Abstract
MRAP, melanocortin 2 (MC2) receptor accessory protein, is required for trafficking by the MC2 (ACTH) receptor. MRAP is a single transmembrane protein that forms highly unusual antiparallel homodimers. We used molecular complementation to ask where MRAP achieves dual topology. Fragments of yellow fluorescent protein (YFP) were fused to the NH2 or COOH terminus of MRAP such that YFP fluorescence could occur only in antiparallel homodimers; fluorescence was present in the endoplasmic reticulum. MRAP retained dual topology after deletion of most of the amino terminus. In contrast, deletion of residues 31-37, just NH2-terminal to the transmembrane domain, forced MRAP into a single Nexo/Ccyt orientation and blocked its ability to promote MC2 receptor trafficking and homodimerize. When the transmembrane domain of MRAP was replaced with the corresponding region from RAMP3, dual topology was retained but MRAP was inactive. Insertion of MRAP residues 29-37 conferred dual topology to RAMP3, normally in an Nexo/Ccyt orientation. When expressed with MRAPΔ1-30, MRAPΔ10-20, or MRAPΔ21-30, MC2 receptor was localized on the plasma membrane but unable to respond to ACTH. Residues 18-21 of MRAP were critical; MC2 receptor expressed with MRAP(18-21A) localized to the plasma membrane but did not bind 125I-ACTH or increase cAMP in response to ACTH. A newly identified MRAP homolog, MRAP2, lacks amino acids 18LDYI21 of MRAP and, like MRAP(18-21A), allows MC2 receptor trafficking but not signaling. MRAP2 with an LDYI insertion functions like MRAP. These results demonstrate that MRAP not only facilitates MC2 receptor trafficking but also allows properly localized receptor to bind ACTH and consequently signal.
Pituitary adrenocorticotropic hormone (ACTH)2 activates melanocortin 2 (MC2) receptors in the adrenal cortex, stimulating the biosynthesis of glucocorticoids. In familial glucocorticoid deficiency, patients are resistant to ACTH and unable to make sufficient glucocorticoids. Unless adrenal corticosteroids are replaced, the failure to respond to ACTH can lead to hypoglycemia, infection, and death. Some individuals with familial glucocorticoid deficiency (type 1) have inactivating mutations in the MC2 receptor (1-3). As shown by Metherell et al. (4), another group of patients with familial glucocorticoid deficiency (type 2) has mutations in a protein needed for MC2 receptor function, termed MRAP (melanocortin 2 receptor accessory protein) (1, 2).
MRAP is required for MC2 receptor maturation and trafficking to the plasma membrane (4-6). It is a small protein containing a single transmembrane domain with no signal peptide. In the absence of MRAP, MC2 receptor is retained in the endoplasmic reticulum (ER), lacks mature carbohydrate, and is rapidly degraded. In the presence of MRAP, MC2 receptor is glycosylated and localized on the plasma membrane, where it binds ACTH and activates adenylyl cyclase (6).
The NH2-terminal and transmembrane segments of MRAP are strongly conserved, whereas the COOH-terminal domains are highly divergent and apparently nonessential. Two splice variants of human MRAP that differ completely in the region COOH-terminal to the transmembrane region (5) and a truncated mouse MRAP lacking the entire COOH terminus (6) promote surface expression and signal transduction by the MC2 receptor. MRAP forms a stable, immunoprecipitable complex with the MC2 receptor (4, 6, 7). The MC2 receptor is one of five melanocortin receptors, class A G protein-coupled receptors (GPCRs) that are coupled to G proteins and cause an increase in cAMP when activated (8). ACTH is the natural agonist for the MC2 receptor, whereas the various melanocyte-stimulating hormone peptides bind with high affinity to all other melanocortin receptors. Most melanocortin receptors can be expressed in heterologous systems, but the MC2 receptor is not functional when expressed in commonly used model systems. The MC2 receptor is not unique in requiring an accessory protein for trafficking to the plasma membrane and signaling. For example, members of the large odorant receptor family cannot be expressed in heterologous cells without the co-expression of accessory proteins (RTPs or REEP) (9). Receptor activity modifying proteins (RAMPs) alter the ligand specificity and sometimes trafficking of a number of class B receptors (10, 11). These accessory proteins are all small proteins with single transmembrane domains; the RAMPs have an Nexo/Ccyt orientation, whereas RTP reportedly has an Ncyt/Cexo topology. Recent work suggests that RAMPs may interact with a much broader set of class B GPCRs than previously recognized (10, 11), and with at least one class C GPCR, the calcium-sensing receptor (12). RAMPs are tightly coupled to their cognate receptors throughout the life cycle of the GPCR.
