Abstract
The transient receptor potential canonical (TRPC) family channels are proposed to be essential for store-operated Ca2+ entry in endothelial cells. Ca2+ signaling is involved in NF-κB activation, but the role of store-operated Ca2+ entry is unclear. Here we show that thrombin-induced Ca2+ entry and the resultant AMP-activated protein kinase (AMPK) activation targets the Ca2+-independent protein kinase Cδ (PKCδ) to mediate NF-κB activation in endothelial cells. We observed that thrombin-induced p65/RelA, AMPK, and PKCδ activation were markedly reduced by knockdown of the TRPC isoform TRPC1 expressed in human endothelial cells and in endothelial cells obtained from Trpc4 knock-out mice. Inhibition of Ca2+/calmodulin-dependent protein kinase kinase β downstream of the Ca2+ influx or knockdown of the downstream Ca2+/calmodulin-dependent protein kinase kinase β target kinase, AMPK, also prevented NF-κB activation. Further, we observed that AMPK interacted with PKCδ and phosphorylated Thr505 in the activation loop of PKCδ in thrombin-stimulated endothelial cells. Expression of a PKCδ-T505A mutant suppressed the thrombin-induced but not the TNF-α-induced NF-κB activation. These findings demonstrate a novel mechanism for TRPC channels to mediate NF-κB activation in endothelial cells that involves the convergence of the TRPC-regulated signaling at AMPK and PKCδ and that may be a target of interference of the inappropriate activation of NF-κB associated with thrombosis.
Activation of the transcription factor nuclear factor-κB (NF-κB)3 is a double-edged sword. NF-κB is known to play an important role both in the mechanism of host defense as well as in the pathogenesis of inflammation and tissue injury(1).NF-κB-dependent expression of intercellular adhesion molecule-1 (ICAM-1; CD54) on the surface of endothelial cells mediates stable polymorphonuclear leukocyte adhesion and transendothelial migration of polymorphonuclear leukocyte (1). Mediators as diverse as TNF-α, lipopolysaccharide, and thrombin induce ICAM-1 expression by activating NF-κB signaling in endothelial cells (1-4). There are, however, differences in the activation mechanism. Thrombin, in contrast to TNF-α and lipopolysaccharide, signals NF-κB activity by activating its G protein-coupled receptor, PAR-1 (protease-activated receptor-1) (3, 4). Also, thrombin may be crucial in linking the activation of the coagulation cascade to the innate immune and inflammatory responses regulated by NF-κB (5, 6).
NF-κB is composed of dimers of five proteins (p50, p52, p65/RelA, RelB, and c-Rel) (7-9) that exist in the cytoplasm in inactive forms bound to the inhibitory protein IκB (7-9). IκB kinase (IKK) complex consists of two catalytic IKKα and IKKβ, and a regulatory subunit, IKKγ (or NEMO (NF-κB essential modulator)) (10). A variety of signals activate IκB kinases α and β (9), which in turn phosphorylate serines 32 and 36 on IκBα and serines 19 and 23 on IκBβ (9). Phosphorylation of IκBα and IκBβ leads to proteolytic degradation of IκB and dissociation of NF-κB, and NF-κB translocates to the nucleus to induce gene transcription (7, 9). Thrombin was shown to mediate RelA homodimer nuclear localization and its phosphorylation at Ser536 to induce gene transcription in endothelial cells (3, 11-14). These studies have also demonstrated that PKCδ signaling was involved in the mechanism of NF-κB activation (4, 5, 14, 15); however, the upstream signaling pathway responsible for PKCδ activation in endothelial cells has not been delineated.
A rise in [Ca2+]i was found to signal the activation of NF-κB (16, 17). However, the targets of Ca2+ signaling that mediate NF-κB activation are unknown. PAR-1 activation in endothelial cells mediates increase in [Ca2+]i by activating the Gq/11-phospholipase C pathway that results in depletion of endoplasmic reticulum (ER) Ca2+ stores and the subsequent store depletion-dependent Ca2+ entry across the plasma membrane (18). This accounts for the sustained rise in [Ca2+]i required for the activation of NF-κB. We (13, 19, 20) and others (21-23) have identified members of the transient receptor potential canonical (TRPC) family of channels that are essential for Ca2+ entry induced by PAR-1 agonist. The TRPC family contains seven isoforms (TRPC1 to -7). Recent studies have shown that TRPC1 forms a complex with STIM1 and ORAI1 and is involved in store-operated Ca2+ entry (24, 25). Primary endothelial cells in culture express multiple TRPC isoforms, TRPC1 to -7 (18, 19). We have shown that human endothelial cells predominantly express TRPC1 and contribute to thrombin-induced Ca2+ entry (18, 19). Mouse lung endothelial cells predominantly express Trpc4 (20, 21). Further, we have shown that in Trpc4 knock-out (Trpc4-/-) mouse lung endothelial cells, the thrombin-induced Ca2+ entry was markedly reduced (20). Here, we show that thrombin-induced Ca2+ entry via TRPC channels activates the calmodulin (CaM) kinase kinase β (CaMKKβ), AMPK, and PKCδ signaling pathway to induce p65/RelA nuclear translocation and transactivation. Further, we show that AMPK activation is required for PKCδ phosphorylation at Thr505 in its activation loop to mediate NF-κB activation. Thus, Ca2+ entry via TRPC channels is an essential signal responsible for activating NF-κB in response to thrombin and thereby serves as a crucial link between activation of the coagulation cascade and innate immunity and inflammation.
EXPERIMENTAL PROCEDURES
Materials—Human α-thrombin was obtained from Enzyme Research Laboratories (South Bend, IN). Endothelial growth medium-2 was purchased from Cambrex Bio Science (Walkersville, MD). Fetal bovine serum was from Hyclone (Logan, UT). Hanks' balanced salt solution, l-glutamine, phosphate-buffered saline, and trypsin were obtained from Invitrogen. Fura-2/AM was from Molecular Probes, Inc. (Eugene, OR). TRIzol reagent, TaqDNA polymerase and Lipofectamine Plus reagent were from Invitrogen. NF-κB reporter plasmid pNF-κB-LUC containing five copies of consensus NF-κB sequences linked to a minimal E1B promoter-firefly luciferase gene was purchased from Stratagene. pRL (sea pansy, Renilla reniformis) luciferase and the dual luciferase assay system were from Promega (Madison, WI). PCR primers were custom synthesized from IDT (Coralville, IA). [γ-32P]ATP (specific activity 6000 Ci/mmol) was obtained from ICN Biomedicals, Inc. (Irvine, CA). Anti-50 and anti-IκB-α polyclonal antibodies, scrambled small interfering RNA (Sc-siRNA), TRPC1 siRNA, and AMPKα1 siRNA were obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Anti-p65 polyclonal antibody was from Chemicon International, Inc. Polyclonal antibody raised against the MCM3 (mini chromosome maintenance 3) protein (nuclear marker) was purchased from Abcam, Inc. (Cambridge, MA). W-7, KN93, and 6-[4-(2-piperidin-1-yl-ethoxy)-phenyl]-3-pyridin-4-yl-pyrrazolo[1,5-a]-pyrimidine (compound C) were purchased from Calbiochem. 7-Oxo-7H-benzimidazo[2,1-a]benz[de]isoquinoline-3-carboxylic acid acetate (STO-609 acetate) was from Tocris Cookson Inc. (Ellisville, MO). Antibodies against AMPKα1 (monoclonal), phospho-Thr172-AMPKα (polyclonal), p38 MAP kinase (polyclonal), phospho-(Thr180/Tyr182)-p38 MAP kinase (monoclonal), phospho-Ser32-IκB-α (polyclonal), phospho-Ser536-p65 (monoclonal), nonphosphorylated PKCδ (polyclonal), phospho-PKCδ (Thr505) (polyclonal), phospho-PDK1 (Ser241)-specific (polyclonal), AKT (polyclonal), and phospho-AKT (Ser473)-specific (polyclonal) were from Cell Signaling Technology (Danvers, MA). Antibody against β-actin (monoclonal) was from Sigma. Adenovirus expressing constitutively active Myc epitope tagged at the N terminus of AMPKα (Ad-CA-AMPKα), in which Thr172 was substituted with aspartate in the truncated 1-312 AMPKα subunit was a generous gift from Dr. Kenneth Walsh (Boston University). PKCδ wild type (PKCδ-WT) and PKCδ Thr505 → Ala (PKCδ-T505A) mutant expression constructs were made as described previously (26).
Animals—Trpc4 gene-disrupted mice was generated as described previously by Freichel et al. (21), using the homologous recombination method in 129SvJ mice. Trpc4 transcript and protein expression were confirmed to be absent in these Trpc4 knock-out (Trpc4-/-) mice (20, 21).
