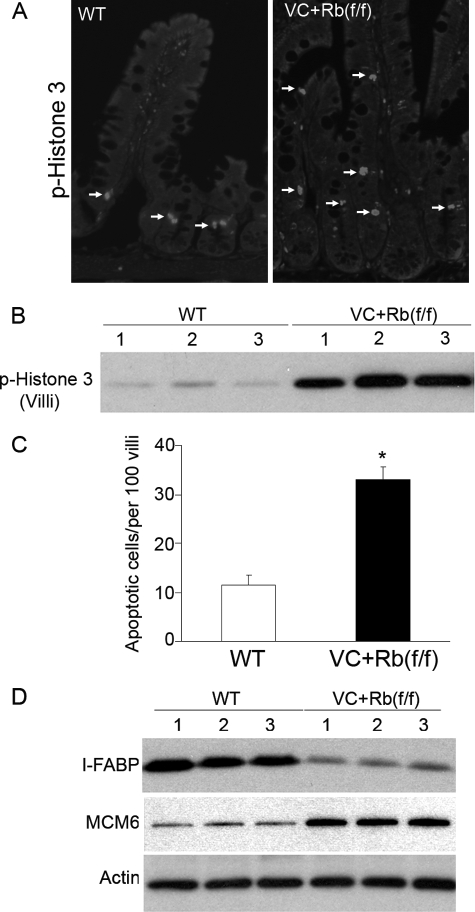
Ectopic Proliferating Villus Cells in VC+Rb(f/f) Mice Progress to Mitosis, Have Greater Rates of Apoptosis, and Have Impaired Absorptive Enterocyte Differentiation Programming—Because villus cells were still in S phase in VC+Rb(f/f) mice, we wondered if they could progress to mitosis. To this end, phosphorylation of histone 3 at Ser-10 was used as an indication for active mitosis. Mitosis in the crypts of WT and pRb-deficient mice could not be distinguished by either immunostaining (Fig. 5A) or Western blot analysis (data not shown). However, pRb deficiency greatly increased this M phase marker in villus cells, indicating that most of the cells were able to complete mitosis in the absence of pRb in villi (Fig. 5B). This may account for the hyperplasia seen in the villus of pRb-null intestine. The continued cycling of villus cells in pRb-null small intestine also led us to question whether these cells could have abnormal apoptosis, because pRb has been shown to regulate apoptosis in other tissues (22, 23). As revealed in Fig. 5C, pRb deletion resulted in about 3-fold increased rates of apoptosis in cycling villus cells as indicated by immunohistochemistry staining for cleaved caspase-3 on the paraffin-embedded small intestine. In summary, these data suggest that pRb is required for maintaining a quiescent status and protecting villus enterocytes from abnormal apoptosis.
FIGURE 5.
pRb-deficient villi have increased rates of mitosis, apoptosis, and impaired differentiation. A, immunofluorescence was performed using p-histone 3 antibody to detect mitosis in the small intestine of wild type (WT) and pRb-null (VC+Rb(f/f)) mice. Arrows indicate p-histone 3-positive cells. p-Histone 3-positive cells were identified in VC+Rb(f/f)) villi but not in the villi from WT mice. B, Western blot analysis of p-histone 3 expression in WT and VC+Rb(f/f)) villus enterocytes. C, apoptotic index of cleaved caspase 3-positive cells in WT and VC+Rb(f/f) villi (* denotes p < 0.05 versus WT, Student's t test). D, Western blot of absorptive cell differentiation marker I-FABP and undifferentiated marker MCM6 in WT and pRb-null villi. Actin was the loading control.