We previously reported that MRAP forms antiparallel homodimers, and is found in both the Nexo/Ccyt and Ncyt/Cexo orientations in similar quantities. Both ends of epitope-tagged MRAP are expressed on the exoplasmic face of the membrane in heterologous cells (5, 6), and both ends of endogenous MRAP can be detected on the surface in adrenal cells (6). Glycosylation sites inserted in either the amino- or carboxyl-terminal domains of MRAP are utilized to roughly equal extents, further supporting dual topology of the protein (6). Transfected MRAP forms antiparallel homodimers, or possibly higher order oligomers (6), and endogenous MRAP runs at the size predicted for dimers on SDS-PAGE (7). The topology of MRAP may be unique among single membrane-spanning proteins.
The remarkable structure of MRAP raises a number of issues. Is MRAP in a dual orientation throughout the cell? What regions of MRAP are important for dual topology? What regions are essential for MRAP functions? Here we show that MRAP forms antiparallel dimers in the ER, and identify distinct regions of the molecule responsible for dual orientation, MC2 receptor trafficking, and MC2 receptor signaling.
EXPERIMENTAL PROCEDURES
Materials—hMC2 receptor with three NH2-terminal HA tags and RAMP3 were obtained from Missouri S&T cDNA Resource Center, RAMP1 constructs from Dr. Ian Dickerson (University of Rochester, Rochester, NY), and YFP-F1 and YFP-F2 constructs from Dr. Catherine Berlot (Weis Center for Research, Geisinger Clinic, Danville, PA) (13). ER-tracker blue-white DPX was purchased from Invitrogen. Antibodies were from AbDSerotec (Kidlington, UK) (monoclonal anti-V5), Sigma (monoclonal M2 anti-FLAG), Covance (Princeton, NJ) (monoclonal HA11 anti-HA), Bio-Rad (horseradish peroxidase-coupled anti-mouse), or Molecular Probes (Carlsbad, CA) (Alexa 488- and 546-coupled anti-mouse). ACTH was purchased from Phoenix Pharmaceuticals (Burlingame, CA). [125I-Tyr23]ACTH-(1-39) (2200 Ci/mmol) was from PerkinElmer Life Sciences. MRAP and RAMP3 mutants and fusion proteins were prepared using QuikChange from Stratagene (La Jolla, CA) or by PCR and verified by sequencing. Sequences of proteins studied are listed in supplemental Fig. S1. Primer sequences are available on request.
Cell Culture and Transfection—CHO cells were grown in Dulbecco's modified Eagle's medium/F-12 supplemented with 5% fetal bovine serum. Plasmids were transiently transfected 24-48 h before experiments using FuGENE 6 (Roche).
Surface Epitope Detection by Fixed Cell ELISA—To measure epitopes on the extracellular side of the plasma membrane, cells in 12-well plates were washed with PBS, fixed for 10 min with 2% paraformaldehyde, washed, blocked in 5% milk in PBS, and processed for ELISA as described (6) using 1:5000 monoclonal anti-V5, anti-FLAG, and anti-HA antibodies.
Total Epitope Detection by Fixed Permeabilized Cell ELISA—To measure total epitopes, cells in 12- or 24-well plates were washed with PBS, fixed for 10 min with 2% paraformaldehyde, washed, blocked in 5% milk in RIPA (150 mm NaCl, 50 mm Tris, 1 mm EDTA, 10 mm sodium fluoride, 1% Triton X-100, 0.1% SDS, 0.5% sodium deoxycholate, pH 8.0) and processed for ELISA as described (6) using 1:5000 monoclonal anti-V5, anti-FLAG, or anti-HA antibodies.