Cell Culture—Primary human pulmonary arterial endothelial cells (HPAECs) obtained from Cambrex Bio Science (Walkersville, MD) were grown in endothelial growth medium-2 supplemented with 10% FBS as described (13, 19). HPAECs between passages 4 and 7 were used for all described experiments. Lung endothelial cells from 129SvJ (wild type) and Trpc4-/- mice were isolated and cultured as described by us (20). Cells passaged between 3 and 4 times were used in experiments. Mouse lung endothelial cells were characterized by their cobblestone morphology, PECAM-1 (platelet/endothelial cell adhesion molecule-1) (or CD31) expression, and Dil-Ac-LDL uptake (20).
Cytoplasmic Ca2+ Measurements—The cytoplasmic Ca2+ concentration ([Ca2+]i) in single endothelial cells was measured using Fura-2/AM (13, 20).
Nuclear Protein Extraction—HPAECs grown to confluence were incubated with 1% FBS-containing medium for 2 h prior exposure to different pharmacological inhibitors for 30 min. Nuclear extracts were prepared from endothelial cells and mouse lung tissue using the method described before (13). After thrombin exposure, cells were scraped and washed twice with ice-cold Tris-buffered saline. Cells were then homogenized with 400 μl of solution A (10 mm KCl, 10 mm Hepes, pH 7.9, 0.1 mm EDTA, pH 8.0, 0.1 mm EGTA, 1 mm dithiothreitol, 0.5 mm phenylmethylsulfonyl fluoride, 5 μg/ml aprotinin, and 5 μg/ml leupeptin). The cells were lysed with 10 strokes using a Dounce homogenizer and centrifuging at 14,000 rpm for 1 min. The supernatant was transferred to a new Eppendorf tube (the cytoplasmic fraction). Nuclear pellets were then resuspended in 100 μl of solution B (20 mm Hepes, pH 7.9, 0.4 NaCl, 1 mm EDTA, pH 8.0, 1 mm EGTA, 1 mm dithiothreitol, 0.5 mm phenylmethylsulfonyl fluoride, 5 μg/ml aprotinin, and 5 μg/ml leupeptin) and then incubated on ice for 20 min. The nuclei were pelleted by centrifugation at 14,000 rpm for 1 min. Supernatants containing the nuclear proteins were aliquoted in small fractions and stored at -70 °C. Nuclear extract from mouse lung tissue was also prepared for electrophoretic mobility shift assay. Frozen lung tissue was thawed on ice and homogenized in Buffer A. Other steps used were similar to endothelial cells described above.
Electrophoretic Mobility Shift and Supershift Assays—NF-κB oligonucleotide containing the NF-κB consensus sequence 5′-AGTTGAGGGGACTTTCCCAGGC-3′ was labeled with [γ-32P]ATP using T4 polynucleotide kinase for 20 min at 37 °C in the presence of 50 μg of poly(dI-dC) and 10 mm Tris-HCl buffer, pH 7.5, containing 50 mm NaCl, 0.5 mm EDTA, 0.5 mm dithiothreitol, 4% (w/v) glycerol, and 1 mm MgCl2. The nuclear extracts (15 μg of protein) were incubated with the radiolabeled NF-κB oligonucleotide (80,000 cpm/reaction) and subjected to electrophoresis on a 6% native gel, dried onto Whatman paper, and then exposed to autoradiography. For supershift assays, nuclear extracts were incubated with indicated antibodies for 30 min subsequent to the addition of radiolabeled oligonucleotide probe (13).
Expression of Reporter Constructs—HPAECs grown to ∼80% confluence in 6-well culture plates were used for reporter construct transfection. Plasmid DNA mixtures containing 1 μg of NF-κB-Luc in a minimal E1B vector and 0.035 μg of pRL/TK were transfected using Lipofectamine (19). In some experiments, PKCδ-WT or PKCδ-T505A mutant plasmid DNA (1 μg/well) co-transfected with NF-κB-Luc and pRL/TK. The mixture containing Lipofectamine-DNA complexes was diluted with 0.8 ml of Opti-MEM I before being added to HPAECs and prewashed two times with Opti-MEM I for 2-4 h. To end transfection, 2 ml of endothelial growth medium supplemented with 10% FBS was added to each well.
Dual Luciferase Reporter Assay—At 24 h after transfection, the cells were incubated in 1% FBS medium containing for 2 h and then stimulated with thrombin. In some experiments, cells were pretreated with 40 μm compound C for 30 min before thrombin treatment. After stimulation, cells were lysed, and 20 μl of lysate was used to measure reporter gene expression. Firefly (Photinus pyralis) and sea pansy (R. reniformis) luciferase activity were assayed by the dual luciferase reagent assay system (19).
siRNA Transfection—HPAECs grown to ∼70% confluence on gelatin-coated culture dishes were transfected with either 100 nm AMPKα1 siRNA, TRPC1 siRNA, or Sc-siRNA using Santa Cruz Biotechnology transfection reagents. Seventy-two hours after transfection, cells were used for experiments.
Immunoblotting—Cells grown to confluence were incubated in Ca2+-containing Hanks' balanced salt solution for 2 h at 37 °C. Following thrombin treatment in the presence or absence of Ca2+ in Hanks' balanced salt solution, cells were washed, and then cytoplasmic and nuclear fractions were isolated separately as described above. Equal amounts of protein were resolved using SDS-PAGE on a 10% separating gel and subsequently transferred to polyvinylidene difluoride membrane. Membranes were incubated in blocking buffer (5% nonfat milk in 10 mm Tris-HCl, pH 7.4, 150 mm NaCl, and 0.05% Tween 20) for 60 min at room temperature. Membranes were then incubated with the indicated antibody in diluted blocking buffer overnight at 4 °C. After two washes, the membranes were incubated with horseradish peroxidase-conjugated goat anti-mouse or -rabbit for 60 min at room temperature. Protein bands were detected by enhanced chemiluminescence.
Reverse Transcription (RT)-PCR—Confluent endothelial cell monolayers were washed with serum-free medium and then incubated with 1% FBS-containing medium for 2 h. After this period, the cells were treated with thrombin in 1% FBS-containing medium for various time intervals. Following treatment, total RNA was isolated using TRIzol reagent. RT was performed using oligo(dT) primers and superscript reverse transcript (Invitrogen), following the manufacturer's recommendation. ICAM-1 transcript expression in human and mouse endothelial cells was amplified using the following primer sets: human (forward, 5′-AGCAATGTGCAAGAAGATAGCCAA-3′; reverse, 5′-CGGCCTGCGTGTCCACC-3′) and mouse (forward, 5′-AGCATTTACCCTCAGCCACTTCCT-3′; reverse, 5′-TGAGGCTCGATTGTTCAGCTGCTA-3′). PCR product was 459 bp for human and 630 bp for mouse ICAM-1. The reaction conditions included an initial 95 °C for 5 min and then 30 cycles of (94 °C for 30 s, 59 °C for 30 s, and 68 °C for 1 min). Amplification of GAPDH used the following primers: forward, 5′-TATCGTGGAAGGACTCATGACCC-3′; reverse, 5′-TACATGGCAACTGTGAGGGG-3′. Reaction conditions were as follows: 30 cycles (94 °C for 45 s, 60 °C for 45 s and 72 °C for 2 min). PCR product were resolved by using 1.5% agarose gel and identified by ethidium bromide staining. Normalization of ICAM-1 expression was achieved by comparing the expression of GAPDH for the matching sample (19).
Perfused Mouse Lung Preparation—According to the approved protocol of University of the Illinois Animal Care Committee, male 129SvJ (wild type) and Trpc4 knock-out (Trpc4-/-) mice weighing 30-35 g were used to make isolated perfused lung preparation as described (27). Lungs were perfused for 20 min to attain the isogravimetric condition, and then lungs were perfused for an additional 30 min with or without thrombin. At the end of this period, lungs were frozen in liquid nitrogen and then used for nuclear extract preparation, as described above.
Immunoprecipitation—HPAECs grown to confluence exposed to thrombin were washed three times with phosphate-buffered saline and lysed in lysis buffer as described (19). Insoluble material was removed by centrifugation (13,000 × g for 15 min) prior to overnight immunoprecipitation with 1 μg/ml antibody (as indicated) at 4 °C. Protein AG-agarose beads were added to each sample and incubated for 1 h at 4 °C. Immunoprecipitates were washed three times with wash buffer (Tris-buffered saline containing 0.05% Triton X-100, 1 mm Na3VO4, 1 mm NaF, 2 μg/ml leupeptin, 2 μg/ml pepstatin A, 2 μg/ml aprotinin, and 44 μg/ml phenylmethylsulfonyl fluoride). Immunoprecipitated proteins were resolved on SDS-PAGE and immunoblotted with the appropriate antibodies.