After migrating to the villus region, enterocytes exit the cell cycle and differentiate into either secretory or absorptive cell lineages. We therefore wondered if villus cell differentiation programming is affected by the ectopic cell cycle in pRb-deficient mice. The absorptive enterocyte differentiation was evaluated by measuring I-FABP protein expression. As expected, I-FABP was heavily expressed in the villi with very little expression in the crypts (data not shown). We then compared villus I-FABP expression of WT littermates with pRb-nulls. As shown in Fig. 5D, I-FABP expression was dramatically reduced in pRb-null villus cells. Yet at the same time, the expression of a marker for undifferentiated enterocytes, minichromosome maintenance deficient 6 (MCM6), was significantly increased in all pRb-null villi. These results strongly support the notion that pRb is required for absorptive cell differentiation in the villi. The role of p107 and p130 in absorptive cell differentiation was also examined and found not to be involved (data not shown). The secretory cell lineage was also examined and found to be unaffected by pRb ablation (data not shown). These data indicate that pRb is the only pocket protein family member required for absorptive cell differentiation in the small intestine.
DISCUSSION
In this study, we have clearly demonstrated a critical role for pRb, but not the other pocket proteins, as being indispensable for normal exit from the cell cycle as crypt enterocytes emerge to the villus in small intestine. We also reveal a unique pattern of phosphorylation of pRb in small intestine and cell cycle regulatory protein expression as a direct consequence of pRb deficiency. Finally, we demonstrate impaired enterocyte differentiation in response to pRb deficiency. Taken together, these data serve to reveal a crucial role for pRb in the regulation of normal enterocyte turnover in the mouse small intestine.
The clinical significance of these findings is the potential involvement of pRb in the pathogenesis of conditions in which normal enterocyte proliferation is perturbed. One such condition is the adaptation response of the remnant bowel to massive intestinal loss (24). During adaptation, enterocyte proliferation is triggered for the generation of taller villi, thereby augmenting the intestinal surface area for digestion and absorption. The involvement of pRb during adaptation-induced enterocyte proliferation and differentiation is presently unknown, but it may be critical for this important clinical response. Another obvious clinical condition in which enterocyte proliferation is enhanced is neoplasia. A recent finding that the loss of pRb in the gastrointestinal tract of Apc1638N mice promotes tumors of the cecum and proximal colon (25) would support the need for further investigation of the role for pRb and other pocket family proteins in enterocytes.
Specific deletion of pRb in the intestine has been attempted using different transgenic mice bred with Rb (flox/flox). One group used Villin Cre transgenic mice as we did. Their knockout mouse developed tumors outside the intestine after 12–17 months of age (11). However, the small intestine phenotype in these mice was not described. The second group used collagen A1A Cre transgenic mice. The generated knock-out mice displayed enhanced proliferation in the putative crypt region and ectopic proliferation in villi (10). The contribution of epithelial pRb to this phenotype, however, was not clear because of the fact that collagen A1A Cre is not expressed solely in the small intestine. The last group used I-FABP Cre transgenic mice (9). They found that pRb deletion induced ectopic S phase entry in colon enterocytes, but the cell cycle defects within the small intestine were not described. We used Villin Cre transgenic mice, which yielded the following distinctive results. 1) pRb is near-completely deleted in both crypts and villi in small intestine. 2) Ablation of pRb alone is sufficient to induce small intestine mucosal hyperplasia. 3) pRb is required for maintaining villus cell quiescence status. 4) pRb is required for absorptive enterocyte differentiation.
The cell cycle re-entry upon pRb ablation has been documented in many tissues such as liver and hair cells using a conditional knock-out strategy (13, 23). In the small intestine, the ectopic proliferation in villi has also been reported in situations with diminished pRb expression or function. In previous work, overexpression of SV40 large T antigen, under the control of the I-FABP promoter, resulted in ectopic cell proliferation in SV40 T antigen expressing villi (27). Even though the blunted pRb function by SV40 large T antigen was not the only factor accounting for this phenotype (28, 29), it more than likely did contribute. In that study, the villus-associated cycling enterocytes appeared to have a normal differentiation program. In another study, pRb was specifically deleted in gastrointestinal endocrine cells by using the promoter Neurogenin 3 Cre. pRb was found to be required for gastrointestinal endocrine cells exiting the cell cycle but not hormone expression (30). By efficiently deleting pRb specifically in small intestine enterocytes, we provide direct and conclusive evidence that pRb is necessary to keep villus cells from abnormal cell cycle entry, mitosis, and apoptosis. Furthermore, pRb is also required for the completion of terminal differentiation of absorptive enterocytes.