mMRAP2 Cloning—Mouse MRAP2 (mMRAP2) was cloned from mRNA isolated from mouse adrenal glands, reverse transcribed, and amplified using a forward primer containing an NheI site on the 5′ end followed by the V5 epitope tag sequence and the first 24 nucleotides of mMRAP2 (5′-ATA TGC TAG CGC CAC CAT GGG TAA GCC TAT CCC CAA CCC TCT GCT CGG TCT TGA TTC TAC TTC TGC CCA GAG GCT GGC TTC TAA C-3′) and a reverse primer containing the end of the mMRAP2 sequence without the stop codon and an MluI site (5′-ATA TAC GCG TGT CCA GGT CTA TGC GTG ATG-3′). The product of the PCR was inserted in pCR2.1-TOPO. The natural NheI site within the mMRAP2 sequence was silently mutated using QuikChange Lightning and the following primers: sense (5′-GGA GGC CTG AGG AGG AAC TAG CCA GGT TCA TGA AG-3′), antisense (5′-CTT CAT GAA CCT GGC TAG TTC CTC CTC AGG CCT CC-3′). The resulting DNA was digested using NheI and MluI and inserted in-frame in a pCI-neo vector containing the 3× FLAG epitope sequence resulting in a plasmid coding for V5-mMRAP2-3Flag.
Live Cell Imaging—Cells on glass coverslips were rinsed and incubated with primary antibodies at 1:100 in F-12 media with 20 mm HEPES and 5% goat serum for 1 h at 37 °C, washed, and incubated with 1:100 secondary antibody and 3 μg/ml Hoechst 33342 for 5 min at room temperature. Secondary antibodies were Alexa 546 anti-mouse or Alexa 488 anti-mouse. For YFP fluorescence localization, CHO cells transfected with YFP-F1-V5-MRAP and V5-MRAP-YFP-F2 were incubated with 1 μm ER-tracker blue-white DPX for 15 to 30 min at 37 °C before imaging. Where noted, nuclei were counterstained with 3 μg/ml Hoechst 33342 for 5 min at room temperature. A Nikon Diaphot inverted microscope with ×100/1.3 NA oil objective, Photometrics CoolSNAP ES camera, and appropriate filter sets from Chroma were used. Images were captured with Metamorph software from Universal Imaging and transferred to Powerpoint for labeling. Micrographs displayed in a group were exposed and processed identically.
Surface Epitope Immunoprecipitation and Western Blotting—Cells were washed, incubated with 1:2000 immunoprecipitating antibodies (anti-V5 and anti-FLAG) in F-12 media with 20 mm HEPES and 5% goat serum for 2 h at room temperature, washed, and lysed for 20 min at 4 °C with 0.1% N-dodecyl-β-maltoside in PBS with protease inhibitors. Lysates were centrifuged and immune complexes collected with protein A/G beads at 4 °C. Beads were washed three times, suspended in loading buffer with 100 mm dithiothreitol, boiled 5 min, and centrifuged. Proteins were resolved by SDS-PAGE on 15% gels from Lonza (Rockland, ME). Western blotting was performed as described (6).
cAMP Assay—CHO cells in 12-well plates were incubated with 0.1 mm isobutylmethylxanthine and vehicle or 100 nm ACTH in Dulbecco's modified Eagle's medium/F-12 medium for 20 min at 37 °C. cAMP was assayed using the Assay Design (Ann Arbor, MI) cAMP EIA Direct kit or PerkinElmer LANCE cAMP assay kit.
125I-ACTH Binding—Cells in 12-well dishes were incubated for 20 min in binding buffer (Dulbecco's PBS with 20 mm HEPES, 0.1% bovine serum albumin, pH 7.5) and then for 1 h in 0.4 ml/well of binding buffer containing ∼120,000 cpm of 125I-ACTH. Dishes were placed on ice and cells washed three times with cold PBS, solubilized in 0.1% SDS, and quantified in a γ-counter.
Data Analysis—All experiments were repeated a minimum of two times on different days in separate experiments. Points in ELISA are the mean and range of duplicate or mean ± S.E. of 3 to 6 determinations, respectively. Statistical significance of differences were analyzed by unpaired Student's t test or analysis of variance with Dunnett's post-test analysis.
RESULTS
Subcellular Localization of MRAP with Dual Topology—We envision two general models to explain how MRAP assumes its novel dual topology. In one, MRAP is initially synthesized in two different orientations in the ER. In the other, MRAP first inserts in one orientation and a fraction of the protein subsequently changes topology, possibly in a later compartment. To assess MRAP topology at the microscopic level, we used bimolecular fluorescence complementation (14). We fused fragments of yellow fluorescent protein (YFP) to either the NH2 or COOH terminus of MRAP (supplemental Fig. S1). If sufficiently close, the F1 and F2 fragments of YFP can interact to form a fluorescent molecule. Because the two fragments of YFP were fused to opposite ends of MRAP, and reconstituting the YFP requires the two fragments to be on the same side of the membrane, YFP fluorescence solely reports the antiparallel dimeric form of MRAP (Fig. 1A). Constructs also contained V5 epitope tags. As a control, we fused the split YFP fragments to the NH2 and COOH termini of RAMP3 (supplemental Fig. S1). RAMP3 is a single transmembrane accessory protein for certain other GPCRs that has an Nexo/Ccyt orientation even without its natural signal peptide (Fig. 3, below).