In Vitro Kinase Assay—In vitro kinase assays were performed using immunoprecipitated recombinant PKCδ (26) and AMPK from endothelial cells. Briefly, HEK 293 cells in 100-mm dishes were transfected with the hemagglutinin-tagged expression constructs of PKCδ-WT or PKCδ-T505A (1 μg/ml). At 24 h after transfection, cells were lysed and immunoprecipitated using an anti-hemagglutinin antibody. The precipitated proteins were used as substrate for an in vitro kinase assay (26). HPAECs treated with and without thrombin were washed and lysed, and the lysate (800 μg protein) was incubated with anti-AMPK-α antibody for 60 min and then with 30 μl of protein A/G-Sepharose for 3 h at 4 °C. The immunoprecipitated proteins were washed twice with kinase buffer (50 mm Hepes, pH 7.5, 10 mm MgCl2, 1 mm dithiothreitol, 2.5 mm EGTA, 1 mm NaF, 0.1 mm Na3VO4, 10 mm β-glycerophosphate) and resuspended in 20 μl of kinase buffer. The kinase assay was initiated by adding 20 μl of the kinase buffer to a total volume of 40 μl containing PKCδ (PKCδ-WT or PKCδ-T505A), anti-AMPK-α antibody-precipitated proteins from endothelial cells, and 5 μCi of [γ-32P]ATP. The reactions were continued at 30 °C for 30 min and then terminated by adding Laemmli sample buffer. Samples were boiled for 5 min, the proteins were separated on 10% SDS-polyacrylamide gel and dried, and then an autoradiogram was performed. We also used recombinant constitutively active AMPKα (CA-AMPKα) for an in vitro kinase assay. In this case, HEK293 cells were infected with Ad-CA-AMPKα (100 plaque-forming units/cell). At 36 h after the infection, the cells were lysed and immunoprecipitated using anti-Myc mAb to precipitate Myc-tagged-Ad-CA-AMPKα, and the immunoprecipitate was used for an in vitro kinase assay as described above.
Statistical Analysis—Statistical comparisons were made using a two-tailed Student's t test. Experimental values were reported as the means ± S.E. Differences in mean values were considered significant at p < 0.05.
RESULTS
Thrombin-induced Increase in [Ca2+]i Mediates NF-κB Activation in Endothelial Cells—Inositol 1,4,5-trisphosphate receptor antagonist, 2-aminoethoxydiphenyl borate (2-APB) is known to inhibit stored Ca2+ release as well as Ca2+ entry (28). We first tested 2-APB for its ability to inhibit a thrombin-induced Ca2+ rise in HPAECs. Cells were loaded with Fura-2/AM in the presence or absence of different concentrations of 2-APB and then stimulated with thrombin. In control cells, thrombin produced an increase in [Ca2+]i, followed by a gradual decline to base line (see supplemental Fig. 1A), whereas thrombin-induced increases in [Ca2+]i transients were inhibited in 2-APB-treated HPAEC in a dose-dependent manner. At 75 μm, 2-APB completely prevented thrombin-induced rise in [Ca2+]i.
Next we examined the effect of 2-APB on thrombin-induced nuclear localization of NF-κB utilizing EMSA. We incubated HPAECs in the presence and absence of 2-APB (75 μm) for 30 min and stimulated the cells with thrombin. Thrombin exposure increased DNA binding activity of NF-κB in control cells; however, the response was abolished in 2-APB-treated cells (see supplemental Fig. 1B). To determine NF-κB-dependent gene expression, we measured ICAM-1 transcript expression. Thrombin-induced ICAM-1 mRNA expression was reduced by >60% in 2-APB-treated cells compared with control cells not treated with 2-APB (see supplemental Fig. 1C). GAPDH expression was not affected by exposure of cells to 2-APB.
Store-operated Ca2+ Entry (SOCE) Is Required for Thrombin-induced NF-κB Activation—We have shown previously that TRPC1 is the dominant TRPC isoform expressed and contributes to thrombin-induced Ca2+ entry in human endothelial cells (13, 18). In this study, we used a gene knockdown approach to address whether Ca2+ entry is required for thrombin-induced NF-κB activation. In TRPC1 siRNA-transfected cells, TRPC1 protein expression was suppressed by >80% compared with control or Sc-siRNA-transfected cells (Fig. 1A). Thrombin-induced Ca2+ store depletion-mediated Ca2+ influx was prevented in TRPC1 siRNA-transfected cells compared with control cells (Fig. 1B). Recent studies have implicated that TRPC1 associates with STIM1 to form a functional SOCE channel complex (24). To address the STIM1 involvement in the mechanism of thrombin-induced Ca2+ entry, we suppressed STIM1 expression by transfecting STIM1-specific siRNA and measured thrombin-induced Ca2+ entry in HPAECs. Like TRPC1 knockdown, STIM1 knockdown also prevented thrombin-induced Ca2+ entry in HPAECs (data not shown). Since the knockdown of either TRPC1 or STIM1 prevented thrombin-induced Ca2+ entry in HPAECs, we used TRPC1 knockdown cells for further experiments to measure thrombin-induced NF-κB activation. In TRPC1 knockdown cells, thrombin-induced p65/RelA binding to DNA was prevented (Fig. 1C), but TNF-α-induced NF-κB activation was not significantly altered (Fig. 1C). We also determined thrombin-induced IκBα degradation and nuclear localization of p65 in control and TRPC1 siRNA-transfected HPAECs. In control cells, thrombin stimulation caused a time-dependent degradation of IκBα and p65 nuclear translocation (Fig. 1D). In contrast to control cells, thrombin-induced IκBα degradation and p65 nuclear translocation were prevented in TRPC1 knockdown cells (Fig. 1D), suggesting that Ca2+ influx signaling is required for thrombin-induced p65/RelA activation in HPAECs.
FIGURE 1.
Store-operated Ca2+ influx in endothelial cells is required for thrombin-induced NF-κB activation. A, HPAECs were transfected with either 100 nm Sc-siRNA or TRPC1 siRNA. At 72 h after transfection, cells were lysed and immunoblotted with anti-TRPC1 polyclonal Ab (top). The membrane was stripped and blotted with anti-β-actin mAb (bottom). B, control HPAECs and HPAECs transfected with Sc-siRNA or TRPC1 siRNA were used to measure thrombin-induced store Ca2+ release and Ca2+ release-activated Ca2+ influx (see details under “Experimental Procedures”). The results were compared with nontransfected control cells. C, control HPAECs and HPAECs transfected with Sc-siRNA or TRPC1 siRNA were exposed to thrombin (50 nm) or TNF-α (1000 units/ml) for the indicated time periods. EMSA was performed as described under “Experimental Procedures.” Experiments were repeated 3-4 times with similar results. D, control HPAECs and HPAECs transfected with TRPC1 siRNA as described under “Experimental Procedures.” At 72 h after transfection, cells were exposed to thrombin for different time intervals. After thrombin treatment, nuclear and cytoplasmic fractions prepared were used for immunoblotting. The cytosolic (Cy) fractions were immunoblotted with anti-IκBα antibody (top panel). The membrane was stripped and probed for cytosolic marker with anti-β actin monoclonal antibody (second panel). The nuclear fractions (Nu) were immunoblotted with anti-p65 Ab (third panel). The membrane was stripped and probed with anti-MCM3 antibody (nuclear marker).
Impairment of p65/RelA Nuclear Translocation in Trpc4-/- Mouse Lung Endothelial Cells—We next investigated the importance of store-operated Ca2+ entry signal in the mechanism of NF-κB activation using Trpc4-/- mouse lungs and endothelial cells. First, we measured thrombin-induced NF-κB-DNA binding activities in the lung preparations (see details under “Experimental Procedures”). In this experiment, lungs were perfused with or without thrombin for 30 min. After perfusion, nuclear extracts were prepared and were used for EMSA. EMSA results showed the absence of NF-κB-DNA complex formation when lungs were perfused only with buffer in both Trpc4-/- and WT mice (Fig. 2A). A strong NF-κB binding signal to DNA was observed in WT lungs perfused with thrombin (Fig. 2A). In contrast, Trpc4-/- lungs exhibited a marked reduction in NF-κB binding to DNA (Fig. 2A). Next, we measured thrombin-induced NF-κB activation in lung endothelial cells (LECs) from WT and Trpc4-/- mice. We observed a marked reduction in thrombin-induced NF-κB-DNA complex formation in Trpc4-/- LECs compared with WT LECs (Fig. 2B). Interestingly, TNF-α-induced NF-κB-DNA complex formation was not altered in Trpc4-/- LECs (Fig. 2B). A supershift assay was carried out utilizing NF-κB-specific antibodies to identify NF-κB proteins translocated to the nucleus. These data showed a shift in the presence of the anti-p65 antibody in thrombin-stimulated LECs, whereas the shift was observed with both anti-p65 antibody and anti-p50 antibody in TNF-α-stimulated cells. These results indicate that thrombin induces nuclear localization of p65 homodimer, but TNF-α induces p65/p50 heterodimer (Fig. 2B). We also determined the NF-κB target gene ICAM-1 expression in mouse LECs in response to thrombin. Total RNA was isolated from mouse LECs, and RT-PCR was performed. In WT LECs, thrombin produced an ∼10-fold increase in ICAM-1 mRNA over basal after 4 h of stimulation (Fig. 2C), and the ICAM-1 mRNA level returned to basal at 6 h after thrombin challenge (Fig. 2C). In Trpc4-/- LECs, no significant increase was seen in response to thrombin (Fig. 2C). We exposed LECs from WT and Trpc4-/- mice with the Ca2+ ionophore ionomycin to increase intracellular Ca2+ and measured ICAM-1 transcript expression. We observed an equal increase in ICAM-1 transcript expression in LECs from WT and Trpc4-/- mice (Fig. 2D). These results show an obligatory role for store-operated Ca2+ entry in signaling thrombin-induced NF-κB activation and ICAM-1 expression in endothelial cells.