Molecular approaches to studies of the small intestine have been hampered by the lack of efficient and high quality methods for isolation of crypts and villi in large quantities. The small intestine is a perfect tissue for studies of cell proliferation, migration, and differentiation, as these events are precisely reflected by their position along the crypt-villus axis. Immunohistochemistry is currently the most effective and widely used method to study alterations in gene expression and/or activity within the various epithelial cell compartments of the gut. However, immunostaining is frequently limited by the lack of appropriate antibodies. Most importantly, the results are usually not quantifiable. Laser capture microdissection microscopy (LCM) has been utilized as a way to separate crypts from villi. Unfortunately, the yield of DNA or RNA by LCM is limited and requires capturing a large number of cells in multiple tissue sections. Most importantly, LCM is not presently feasible to isolate crypt/villus cells for assays such as Western blotting. Traditional crypt/villus isolation methods are available (31–34), but they involve either an incubation step at 37 °C or an everting step, which is technically challenging and requires significant manipulation to the bowel. Both procedures raise concerns for protein and RNA integrity. Our method avoided the higher temperature incubation and bowel eversion, but rather used aggressive vortexing at lower temperatures along with a cell strainer to separate crypts from villi. It allows a greater yield of intact protein and RNA and has been routinely used in our lab for Western blotting, kinase assays, real time PCR, and microarray studies.
Contradictory results have been reported by using different methods to localize cell cycle regulatory protein expression in the small intestine. For example, p27 is a cell cycle inhibitor of the Cip/Kip family and its expression was found to be highest in proliferating crypt cells (35). However, in another report, p27 expression was found to be exclusively in the villi, and its expression in crypts was repressed by Notch signaling to maintain proliferating crypt progenitor cells (36). Conflicting results have also been reported for the pattern of cyclin D1, Cdk4, and cyclin E expression within the small intestine (35, 37). Our new isolation method enables us to conclude with greater certainty that expression of cyclin D1, Cdk4, and cyclin E is highest in the proliferating crypts and is dramatically reduced as cells move to the differentiated villi. In addition, we found p107, cyclin A, cyclin D2, cyclin D3, Cdk2, Cdk6, Cdc2, and PCNA were all highest in crypts and dropped into very low or even undetectable level in villus cells.
We have revealed for the first time that pRb is hypophosphorylated as enterocytes move into the villus region. This might be critical for maintaining the quiescence of villus cells and may promote the terminal differentiation of enterocytes, because accumulation of hypophosphorylated pRb (active form) has been found to coincide with cell differentiation and growth arrest in other tissues such as lung epithelial cells (26). In the small intestine we determined that hypophosphorylated pRb also coincides with the terminally differentiated villus enterocytes. Furthermore, our results suggested that genes associated with cell cycle progression such as cyclin E, cyclin D3, cdk2, and cdc2 have been actively repressed by hypophosphorylated pRb in villus cells. However, whether deprivation of pRb phosphorylation is sufficient to keep villus cells quiescent is still an open question. Planned experiments with conditional constitutive phosphorylated pRb mutant mice (to force pRb phosphorylation in villi) should be able to provide further insight into this consideration.
Even though the mechanism for pRb phosphorylation to regulate villus cell quiescence and terminal differentiation is not clear, our results establish that pRb protein itself is clearly required for this process. Deletion of pRb alone in the small intestine clearly induces villus cell ectopic proliferation/division and impaired differentiation. Interestingly, when compared with cycling crypt cells, ectopic cycling villus cells actually have very low levels of cyclin A, D1, D2, and Cdk4/6. These data indicate the existence of redundant functions for cyclins and Cdks in the regulation of ectopic villus cell proliferation. Alternatively it may suggest that phenotypically similar cycling cells in villi may use different cell cycle machinery. It is possible that absent pRb expression is the major trigger for aberrant cell cycle regulatory protein expression. Or perhaps other factors downstream of the pRb-directed E2F transcriptional regulation may be operative. Further studies will be necessary to determine the exact mechanism for these alterations.