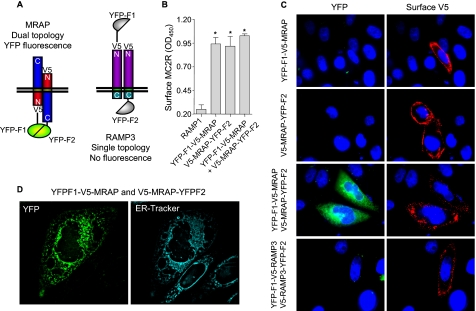
FIGURE 1.
The anti-parallel homodimeric structure of MRAP is achieved in the ER. A, schematic representation of configurations in which the YFP fragments fused to MRAP or RAMP molecules interact in the cell. B, surface MC2 receptor was detected by ELISA using anti-HA antibody in nonpermeabilized CHO cells expressing MC2 receptor with an NH2-terminal HA tag and RAMP1, YFP-F1-V5-MRAP, V5-MRAP-YFP-F2, or both. C, live CHO cells transfected with MRAP or RAMP1 fused to YFP fragments as shown. Left panels, YFP fluorescence. Right panels, surface expression of MRAP detected using anti-V5 antibody. D, live CHO cells transfected with YFP-F1-V5-MRAP and V5-MRAP-YFP-F2 and stained with ER-tracker. *, p < 0.05 versus RAMP1 control.
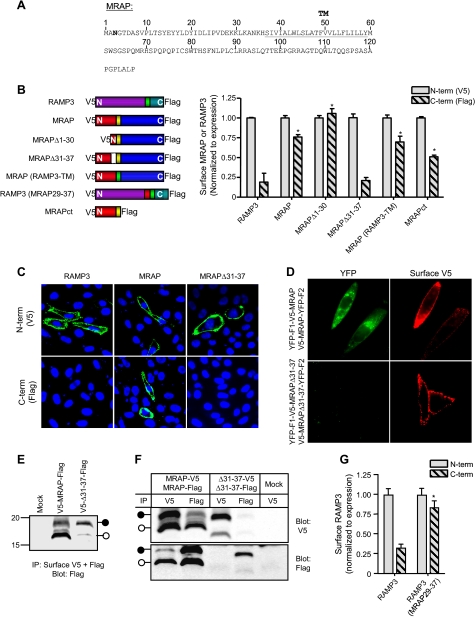
FIGURE 3.
Amino acids 31 to 37 of MRAP are required for dual topology. A, amino acid sequence of mouse MRAP. The predicted transmembrane domain is underlined, and the single natural potential N-linked glycosylation site is shown in bold. B, left panel, schematic representation of constructs studied. Right panels, surface expression of NH2-(V5) and/or COOH-terminal (FLAG) epitopes measured by ELISA in nonpermeabilized CHO cells using anti-V5 or anti-FLAG antibodies. Results for MRAPct are from Ref. 6. C, surface staining of live CHO cells expressing V5-RAMP3-Flag, V5-MRAP-Flag, or V5-MRAPΔ31-37-Flag with anti-V5 (top) or anti-FLAG (bottom) antibodies (shown in green); nuclei are counterstained in blue. D, live CHO cells expressing YFP-F1-V5-MRAP and V5-MRAP-YFP-F2 or the equivalent constructs of MRAPΔ31-37. Left panels show YFP fluorescence and right panels show surface MRAP visualized by staining live cells with anti-V5 antibody. E, CHO cells transfected with V5-MRAP-Flag or V5-MRAPΔ31-37-Flag were incubated with anti-V5 plus anti-FLAG antibodies. Immunoprecipitates (IP) were resolved on SDS-PAGE and the blot probed with anti-FLAG antibody. Filled and open circles show mobility of glycosylated and nonglycosylated species determined after PNGase digestion (6). F, cells expressing MRAP-V5 and MRAP-Flag or equivalent constructs of MRAPΔ31-37 were lysed and anti-V5 or anti-FLAG antibody was added. Immunoprecipitates were resolved on SDS-PAGE and blots probed with anti-V5 or anti-FLAG antibody. G, cells were transfected with V5-RAMP3-Flag or V5-RAMP3(MRAP29-37)-Flag. NH2- or COOH-terminal epitopes on the surface of nonpermeabilized cells were detected by ELISA using anti-V5 or anti-FLAG antibodies. Overall expression of V5-RAMP3(MRAP29-37)-Flag was comparable with that of V5-RAMP3-Flag, but surface expression was lower. In panels B and F, data are normalized to surface expression of the NH2-terminal V5 epitope for each construct. *, p < 0.01 versus RAMP3 control.