FIGURE 2.
Impairment of thrombin-induced p65/RelA nuclear localization in Trpc4 knock-out (Trpc4-/-) mouse lung endothelial cells. A, lungs from WT or Trpc4-/- were perfused with thrombin (50 nm) for 30 min, and nuclear extracts prepared were used to measure NF-κB activation utilizing EMSA (see details under “Experimental Procedures”). The autoradiograph was quantified using a densitometer, and the results were expressed in arbitrary units (left). For WT, n = 6; Trpc4-/-, n = 6. *, p < 0.05, different from WT. B, LECs from WT and Trpc4-/- mice were challenged with thrombin (50 nm) or TNF-α (1000 units/ml) for the indicated periods and then used for EMSA and supershift assays. C, LECs from WT and Trpc4-/- mice type were challenged with thrombin (50 nm) for the indicated times. Total RNA was isolated, and RT-PCR was performed to determine ICAM-1 and GAPDH transcript expression. ICAM-1 mRNA induction -fold in response to thrombin was calculated by measuring the ratio of ICAM-1 to GAPDH. The results are the mean ± S.E. from four separate experiments shown. *, p < 0.05, significantly different from the control. D, effects of TNF-α and ionomycin on ICAM-1 mRNA expression. LECs from WT and Trpc4-/- mice were treated with TNF-α (1000 units/ml) or ionomyocin (5 μm) for 4 h. Total RNA was isolated, and RT-PCR was performed to determine ICAM-1 and GAPDH expression levels. The experiment was repeated three times with similar results.
Requirement for Ca2+/Calmodulin Signaling for Nuclear Localization of NF-κB—We next investigated the role of CaM as a downstream Ca2+ target mediating NF-κB activation. HPAECs were treated with CaM-specific antagonist, W-7, and thrombin-induced NF-κB binding to DNA was measured by EMSA. W-7 inhibited thrombin-induced NF-κB translocation to the nucleus as well as IκBα degradation (see supplemental Fig. 2, A and B). The Ca2+/CaM complex activates CaM-dependent protein kinases, which include CaMKI, CaMKII, CaMKIV, and CaM-kinase kinase (29, 30). Based on studies showing that CaMKII and CaMKIV are involved in signaling NF-κB-dependent gene expression (31, 32), we investigated whether CaMKII or CaMKIV signaling is required for thrombin-induced NF-κB activation. Inhibition of CaMKII and CaMKIV by incubating HPAECs with KN93 before stimulating with thrombin produced no significant effect on thrombin-induced NF-κB nuclear translocation (see supplemental Fig. 2C). We also studied the effects of the CaMKKβ-specific inhibitor STO-609 on thrombin-induced NF-κB nuclear translocation. STO-609 pretreatment markedly reduced thrombin-induced nuclear localization of NF-κB (Fig. 3A).
FIGURE 3.
Inhibition of CaMKKβ or AMPK prevents thrombin-induced NF-κB activation in endothelial cells. A, HPAECs were pretreated with varying concentrations of CaMKKβ inhibitor STO-609 for 30 min and then challenged with thrombin. After thrombin treatment, nuclear extracts prepared were used for EMSA (top). The experiment was repeated four times. NF-κB-DNA binding activity was quantified by scanning the autoradiograph using densitometer and expressed in arbitrary units (bottom). *, p < 0.05, significantly different from thrombin-treated control cells. B, HPAECs were pretreated with varying concentrations of AMPK inhibitor compound C (Comp C) for 30 min, and then cells were challenged with thrombin (50 nm). After thrombin treatment, nuclear extracts prepared were used for EMSA. The experiment was repeated three times. C, HPAECs preincubated with compound C (40 μm) for 30 min were stimulated with thrombin (50 nm) for 0, 15, 30, and 60 min. After thrombin treatment, cells were lysed and immunoblotted with anti-phospho-IκBα antibody. The membrane was stripped and probed with anti-β actin mAb for loading control. D, HPAECs were transfected with NF-κB-luciferase (NF-κB pro-luc) expression constructs (see details under “Experimental Procedures”). At 24 h after transfection, cells were pretreated with 40 μm compound C for 30 min and then stimulated with thrombin (50 nm) for 6 h. Cells were lysed and reporter activity was measured. Data are mean ± S.E.; n = 4 for each condition. *, p > 0.05 significantly different from control cells (not treated with compound C). E, HPAECs exposed to compound C for 30 min challenged with thrombin for 4 and 16 h were used for RT-PCR and immunoblot (IB), respectively, to determine ICAM-1 expression.
Obligatory Role of AMPK in Mediating NF-κB Nuclear Translocation and NF-κB-dependent Gene Expression—Since thrombin may activate AMPK signaling through Ca2+/CaM-dependent CaMKKβ (33), we determined the effects of the AMPK inhibitor compound C on NF-κB activation. Compound C prevented thrombin-induced NF-κB binding to DNA in a dose-dependent manner (Fig. 3B). Further, we observed the inhibition of thrombin-induced IκBα phosphorylation by exposing cells to Compound C (Fig. 3C). Next we determined the effect of Compound C on NF-κB-driven reporter expression in endothelial cells (see details under “Experimental Procedures”). Thrombin-induced NF-κB-driven reporter expression was markedly suppressed in cells exposed to Compound C (Fig. 3D). In addition, the AMPK inhibitor prevented thrombin-induced ICAM-1 mRNA and protein expression (Fig. 3E).
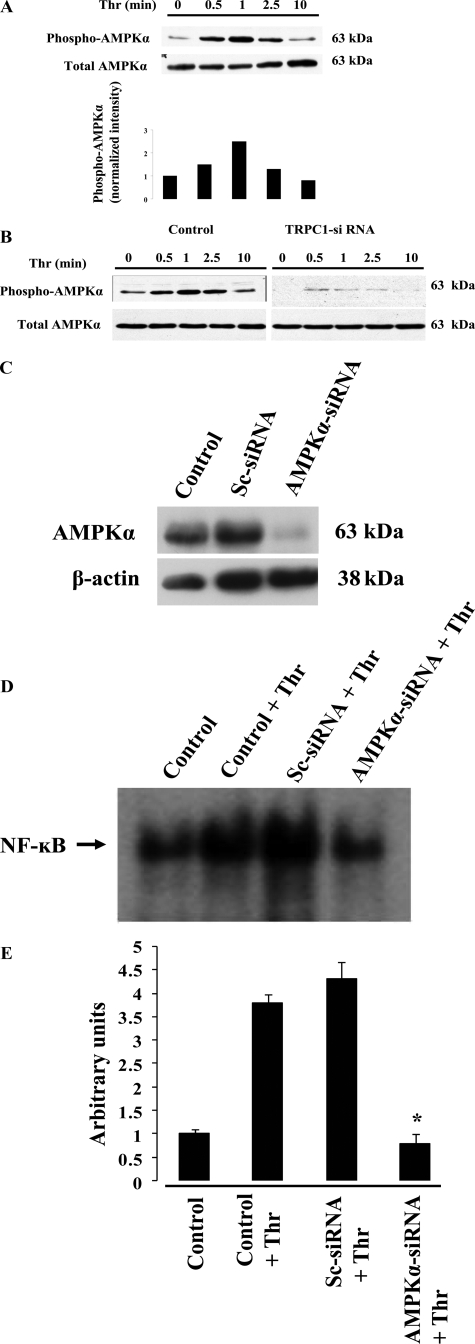
To address mechanisms of thrombin-induced AMPK activation, we determined the effects of PAR-1-specific agonist peptide (TFLLRNPNDK)-induced phosphorylation of AMPKα subunit. We observed a time-dependent PAR-1-mediated phosphorylation of AMPKα (Fig. 4A). Thrombin induced a similar pattern of AMPKα subunit phosphorylation in HPAECs. We also observed that PAR-1 agonist peptide-induced AMPKα phosphorylation was prevented by treating cells with CaM antagonist W-7 or chelation of intracellular Ca2+ with 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid acetoxymethyl ester (data not shown). Further, we observed that PAR-1 agonist peptide-induced AMPKα phosphorylation was largely reduced in TRPC1 siRNA-transfected HPAECs (Fig. 4B). These results suggest that Ca2+/CaM signaling occurs upstream of AMPK in endothelial cells.
FIGURE 4.