Supplementary Material
This work was supported, in whole or in part, by National Institutes of Health Grants RO1 DK53234 (to B. W. W.), T32 CA009621 (to S. L.), and P30DK52574 (to Morphology and Murine Models Cores of the Digestive Diseases Research Core Center of the Washington University School of Medicine). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1 and S2.
Footnotes
The abbreviations used are: pRb, retinoblastoma protein; Rb, retinoblastoma protein; I-FABP, intestinal fatty acid-binding protein; Cdc2, cell division cycle 2; PCNA, proliferating cell nuclear antigen; Cdk, cyclin-dependent kinase; WT, wild type; BrdUrd, bromodeoxyuridine; LCM, laser capture microdissection microscopy.
References
- 1.Reya, T., and Clevers, H. (2005) Nature 434 843–850 [DOI] [PubMed] [Google Scholar]
- 2.Barker, N., van Es, J. H., Kuipers, J., Kujala, P., van den Born, M., Cozijnsen, M., Haegebarth, A., Korving, J., Begthel, H., Peters, P. J., and Clevers, H. (2007) Nature 449 1003–1007 [DOI] [PubMed] [Google Scholar]
- 3.Mills, J. C., and Gordon, J. I. (2001) Proc. Natl. Acad. Sci. U. S. A. 98 12334–12336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Potten, C. S., and Loeffler, M. (1990) Development (Camb.) 110 1001–1020 [DOI] [PubMed] [Google Scholar]
- 5.Cobrinik, D. (2005) Oncogene 24 2796–2809 [DOI] [PubMed] [Google Scholar]
- 6.Dimova, D. K., and Dyson, N. J. (2005) Oncogene 24 2810–2826 [DOI] [PubMed] [Google Scholar]
- 7.Wikenheiser-Brokamp, K. A. (2006) Cell. Mol. Life Sci. 63 767–780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vidal, A., Carneiro, C., and Zalvide, J. B. (2007) Front. Biosci. 12 4483–4496 [DOI] [PubMed] [Google Scholar]
- 9.Haigis, K., Sage, J., Glickman, J., Shafer, S., and Jacks, T. (2006) J. Biol. Chem. 281 638–647 [DOI] [PubMed] [Google Scholar]
- 10.Yang, H. S., and Hinds, P. W. (2007) BMC Dev. Biol. 7 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kucherlapati, M. H., Nguyen, A. A., Bronson, R. T., and Kucherlapati, R. S. (2006) Cancer Res. 66 3576–3583 [DOI] [PubMed] [Google Scholar]
- 12.Marino, S., Vooijs, M., van Der, G. H., Jonkers, J., and Berns, A. (2000) Genes Dev. 14 994–1004 [PMC free article] [PubMed] [Google Scholar]
- 13.Mayhew, C. N., Bosco, E. E., Fox, S. R., Okaya, T., Tarapore, P., Schwemberger, S. J., Babcock, G. F., Lentsch, A. B., Fukasawa, K., and Knudsen, E. S. (2005) Cancer Res. 65 4568–4577 [DOI] [PubMed] [Google Scholar]
- 14.el Marjou, F., Janssen, K. P., Chang, B. H., Li, M., Hindie, V., Chan, L., Louvard, D., Chambon, P., Metzger, D., and Robine, S. (2004) Genesis 39 186–193 [DOI] [PubMed] [Google Scholar]
- 15.Clevers, H. (2006) Cell 127 469–480 [DOI] [PubMed] [Google Scholar]
- 16.Haegebarth, A., Bie, W., Yang, R., Crawford, S. E., Vasioukhin, V., Fuchs, E., and Tyner, A. L. (2006) Mol. Cell. Biol. 26 4949–4957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Taylor, J. A., Martin, C. A., Nair, R., Guo, J., Erwin, C. R., and Warner, B. W. (2008) J. Pediatr. Surg. 43 1018–1024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ruiz, S., Santos, M., Segrelles, C., Leis, H., Jorcano, J. L., Berns, A., Paramio, J. M., and Vooijs, M. (2004) Development (Camb.) 131 2737–2748 [DOI] [PubMed] [Google Scholar]