To confirm that the fusion proteins were functional, we expressed YFP-F1-V5-MRAP and V5-MRAP-YFP-F2 with HA-tagged MC2 receptor and then quantified MC2 receptor on the plasma membrane by a previously described ELISA (6) that detects only the cell surface epitope. MC2 receptor was not localized on the plasma membrane when expressed with RAMP1, used as a negative control, but was able to traffic to the cell surface when expressed with either of the split YFP-MRAP constructs or the combination of the two (Fig. 1B).
Cells expressing MRAP fused to either the F1 (YFP-F1-V5-MRAP) or F2 (V5-MRAP-YFP-F2) fragments of YFP were not fluorescent, but surface staining of MRAP using the anti-V5 antibody confirmed that these two fusion proteins were expressed and present on the plasma membrane (Fig. 1C). Cells expressing both constructs displayed clear YFP fluorescence, providing strong evidence for the existence of MRAP as an antiparallel homodimer. YFP fluorescence partially overlapped with an ER marker (Fig. 1D), proving that the unique antiparallel structure of MRAP is already achieved in the ER. MRAP fluorescence was also observed on the plasma membrane.
Fluorescence was not seen in cells co-expressing YFP-F1-V5-RAMP3 and V5-RAMP3-YFP-F2, consistent with the single orientation of RAMP3 (Fig. 1, A and C). In an additional control, we co-expressed YFP-F1-V5-MRAP with RAMP3 fused to YFP-F2 at the COOH terminus or V5-MRAP-YFP-F2 with RAMP3 fused to YFP-F1 at the NH2 terminus. The average YFP fluorescence intensity in cells expressing the two MRAP-RAMP3 pairs was less than 20% of the YFP intensity in cells expressing an MRAP-MRAP pair in the same experiment (p < 0.001) (supplemental Fig. S2).
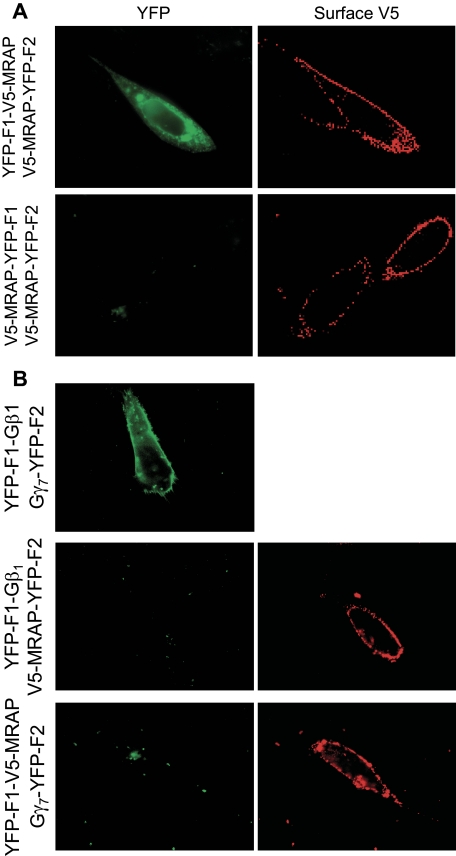
To determine whether MRAP can form parallel homodimers, we fused both fragments of YFP to the COOH terminus of MRAP. When V5-MRAP-YFP-F1 and V5-MRAP-YFP-F2 were coexpressed, no fluorescence was detected, showing that MRAP does not form parallel homodimers in which YFP fragments can interact (Fig. 2A). To demonstrate that association of YFP fragments does not force nonspecific dimerization, we expressed the MRAP-YFP fusion proteins with G protein-YFP fusion proteins, because we did not expect the G protein subunits to interact with MRAP on their own. We expressed YFP-F1-V5-MRAP with the G protein γ7 subunit fused to YFP-F2 or V5-MRAP-YFP-F2 with the G protein β1 subunit fused to YFP-F1. Neither of these combinations resulted in fluorescence. When Gβ1-YFP-F1 and Gγ7-YFP-F2 were co-transfected, however, YFP fluorescence was detected, confirming that those fusion proteins were expressed and formed Gβ1γ7 dimers (Fig. 2B).