Ca2+-dependent AMPK activation signals NF-κB activation in endothelial cells. A, HPAECs were exposed to PAR-1 agonist peptide (40 μm) for different time intervals. After PAR-1 agonist peptide treatment, cells were lysed, and the lysates were immunoblotted with anti-phosphospecific Thr172 AMPKα antibody. The membrane was stripped and blotted with AMPKα-specific antibody. The phosphorylation of AMPKα was quantified densitometrically, and the results were normalized to AMPKα level (bottom). Representative immunoblot and the densitometry mean data of four independent experiments are shown. B, HPAECs transfected with TRPC1 siRNA (see details under “Experimental Procedures”) were challenged with PAR-1 agonist peptide, and phosphorylation of AMPKα was determined as described above. C, HPAECs were transfected with the Sc-siRNA (100 nm) or AMPKα siRNA (100 nm). At 72 h after transfection, cells were lysed and immunoblotted with anti-AMPKα antibody. The membrane was stripped and probed with anti-actin mAb for to monitor loading. D, HPAECs transfected with the Sc-siRNA (100 nm) or AMPKα siRNA (100 nm) were challenged with thrombin (50 nm) for 60 min. After thrombin treatment, nuclear extracts prepared were used for EMSA. The experiment was repeated four times. The autoradiograph from four independent experiments were quantified by densitometry and expressed in arbitrary units (E). *, p < 0.05, significantly different from control or Sc-siRNA-transfected cells.
To further address the role of AMPK in signaling NF-κB activation, we used siRNA to knockdown the endogenous AMPKα subunit in HPAECs. Transfection of HPAECs with siRNA specific to AMPKα inhibited >85% of AMPKα expression compared with control, whereas scrambled siRNA (control siRNA) had no effect (Fig. 4C). Thrombin-induced nuclear localization of NF-κB was prevented in AMPKα-knockdown cells compared with control cells (Fig. 4, D and E). Thus, AMPK signaling is critical in the regulation of thrombin-induced IκBα degradation and NF-κB binding to nuclear DNA.
Inhibition of AMPK Prevents Thrombin-induced p38 MAP Kinase Activation—Studies have shown that p38 MAP kinase activation can increase the binding of TATA-binding protein to DNA as well as increase the phosphorylation of p65/RelA to induce NF-κB-dependent gene expression (34, 35). To study whether AMPK is upstream of p38 MAP kinase, we exposed HPAECs with AMPK inhibitor compound C and measured thrombin-induced p38 MAP kinase phosphorylation. Thrombin increased phosphorylation of p38 MAP kinase in a time-dependent manner, whereas AMPK inhibitor treatment prevented the response (Fig. 5A). We also showed that knockdown of AMPKα using siRNA markedly reduced thrombin-induced p38 MAP kinase phosphorylation, whereas the control siRNA had no effect (Fig. 5B). In addition, we measured thrombin-induced phosphorylation of p65/RelA in HPAECs transfected with AMPKα-specific siRNA. We observed that thrombin-induced phosphorylation of p65 at Ser536 was prevented in cells transfected with AMPKα-specific siRNA compared with scrambled siRNA (Fig. 5C), indicating that an AMPK-dependent p38 MAP kinase activation signal mediates p65/Rel phosphorylation.
FIGURE 5.
Thrombin-induced AMPK activation phosphorylates p38 MAP kinase, p65/RelA, and PKCδ in endothelial cells. A, HPAECs were incubated in the presence and absence of compound C (40 μm) for 30 min and then stimulated with thrombin (50 nm) for different time intervals. After thrombin treatment, cells were lysed and immunoblotted with anti-phospho-p38 MAP kinase mAb. The membrane was stripped and blotted with anti-p38 MAP kinase Ab for total p38 MAP kinase. B, HPAECs transfected with the Sc-siRNA or AMPKα siRNA were challenged with thrombin (50 nm) for different time intervals. After thrombin treatment, cells were lysed and immunoblotted with anti-phospho-p38 MAP kinase mAb and p38 MAP kinase Ab. The experiment was repeated three times. The results from representative experiments are shown. C, HPAECs transfected with the Sc-siRNA or AMPKα siRNA were challenged with thrombin (50 nm) for 0, 10, and 30 min. After thrombin treatment, cells were lysed and immunoblotted with anti-phospho-Ser536-p65 mAb and anti-p65 Ab. D, control HPAECs and HPAECs transfected with TRPC1 siRNA were challenged with thrombin for different time intervals. After thrombin challenge, cells were lysed and immunoblotted with anti-phospho-PKCδ (Thr505) Ab. The blots were stripped and probed with anti-PKCδ Ab. The experiment was repeated three times. The results from representative experiments are shown. E, lung endothelial cells from WT and Trpc4-/- mice type were challenged with thrombin (50 nm) for the indicated times. After thrombin challenge, cells were lysed and immunoblotted with anti-phospho-PKCδ (Thr505) Ab. The experiment was repeated three times. F, control HPAECs and HPAECs transfected with AMPKα siRNA were challenged with thrombin for different time intervals. After thrombin challenge, cells were lysed and immunoblotted with anti-phospho-PKCδ (Thr505) or anti-PKCδ antibodies. The experiment was repeated three times. The results from representative experiments are shown.
Ca2+ Influx-dependent AMPK Activation Leads to Thrombin-induced PKCδ Activation—Since we observed that thrombin-induced p65/RelA activation was prevented in both TRPC1 and AMPKα knockdown HPAECs, we investigated the possibility that thrombin-induced Ca2+ influx signal is a prerequisite for PKCδ activation in endothelial cells. PKCδ activation is shown by agonist-induced phosphorylation of Thr505 in its activation loop (26, 36). In control cells, thrombin induced PKCδ phosphorylation at Thr505 in a time-dependent manner (Fig. 5D). In contrast, thrombin-induced PKCδ phosphorylation was abolished in TRPC1 siRNA-transfected endothelial cells (Fig. 5D), which lacked Ca2+ influx following thrombin stimulation (Fig. 1B). A similar observation was made in thrombin-induced PKCδ phosphorylation in LECs obtained from WT and Trpc4-/- mice. Thrombin-induced PKCδ phosphorylation was markedly reduced in Trpc4-/- LECs compared with WT LECs (Fig. 5E). To determine whether Ca2+-dependent AMPK activation is upstream of PKCδ activation, we measured thrombin-induced PKCδ phosphorylation in AMPKα siRNA-transfected cells. As expected, thrombin-induced PKCδ phosphorylation was prevented in these cells compared with control cells (Fig. 5F). Moreover, inhibition of AMPK with Compound C also prevented thrombin-induced PKCδ phosphorylation (data not shown).
Next we determined the effect of the PKCδ-specific inhibitor rottlerin on thrombin-induced AMPKα phosphorylation (see above). Rottlerin had no effect on thrombin-induced AMPKα phosphorylation (data not shown), but rottlerin prevented thrombin-induced p65/RelA binding to DNA (nuclear translocation) in endothelial cells (data not shown). These results place PKCδ downstream of AMPK and upstream of the NF-κB activation pathway, including IKKβ and p38 MAP kinase. Therefore, Ca2+ influx-dependent AMPK activation mediates PKCδ activation and subsequent NF-κB activation in endothelial cells. Furthermore, AMPK-mediated PKCδ activation also can induce p65/RelA transactivation via p38 MAP kinase activation.
Thrombin-induced PKCδ Phosphorylation at Thr505 Is Mediated by AMPK and Not by PDK1—To confirm the relationship between AMPK and PKCδ in thrombin-induced NF-κB activation in endothelial cells, first, we determined thrombin-induced association of AMPK with PKCδ by immunoprecipitation. We observed the co-immunoprecipitation of PKCδ with AMPKα in thrombin-stimulated cells (Fig. 6A). Since PKCδ phosphorylation in its activation loop where Thr505 is located is a mechanism of regulation of the kinase activity (26, 36), we performed an in vitro kinase assay to determine thrombin-induced and AMPK-mediated PKCδ phosphorylation (see details under “Experimental Procedures”). Thrombin activation of HPAECs induced phosphorylation of PKCδ-WT protein (Fig. 6B); however, phosphorylation was not observed in PKCδ-T505A mutant protein (Fig. 6B). We also used recombinant constitutively active AMPKα for an in vitro kinase assay, and we observed phosphorylation in PKCδ-WT protein but not in PKCδ-T505A mutant protein (Fig. 6C). These results collectively suggest that AMPK activation is required for PKCδ phosphorylation at Thr505 in the activation loop in endothelial cells.
FIGURE 6.