- 19.MacLellan, W. R., Garcia, A., Oh, H., Frenkel, P., Jordan, M. C., Roos, K. P., and Schneider, M. D. (2005) Mol. Cell. Biol. 25 2486–2497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Markey, M. P., Bergseid, J., Bosco, E. E., Stengel, K., Xu, H., Mayhew, C. N., Schwemberger, S. J., Braden, W. A., Jiang, Y., Babcock, G. F., Jegga, A. G., Aronow, B. J., Reed, M. F., Wang, J. Y., and Knudsen, E. S. (2007) Oncogene 26 6307–6318 [DOI] [PubMed] [Google Scholar]
- 21.Camarda, G., Siepi, F., Pajalunga, D., Bernardini, C., Rossi, R., Montecucco, A., Meccia, E., and Crescenzi, M. (2004) J. Cell Biol. 167 417–423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wikenheiser-Brokamp, K. A. (2004) Development (Camb.) 131 4299–4310 [DOI] [PubMed] [Google Scholar]
- 23.Sage, C., Huang, M., Vollrath, M. A., Brown, M. C., Hinds, P. W., Corey, D. P., Vetter, D. E., and Chen, Z. Y. (2006) Proc. Natl. Acad. Sci. U. S. A. 103 7345–7350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Drozdowski, L., and Thomson, A. B. (2006) World J. Gastroenterol. 12 4614–4627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kucherlapati, M. H., Yang, K., Fan, K., Kuraguchi, M., Sonkin, D., Rosulek, A., Lipkin, M., Bronson, R. T., Aronow, B. J., and Kucherlapati, R. (2008) Proc. Natl. Acad. Sci. U. S. A. 105 15493–15498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levine, R. A., Hopman, T., Guo, L., Chang, M. J., and Johnson, N. (1998) Exp. Cell Res. 239 264–276 [DOI] [PubMed] [Google Scholar]
- 27.Hauft, S. M., Kim, S. H., Schmidt, G. H., Pease, S., Rees, S., Harris, S., Roth, K. A., Hansbrough, J. R., Cohn, S. M., Ahnen, D. J., Wright, N. A., Goodlad, R. A., and Gordon, J. I. (1992) J. Cell Biol. 117 825–839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rathi, A. V., Saenz Robles, M. T., and Pipas, J. M. (2007) J. Virol. 81 9481–9489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saenz-Robles, M. T., Markovics, J. A., Chong, J. L., Opavsky, R., Whitehead, R. H., Leone, G., and Pipas, J. M. (2007) J. Virol. 81 13191–13199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang, Y., Ray, S. K., Hinds, P. W., and Leiter, A. B. (2007) Dev. Biol. 311 478–486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Flint, N., Cove, F. L., and Evans, G. S. (1991) Biochem. J. 280 331–334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Traber, P. G., Gumucio, D. L., and Wang, W. (1991) Am. J. Physiol. 260 G895–G903 [DOI] [PubMed] [Google Scholar]
- 33.Weiser, M. M. (1973) J. Biol. Chem. 248 2542–2548 [PubMed] [Google Scholar]
- 34.Weiser, M. M. (1973) J. Biol. Chem. 248 2536–2541 [PubMed] [Google Scholar]
- 35.Smartt, H. J., Guilmeau, S., Nasser, S. V., Nicholas, C., Bancroft, L., Simpson, S. A., Yeh, N., Yang, W., Mariadason, J. M., Koff, A., and Augenlicht, L. H. (2007) Gastroenterology 133 232–243 [DOI] [PubMed] [Google Scholar]
- 36.Riccio, O., van Gijn, M. E., Bezdek, A. C., Pellegrinet, L., van Es, J. H., Zimber-Strobl, U., Strobl, L. J., Honjo, T., Clevers, H., and Radtke, F. (2008) EMBO Rep. 9 377–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chandrasekaran, C., Coopersmith, C. M., and Gordon, J. I. (1996) J. Biol. Chem. 271 28414–28421 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.