FIGURE 2.
Specificity of MRAP bimolecular fluorescence complementation. A, live CHO cells transfected with MRAP fused to YFP fragments on opposite sides or the same side of the MRAP protein. B, live CHO cells transfected with V5-MRAP-YFP-F2 and Gβ1-YFP-F1, YFP-F1-V5-MRAP, and Gγ7-YFP-F2, or Gβ1-YFP-F1 and Gγ7-YFP-F2 as shown. Left panels, YFP fluorescence. Right panels, surface expression of MRAP detected by staining live cells with anti-V5 antibody.
Structural Requirements for Dual Topology of MRAP—To establish what regions of MRAP are important for dual orientation, we made a series of deletion and replacement mutants along the length of the 127-amino acid protein (supplemental Fig. S1 and Fig. 3A). Deletion of amino acids 1 to 30, corresponding to most of the amino terminus, did not significantly alter dual topology, because both NH2- and COOH-terminal ends of the truncated MRAP were detectable on the extracellular side of the membrane (Fig. 3B). Likewise, MRAP retained dual orientation when its 23-residue transmembrane domain was replaced with the corresponding region of RAMP3, a single membrane spanning protein with an exclusively Nexo/Ccyt orientation (10, 11). Deletion of 64 of the 67 residues in the MRAP COOH terminus reduced the fraction of MRAP in an Ncyt/Cexo orientation by about half but did not eliminate dual topology (6).
In contrast, deletion of residues 31-37 of the amino terminus (LKANKHS), just proximal to the transmembrane domain, forced MRAP into a Nexo/Ccyt topology, showing that this positively charged region is required for MRAP to be inserted in opposite orientations. The same result was obtained when MRAP lacking residues 29-37 was studied (data not shown). As a further test of orientation, we incubated live cells with antibodies against either NH2- or COOH-terminal epitope tags and analyzed staining by immunofluorescence microscopy. Both ends of intact MRAP were detected on the external side of the plasma membrane, but only the NH2-terminal epitopes of RAMP3, without a signal peptide, or MRAPΔ31-37 could be detected (Fig. 3C).
MRAPΔ31-37 topology was also tested using the bimolecular fluorescence complementation protocol. As expected for a single orientation mutant, no fluorescence was detected when YFP-F1-V5-MRAPΔ31-37 and V5-MRAPΔ31-37-YFP-F2 were co-expressed in CHO cells (Fig. 3D). These findings corroborate the exclusive Nexo/Ccyt orientation of MRAPΔ31-37.
Because the only N-linked glycosylation site in MRAP is Asn3 in the NH2 terminus and glycosylation can only occur on the luminal side of the ER, the side that eventually will face the extracellular space, we used glycosylation of MRAP at the plasma membrane as a marker of an Nexo/Ccyt orientation. MRAP or MRAPΔ31-37, tagged at the NH2 terminus with V5 and at the COOH terminus with FLAG, were expressed in CHO cells and MRAP on the cell surface was selectively immunoprecipitated by adding anti-V5 and anti-FLAG antibodies to intact cells. We added both antibodies to isolate all surface MRAP regardless of orientation. Immunoprecipitated MRAP was resolved by SDS-PAGE and the membrane immunoblotted with anti-FLAG antibody (Fig. 3E). Cell surface MRAP was a mixture of glycosylated and nonglycosyated forms, indicating that it was present in both Nexo/Ccyt and Ncyt/Cexo orientations. In contrast, almost all of the MRAPΔ31-37 at the cell surface was glycosylated, confirming that this mutant has an almost exclusively Nexo/Ccyt topology.