AMPK-mediated Thr505 phosphorylation in the activation loop of PKCδ signals NF-κB activation in endothelial cells. A, HPAECs exposed to thrombin (50 nm) for the indicated time intervals were lysed and immunoprecipitated (IP) with control IgG or anti-PKCδ Ab. The precipitated proteins were immunoblotted (IB) with anti-AMPKα Ab (top). The membrane was stripped and probed with anti-PKCδ Ab (bottom). B, AMPK-mediated PKCδ phosphorylation at Thr505 was determined by in vitro kinase assay (see details under “Experimental Procedures”). Hemagglutinin-tagged PKCδ proteins (PKCδ-WT and PKCδ-T505A) expressed in HEK293 cells were immunoprecipitated and used as substrates. HPAECs were stimulated with thrombin, lysed, and immunoprecipitated using anti-AMPKα Ab or control IgG. The anti-AMPKα Ab or control IgG precipitated proteins served as kinase for in vitro phosphorylation of PKCδ. The autoradiograph (top) shows 32P incorporation in PKCδ-WT but not in PKCδ-T505A mutant. An equal volume of the assay mixture was immunoblotted with anti-AMPKα (middle) and anti-PKCδ (bottom) antibodies. The experiment was repeated three times. The results from representative experiments are shown. C, recombinant constitutively active AMPKα-mediated PKCδ phosphorylation at Thr505 was determined by in vitro kinase assay. HEK293 cells infected with adenovirus expressing Myc epitope-tagged CA-AMPKα was immunoprecipitated with anti-Myc mAb as described under “Experimental Procedures.” The anti-Myc mAb precipitated protein was used for in vitro phosphorylation of PKCδ. The autoradiograph (top) shows 32P incorporation in PKCδ-WT but not in the PKCδ-T505A mutant. Equal volume of the assay mixture was immunoblotted with anti-PKCδ (middle) and anti-Myc (bottom) antibodies. The results from representative experiments are shown. D and E, HPAECs grown to 80% confluence were transfected with PKCδ-WT or PKCδ-T505A mutant and NF-κB pro-luc constructs (see details under “Experimental Procedures”). At 24 h after transfection, cells were stimulated with thrombin (50 nm) or TNF-α (1000 units/ml) for 6 h. After this treatment, cells were lysed, and reporter activity was measured. Also, the cell lysates were immunoblotted with anti-PKCδ Ab to determine PKCδ expression. The mean ± S.E. from four experiments repeated in triplicate is shown in D and E. The asterisk indicates statistically significant different from PKCδ-WT-expressing cells stimulated with thrombin (*, p < 0.001).
PDK1 (phosphoinositide-dependent protein kinase-1) is known to phosphorylate PKCδ at Thr505 in the activation loop (36). Phosphoinositide 3-kinase is an upstream kinase that phosphorylates PDK1 and also activates AKT either directly or indirectly via activating PDK1 (37, 38). Thrombin is known to activate phosphoinositide 3-kinase and thus presumably can mediate phosphorylation of PDK1 (39, 40). To address whether PDK1 is involved in thrombin-induced phosphorylation of PKCδ at Thr505, first, we measured thrombin-induced PDK1 phosphorylation at Ser241 (which is required for its activity (41)) in HPAECs. Surprisingly, we did not observe inducible phosphorylation of PDK1 in response to thrombin; however, we observed the expression of constitutively phosphorylated PDK1 in HPAECs (supplemental Fig. 3A). As a positive control, we measured thrombin-induced AKT phosphorylation in HPAECs. We observed phosphorylation of AKT in a time-dependent manner in thrombin-stimulated cells, and this effect was blocked by the phosphoinositide 3-kinase inhibitor LY294002 (supplemental Fig. 3A). The phosphoinositide 3-kinase inhibitor had no effect on the constitutive phosphorylation of PDK1 in endothelial cells (supplemental Fig. 3A). In addition, we observed that thrombin-induced PKCδ phosphorylation was not altered by preexposure of endothelial cells to phosphoinositide 3-kinase inhibitor LY294002. Next, we determined the association of PDK1 with PKCδ and AMPKα in thrombin-stimulated HPAECs by immunoprecipitation. We observed that PDK1 was not immunoprecipitated with either PKCδ or AMPK (supplemental Fig. 3B). Thus, these results suggest that PDK1 is unlikely to mediate PKCδ phosphorylation at Thr505 in thrombin-stimulated endothelial cells.
To address whether AMPK-induced Thr505 phosphorylation in the activation loop of PKCδ is critical for NF-κB activation, we measured thrombin-induced NF-κB-driven reporter (luciferase) expression in PKCδ-WT- or PKCδ-T505A mutant-transfected endothelial cells. Thrombin stimulation induced NF-κB-driven reporter expression >6-fold in control cells compared with cells not stimulated with thrombin (Fig. 6D). In PKCδ-WT transfected cells, thrombin-induced NF-κB-driven reporter expression was increased compared with control cells (Fig. 6D). Interestingly, thrombin-induced NF-κB-driven reporter expression was markedly reduced in PKCδ-T505A mutant-transfected endothelial cells (Fig. 6D), indicating that the thrombin-induced Thr505 phosphorylation in the activation loop of PKCδ is essential for thrombin-induced NF-κB activation in endothelial cells. This activation mechanism appears to be specific for the thrombin-induced NF-κB activation, since expression of PKCδ-T505A mutant in endothelial cells failed to prevent TNF-α-induced NF-κB activation (Fig. 6E), suggesting that thrombin-induced Ca2+ signaling specifically targets the PKCδ signaling pathway to activate NF-κB in endothelial cells.
DISCUSSION
PAR-1 activation by thrombin induces Ca2+ mobilization in endothelial cells that occurs in two stages, initially ER-Ca2+ store depletion followed by Ca2+ entry (also called SOCE) in endothelial cells (13, 20). The second phase of Ca2+ mobilization accounts for the sustained increase in intracellular Ca2+ required for signaling the increase in endothelial permeability and other responses generally classified as “endothelial activation.” We have shown that the canonical TRPC isoforms are essential for thrombin-induced Ca2+ entry in endothelial cells (13, 18, 20). Here we describe a fundamental role of Ca2+ entry in signaling NF-κB activation in endothelial cells secondary to CaMKKβ-mediated activation of the AMPK/PKCδ pathway.
Thrombin elicits multiple NF-κB-mediated responses, including the expression of ICAM-1, VCAM-1, and TRPC1 in endothelial cells (3, 4, 12, 13). The role of increased intracellular Ca2+ in the mechanism of NF-κB activation and gene expression is not well understood. In the present study, we showed this using pharmacological agent 2-APB, which prevented both stages of Ca2+ mobilization in HPAECs. Thrombin-induced NF-κB activation as well as ICAM-1 transcript expression were also markedly reduced by 2-APB. However, HPAECs treated with 2-APB showed no effect on TNF-α-induced NF-κB activation and ICAM-1 transcript expression, indicating that the effects of PAR-1 activation cannot be generalized to prototypic inflammatory cytokines, such as TNF-α. We have shown previously that TNF-α failed to induce a rise in [Ca2+]i in endothelial cells (42); thus, TNF-α activates NF-κB through a Ca2+-independent pathway.
All of the TRPC members contain six transmembrane domains, and the Ca2+-permeable pore region is located between transmembrane helices 5 and 6 (43). TRPC members assemble to homo- and heterotetrameric structure to form a putative SOCE channel complex (44). Further, recent findings suggest that TRPC channels also interact with STIM1 and ORAI1 to form SOCE channel or CRAC channel (24, 25). Since the TRPC channels are essential components of the SOCE channel complex, we used TRPC channel knockdown and knock-out strategies to address the role of TRPC channels in the mechanism of thrombin-induced NF-κB activation. Thrombin-induced Ca2+ entry was prevented by knockdown of either TRPC1 or STIM1 in HPAECs. Interestingly, we observed that thrombin-induced p65/RelA nuclear translocation and IκBα degradation were both markedly reduced in TRPC1 knockdown HPAECs. Next, we utilized Trpc4 knock-out mice (20, 21) to investigate the role of TRPC-mediated Ca2+ influx in the mechanism of thrombin-induced NF-κB activation. In this study, we observed that thrombin-induced NF-κB binding to DNA as well as ICAM-1 transcript expression were significantly reduced in Trpc4 knock-out mouse endothelial cells compared with endothelial cells from wild type mice.
CaM, the Ca2+-binding protein expressed in mammalian cells, senses changes in intracellular Ca2+ concentration and thereby participates in Ca2+ signaling (29-32). Ca2+ binding to EF-hand motifs on CaM is responsible for activating downstream target molecules (29, 30). To study the role of CaM in NF-κB activation, we determined the effects of CaM antagonist W-7. W-7 inhibited thrombin-induced NF-κB binding to DNA in a dose-dependent manner and concomitantly prevented IκBα degradation, suggesting a key role of Ca2+/CaM complex formation in signaling NF-κB activation. The CaMKKβ-specific inhibitor STO-609 also prevented thrombin-induced NF-κB activation in endothelial cells, suggesting the role of CaMKKβ downstream of CaM in signaling NF-κB activation.