Because MRAP dimers are antiparallel, we hypothesized that MRAPΔ31-37, being exclusively Nexo/Ccyt, would not homodimerize. To test this idea, we co-expressed MRAPΔ31-37-V5 with MRAPΔ31-37-FLAG, lysed cells, and immunoprecipitated with either anti-V5 or anti-FLAG antibody. No MRAPΔ31-37-V5 was immunoprecipitated with anti-FLAG and no MRAPΔ31-37-FLAG was immunoprecipitated with anti-V5, whereas a significant fraction of wild type MRAP-V5 was detected after anti-FLAG immunoprecipitation and vice versa (Fig. 3F). These results all lead to the conclusion that the 31-37 region is necessary for dual topology and that dual topolgy is required for dimerization.
To determine whether this small region of MRAP is sufficient to force dual topology, we inserted amino acids 29-37 of MRAP into the NH2 terminus of RAMP3 proximal to the transmembrane domain and deleted the RAMP3 signal peptide (supplemental Fig. S1). RAMP3 without signal peptide was in an Nexo/Ccyt orientation (Fig. 3G), as it is with its signal sequence. In contrast, both ends of RAMP3(29-37MRAP) were detected on the extracellular side of the membrane (Fig. 3G), showing that addition of the short MRAP sequence KKLKANKHS caused a protein that normally has a single Nexo/Ccyt topology to assume a dual orientation.
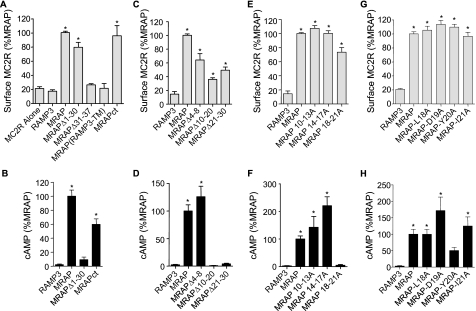
Structural Requirements for Function of MRAP—To learn what regions of MRAP are required for receptor trafficking, we co-expressed MC2 receptor with RAMP3, as a negative control, or with MRAPΔ1-30, MRAPΔ31-37, MRAP(RAMP3TM), or MRAPct (Fig. 4A). As expected, MC2 receptor was not detectable at the plasma membrane when it was co-expressed with RAMP3. The receptor did traffic to the plasma membrane when it was co-expressed with MRAP, MRAPΔ1-30, or MRAPct, demonstrating that amino acids 1 to 30 and the COOH terminus are not required for MRAP to facilitate MC2 receptor surface expression, just as these regions are not required for dual topology. On the other hand, MC2 receptor was retained in the ER when it was expressed with an MRAP in which the transmembrane domain was replaced by the corresponding region of RAMP3. Even though MRAP could assume a dual orientation without its natural transmembrane domain, the chimeric MRAP could not facilitate MC2 receptor trafficking. MC2 receptor was also retained in the ER when expressed with the single topology and monomeric mutant MRAPΔ31-37.
FIGURE 4.
Regions of MRAP required for MC2 receptor trafficking and signaling. HA-MC2 receptor was expressed in CHO cells with empty vector (MC2R alone), RAMP3, MRAP, or MRAP mutants. A, C, E, and G, surface HA-MC2 receptor was detected in nonpermeabilized cells by ELISA using anti-HA antibody. B, D, F, and H, cAMP concentration following incubation with 0.1 mm isobutylmethylxanthine and 100 nm ACTH for 20 min. Results for MRAPct are from Ref. 6. Data are expressed as percent of the value obtained with full-length MRAP. *, p < 0.05 versus RAMP3 control.
To verify that the absence of MC2 receptor at the cell surface with various MRAP mutants was not due to inefficient translation, we showed that high concentrations of total receptor were present in cells expressing MC2 receptor with no accessory protein, RAMP3, MRAPΔ31-37, or MRAP(RAMP3TM) (supplemental Fig. S3). The proteasome inhibitor MG132 increased the total MC2 receptor 2- to 3-fold, suggesting that there is significant degradation of MC2 receptor after synthesis.
To learn whether MRAP has a role in MC2 receptor signaling, we measured ACTH-stimulated cAMP production in cells transfected with the MC2 receptor and RAMP3, MRAP, or those MRAP mutants that allowed surface expression of the MC2 receptor (Fig. 4B). ACTH did not increase cAMP in cells that expressed MC2 receptor with RAMP3. In contrast, ACTH stimulated a 40-fold or greater increase in cAMP concentration when the MC2 receptor was expressed with MRAP and a smaller 25-fold increase with MRAPct (6).