AMPK is a serine/threonine kinase composed of the catalytic α subunit and the regulatory β and γ subunits (45-47). Phosphorylation of the α subunit at threonine 172 is required for the catalytic activity of AMPK (45-47). Ca2+/CaM-dependent CaMKKβ was shown to activate AMPK in multiple cell types (46, 47). Since PAR-1 has been shown to activate AMPK signaling via Ca2+/CaM-dependent CaMKKβ in endothelial cells (33), we surmised that Ca2+/CaM signaling and resultant AMPK activation may regulate NF-κB activation. We observed that an AMPK-specific inhibitor compound C inhibited thrombin-induced NF-κB binding to DNA in a dose-dependent manner. AMPKα subunit was also phosphorylated at Thr172 in response to PAR-1 activation induced by PAR-1 agonist peptide (TFLLRNPNDK) or thrombin. In TRPC1 knockdown endothelial cells, PAR-1 agonist peptide-induced AMPKα phosphorylation was prevented. Further, chelation of intracellular Ca2+ with 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid acetoxymethyl ester or inhibition of CaM function with W-7 blocked thrombin-induced AMPK activation. These results collectively show that CaMKKβ-dependent AMPK activation is an important pathway mediating thrombin-induced NF-κB activation in endothelial cells. This observation was confirmed by depleting endogenous AMPKα expression using AMPKα-specific siRNA, which reduced AMPKα expression (by 85%) and prevented thrombin-induced NF-κB binding to DNA. Previous studies have shown that AMPK activation with pharmacological agents such as metformin and 5-amino-4-imidazole carboxamide riboside prevented TNF-α-induced NF-κB activation in endothelial cells (48, 49). Our results showed that Ca2+-dependent AMPK activation is required for thrombin-induced p65/RelA binding to nuclear DNA. These findings support the notion that distinct signaling pathways are activated in endothelial cells to induce NF-κB signaling by thrombin and TNF-α.
Another component of the thrombin-induced NF-κB activation signaling pathway delineated in these studies is the role of p38 MAP kinase. Li et al., (50) showed that activated AMPK interacts with the scaffold protein TAB1, and the resulting AMPK-TAB1 complex associates with p38 MAP kinase and thus promotes p38 MAP kinase autophosphorylation in ischemic hearts. We observed in the present study that inhibition of AMPK in endothelial cells prevented thrombin-induced p38 MAP kinase activation, demonstrating that AMPK lies upstream of p38 MAP kinase. Further, knockdown of AMPK in endothelial cells prevented the thrombin-induced phosphorylation of p65/RelA mediated by p38 MAP kinase. Thus, from these observations, we can conclude that AMPK-dependent p38 MAP kinase activation plays a key role in thrombin-induced p65/RelA transactivation.
The novel PKC isoform PKCδ has been shown to play an important role in the mechanism of NF-κB activation. Minami et al. (15) showed that thrombin-induced VCAM-1 expression was dependent on PKCδ-mediated NF-κB activation in endothelial cells. We previously demonstrated that the thrombin-induced PKCδ activation signal induced NF-κB activation and ICAM-1 expression in endothelial cells (4). In another study, thrombin-induced PKCδ activation induced p65/RelA transactivation signaling via p38 MAP kinase to up-regulate ICAM-1 (14). PKCδ activation is Ca2+-indedependent but dependent on diacylglycerol (4, 36). Several studies have shown that besides diacylglycerol binding to the regulatory domain, phosphorylation at the catalytic domain of PKCδ is essential for its activity (14, 26, 36, 51). Cheng et al. (26) have recently shown that phosphorylation at Thr505 in the catalytic domain of the activation loop is critical for the catalytic function of PKCδ. Thus, we addressed the possibility that PKCδ activation downstream of the Ca2+ influx signaling pathway elaborated above was involved in regulating thrombin-induced NF-κB activation. We observed that Trpc4 knock-out, TRPC1 knockdown, or AMPKα knockdown in endothelial cells prevented thrombin-induced phosphorylation of PKCδ at the catalytic domain Thr505, indicating that Ca2+ influx-dependent AMPK activation is required for PKCδ activation. We also showed co-immunoprecipitation of PKCδ with AMPK in thrombin-stimulated endothelial cells. Further, we showed that AMPK specifically phosphorylated PKCδ at Thr505 by in vitro kinase assay. In addition, we provide evidence in this study that PDK1 signaling is not involved in thrombin-induced PKCδ phosphorylation at Thr505 in endothelial cells. Moreover, in this study, we addressed the relationship between AMPK-mediated PKCδ phosphorylation and NF-κB activation in endothelial cells. We showed that PKCδ-T505A mutant expression suppressed thrombin-induced but not TNF-α-induced NF-κB activation in endothelial cells. These results demonstrate that the Ca2+ influx-dependent AMPK activation and resultant PKCδ activation is an important pathway mediating NF-κB activity in thrombin-stimulated endothelial cells. This novel mechanism of activation complements another pathway involving thrombin-induced Ca2+-dependent PKCα activation that could induce NF-κB signaling by activating PKCδ via Rho signaling (11, 52, 53).
In summary, we have shown that PAR-1-mediated Ca2+ entry via TRPC channels results in AMPK activation, which is required for activation of the downstream target PKCδ and induction of p65/RelA signaling in endothelial cells (Fig. 7). NF-κB activation by the TRPC channel signaling pathway therefore provides an important link between activation of the coagulation cascade and NF-κB-regulated innate immunity response and inflammatory mechanisms.
FIGURE 7.
Signaling pathway mediating thrombin-induced NF-κB activation in endothelial cells. PAR-1 activation-induced ER store Ca2+ depletion via phospholipase Cβ (PLCβ) activates Ca2+ entry through TRPC channels to cause sustained increase in intracellular Ca2+ in endothelial cells. Ca2+ binding to CaM activates CaMKKβ, which in turn phosphorylates AMPKα (which is required for catalytic function of AMPK). Activated AMPK phosphorylates PKCδ at Thr505 to activate PKCδ, which in turn targets IKKβ to mediate NF-κB binding to nuclear DNA by inducing IκBα degradation. Both the activated AMPK and PKCδ can induce p65/RelA transactivation signaling via p38 MAP kinase.
Supplementary Material
This work was supported, in whole or in part, by National Institutes of Health Grants P01HL077806, GM058531, GM066182, and T32HL072742. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1-3.
Footnotes
The abbreviations and trivial names used are: NF-κB, nuclear factor-κB; SOCE, store-operated Ca2+ entry; AMPK, AMP-activated protein kinase; PKCδ, protein kinase Cδ; HPAEC, human pulmonary arterial endothelial cell; siRNA, small interfering RNA; EMSA, electrophoretic mobility shift assay; TNF, tumor necrosis factor; IKK, IκB kinase; TRPC, transient receptor potential canonical; siRNA, small interfering RNA; Sc-siRNA, scrambled siRNA; FBS, fetal bovine serum; RT, reverse transcription; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; WT, wild type; CA, constitutively active; Ab, antibody; mAb, monoclonal antibody; 2-APB, 2-aminoethoxydiphenyl borate; LEC, lung endothelial cell; CaM, calmodulin; CaMKKβ, CaM kinase kinase β; MAP, mitogen-activated protein; ICAM, intercellular adhesion molecule; compound C, 6-[4-(2-piperidin-1-yl-ethoxy)-phenyl]-3-pyridin-4-yl-pyrrazolo[1,5-a]-pyrimidine; STO-609 acetate, 7-oxo-7H-benzimidazo[2,1-a]benz[de]isoquinoline-3-carboxylic acid acetate.
References
- 1.Fan, J., Ye, R. D., and Malik, A. B. (2001) Am. J. Physiol. 281 L1037-L1050 [DOI] [PubMed] [Google Scholar]
- 2.Ledebur, H. C., and Parks, T. P. (1995) J. Biol. Chem. 270 933-943 [DOI] [PubMed] [Google Scholar]
- 3.Rahman, A., Anwar, K. N., True, A. L., and Malik, A. B. (1999) J. Immunol. 162 5466-5476 [PubMed] [Google Scholar]
- 4.Rahman, A., Ture, A. L., Anwar, K. N., Ye, R. D., Voyno-Yasenetskaya, T. A., and Malik, A. B. (2002) Circ. Res. 91 398-405 [DOI] [PubMed] [Google Scholar]
- 5.Cirino, G., and Vergnolle, N. (2006) Curr. Opin. Pharmacol. 6 428-434 [DOI] [PubMed] [Google Scholar]
- 6.Esmon, C. T. (2005) Br. J. Haematol. 131 417-430 [DOI] [PubMed] [Google Scholar]
- 7.Delhase, M., Hayakawa, M., Chen, Y., and Karin, M. (1999) Science 284 309-313 [DOI] [PubMed] [Google Scholar]
- 8.Ghosh, S., and Karin, M. (2002) Cell 109 S81-S96 [DOI] [PubMed] [Google Scholar]
- 9.Hayden, M. S., and Ghosh, S. (2004) Genes Dev. 18 2195-2224 [DOI] [PubMed] [Google Scholar]
- 10.May, M. J., D'Acquisto, F., Madge, L. A., Glockner, J., Pober, J. S., and Ghosh, S. (2000) Science 289 1550-1554 [DOI] [PubMed] [Google Scholar]
- 11.Anwar, K. N., Fazal, F., Malik, A. B., and Rahman, A. (2004) J. Immunol. 173 6965-6972 [DOI] [PubMed] [Google Scholar]
- 12.Minami, T., and Aird, C. W. (2001) J. Biol. Chem. 276 47632-47641 [DOI] [PubMed] [Google Scholar]
- 13.Paria, B. C., Bair, A. M., Xue, J., Yu, Y., Malik, A. B., and Tiruppathi, C. (2006) J. Biol. Chem. 281 20715-20727 [DOI] [PubMed] [Google Scholar]
- 14.Rahman, A., Anwar, K. N., Uddin, S., Xu, N., Ye, R. D., Platanias, L. C., and Malik, A. B. (2001) Mol. Cell. Biol. 21 5554-5565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Minami, T., Abid, M. R., Zhang, J., King, G., Kodama, T., and Aird, W. C. (2003) J. Biol. Chem. 278 6976-6984 [DOI] [PubMed] [Google Scholar]
- 16.Dolmetsch, R. E., Lewis, R. S., Goodnow, C. C., and Healy, J. I. (1997) Nature 386 855-858 [DOI] [PubMed] [Google Scholar]
- 17.Dolmetsch, R. E., Xu, K., and Lewis, R. S. (1998) Nature 392 933-936 [DOI] [PubMed] [Google Scholar]
- 18.Tiruppathi, C., Ahmmed, G. U., Vogel, S. M., and Malik, A. B. (2006) Microcirculation 13 693-708 [DOI] [PubMed] [Google Scholar]
- 19.Paria, B. C., Malik, A. B., Kwiatek, A. M., Rahman, A., May, M. J., Ghosh, S., and Tiruppathi, C. (2003) J. Biol. Chem. 278 37195-37203 [DOI] [PubMed] [Google Scholar]
- 20.Tiruppathi, C., Freichel, M., Vogel, S. M., and Malik, A. B. (2002) Circ. Res. 91 70-76 [DOI] [PubMed] [Google Scholar]
- 21.Freichel, M., Suh, S. H., Pfeifer, A., Schweig, U., Trost, C., Weissgerber, P., Biel, M., Philipp, S., Freise, D., Droogmans, G., Hofmann, F., Flockerzi, V., and Nilius, B. (2001) Nat. Cell Biol. 3 121-127 [DOI] [PubMed] [Google Scholar]
- 22.Freichel, M., Schweig, U., Stauffberger, S., Freise, D., Schorb, W., and Flockerzi, V. (1999) Cell Physiol. Biochem. 9 270-283 [DOI] [PubMed] [Google Scholar]
- 23.Norwood, N., Moore, T. M., Dean, D., Bhattacharjee, R., Li, M., and Stevens, T. (2000) Am. J. Physiol. 279 L815-L824 [DOI] [PubMed] [Google Scholar]
- 24.Ong, H. L., Cheng, K. T., Liu, X., Bandyopadhyay, B. C., Paria, B. C., Soboloff, J., Pani, B., Gwack, Y., Srikanth, S., Singh, B. B., Gill, D. L., and Ambudkar, I. S. (2007) J. Biol. Chem. 282 9105-9116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheng, K. T., Liu, X., Ong, H. L., and Ambudkar, I. S. (2008) J. Biol. Chem. 283 12935-129340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cheng, N., He, R., Tian, J., Dinauer, M. C., and Ye, R. D. (2007) J. Immunol. 179 7720-7728 [DOI] [PubMed] [Google Scholar]
- 27.Vogel, S. M., Gao, X., Mehta, D., Ye, R. D., John, T. A., Andrade-Gordon, P., Tiruppathi, C., and Malik, A. B. (2000) Physiol. Genomics 4 137-145 [DOI] [PubMed] [Google Scholar]
- 28.Parekh, A. B., and Putney, J. W., Jr. (2005) Physiol. Rev. 85 757-810 [DOI] [PubMed] [Google Scholar]
- 29.Corcoran, E. E., and Means, A. R. (2000) J. Biol. Chem. 276 2975-2978 [DOI] [PubMed] [Google Scholar]
- 30.Stull, J. T. (2001) J. Biol. Chem. 276 2311-2312 [DOI] [PubMed] [Google Scholar]
- 31.Ishiguro, K., Green, T., Rapley, J., Wachtel, H., Giallourakis, C., Landry, A., Cao, Z., Lu, N., Takafumi, A., Goto, H., Daly, M. J., and Xavier, R. J. (2006) Mol. Cell. Biol. 26 5497-5508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jang, M. K., Goo, Y. H., Sohn, Y. C., Kim, Y. S., Lee, S. K., Kang, H., Cheong, J., and Lee, J. W. (2001) J. Biol. Chem. 276 20005-20010 [DOI] [PubMed] [Google Scholar]
- 33.Stahmann, N., Woods, A., Carling, D., and Heller, R. (2006) Mol. Cell. Biol. 26 5933-5945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Craig, R., Larkin, A., Mingo, A. M., Thuerauf, D. J., Andrews, C., McDonought, P. M., and Glembotski, C. C. (2000) J. Biol. Chem. 275 23814-23824 [DOI] [PubMed] [Google Scholar]
- 35.Vanden Berghe, W., Plaisance, S., Boone, E., De Bosscher, K., Schmitz, M. L., Fiers, W., and Haegeman, G. (1998) J. Biol. Chem. 273 3285-3290 [DOI] [PubMed] [Google Scholar]
- 36.Newton, A. C. (2003) Biochem. J. 370 361-371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Le Good, J. A., Ziegler, W. H., Parekh, D. B., Alessi, D. R., Cohen, P., and Parker, P. J. (1998) Science 281 2042-2045 [DOI] [PubMed] [Google Scholar]
- 38.Deb, D. K., Sassano, A., Lekmine, F., Majchrzak, B., Verma, A., Kambhampati, S., Uddin, S., Rahman, A., Fish, E. N., and Platanias, L. C. (2003) J. Immunol. 171 267-273 [DOI] [PubMed] [Google Scholar]
- 39.Cao, H., Dronadula, N., and Rao, G. N. (2006) Am. J. Physiol. 290 C172-C182 [DOI] [PubMed] [Google Scholar]
- 40.Motley, E. D., Eguchi, K., Patterson, M. M., Palmer, P. D., Suzuki, H., and Eguchi, S. (2007) Hypertension 49 577-583 [DOI] [PubMed] [Google Scholar]
- 41.Casamayor, A., Morrice, N. A., and Alessi, D. R. (1999) Biochem. J. 342 287-292 [PMC free article] [PubMed] [Google Scholar]
- 42.Tiruppathi, C., Naqvi, T., Sandoval, R., Mehta, D., and Malik, A. B. (2001) Am. J. Physiol. 281 L958-L968 [DOI] [PubMed] [Google Scholar]
- 43.Liu, X., Singh, B. B., and Ambudkar, I. S. (2003) J. Biol. Chem. 278 11337-11343 [DOI] [PubMed] [Google Scholar]
- 44.Hofmann, T., Schaefer, M., Schultz, G., and Gudermann, T. (2002) Proc. Natl. Acad. Sci. U. S. A. 99 7461-7466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Birnbaum, M. J. (2005) Mol. Cell. Biol. 19 289-296 [Google Scholar]
- 46.Hardie, D. G. (2003) Endocrinology 144 5179-5183 [DOI] [PubMed] [Google Scholar]
- 47.Hawley, S. A., Selber, M. A., Goldstein, E. G., Edelman, A. M., Carling, D., and Hardie, D. G. (1995) J. Biol. Chem. 270 27186-27191 [DOI] [PubMed] [Google Scholar]
- 48.Cacicedo, J. M., Yagihashi, N., Keaney, J. F., Jr., Ruderman, N. B., and Ido, Y. (2004) Biochem. Biophys. Res. Commun. 324 1204-1209 [DOI] [PubMed] [Google Scholar]
- 49.Hattori, Y., Suzuki, K., Hattori, S., and Kasai, K. (2006) Hypertension 47 1183-1188 [DOI] [PubMed] [Google Scholar]
- 50.Li, J., Miller, E., Ninomiya-Tsuji, J., Russell, R. R., III, and Young, L. H. (2005) Circ. Res. 97 872-879 [DOI] [PubMed] [Google Scholar]
- 51.Tan, M., Xu, X., Ohba, M., Ogawa, W., and Cui, M. Z. (2003) J. Biol. Chem. 278 2824-2828 [DOI] [PubMed] [Google Scholar]
- 52.Mehta, D., Rahman, A., and Malik, A. B. (2001) J. Biol. Chem. 276 22614-22620 [DOI] [PubMed] [Google Scholar]
- 53.Trushin, S. A., Pennington, K. N., Carmona, E. M., Asin, S., Savoy, D. N., Billadeau, D. D., and Paya, C. V. (2003) Mol. Cell. Biol. 23 7068-7081 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.