Interestingly, when it was expressed with MRAPΔ1-30, the MC2 receptor could traffic to the plasma membrane but was unable to cause an increase in cAMP in response to ACTH (Fig. 4). This is evidence that MRAP is not only important for the MC2 receptor to exit the ER and reach the plasma membrane, but also to signal, and that these two functions of MRAP are separable.
To establish which amino acids are important for MRAP to support MC2 receptor activation, we deleted smaller regions within the first 30 amino acids of MRAP. The amount of receptor expressed on the plasma membrane was reduced by 40 to 75% after deletion of residues 4-8, 10-20, or 21-30 (Fig. 4C), but the amount of cell surface receptor was still significantly higher than that seen without any MRAP. Based on ELISA measurements, the levels of wild type, Δ4-8, Δ10-20, and Δ21-30 MRAPs on the plasma membrane did not differ greatly. Importantly only MRAPΔ4-8 allowed the MC2 receptor to signal (Fig. 4D). The cAMP response to ACTH was completely abolished when MC2 receptor was expressed with MRAPΔ10-20 or MRAPΔ21-30 (Fig. 4D).
When amino acids 10-13 (PLTS) or 14-17 (YEYY) of MRAP were mutated to alanines, MRAP facilitated both trafficking and signaling of the MC2 receptor (Fig. 4, E and F). In fact mutation of amino acids 14-17 to alanine increased the amplitude of the cAMP response 2-fold. When MC2 receptor was expressed with MRAP in which amino acids 18-21 (LDYI) were mutated to alanines, however, the receptor reached the cell surface but ACTH did not produce a measurable increase in cAMP (Fig. 4, E and F). MRAP remained functional when amino acids 18-21 were mutated to alanine individually, although alanine substitution of Tyr20 resulted in a 50% reduction of the cAMP response to ACTH (Fig. 3, G and H).
To determine whether the 18-21 region of MRAP regulates agonist specificity, we expressed MC2 receptor with MRAP or MRAP(18-21A) and tested the effects of high concentrations of ACTH-(1-24), ACTH-(1-18), which potently stimulates MC2 receptor in adrenal cells, and (Nle4,D-Phe7)α-melanocyte stimulating hormone, which normally activates MC 1, 3, 4, and 5 but not MC2 receptors (8). (Nle4,D-Phe7)α-melanocyte stimulating hormone did not increase cAMP production via the MC2 receptor with either MRAP. Both ACTH peptides were active with MRAP but not MRAP(18-21A) (supplemental Fig. S4). MC2 receptor had no constitutive activity with any MRAP.
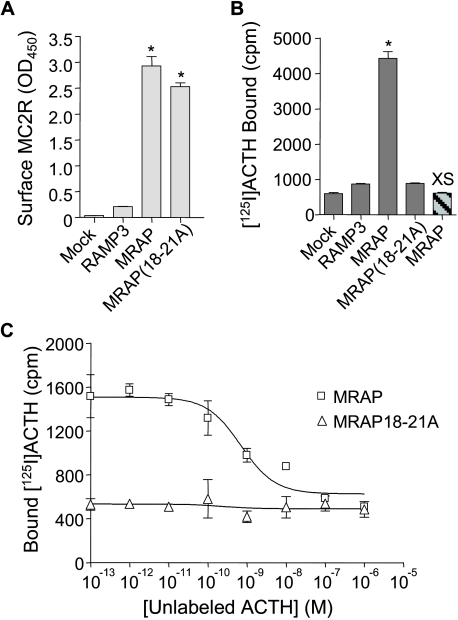
125I-ACTH Binding—We tested the ability of MC2 receptors to bind 125I-ACTH in cells expressing either MRAP or MRAP(18-21A) (Fig. 5). Cell surface expression of the MC2 receptor was nearly identical in cells expressing MRAP or MRAP(18-21A). Nonspecific binding of 125I-ACTH, measured in either mock-transfected cells or cells incubated with 125I-ACTH and excess unlabeled ACTH, was low. 125I-ACTH binding was robust in cells expressing MC2 receptor with MRAP, and ACTH-(1-24) displaced bound 125I-ACTH with an IC50 of 0.7 nm. On the other hand, 125I-ACTH binding was negligible in cells expressing receptor and MRAP(18-21A). These results highlight the importance of residues 18-21 of MRAP in allowing the MC2 receptor to achieve a conformation in which it can bind ACTH and consequently signal.
FIGURE 5.
Effect of MRAP on 125I-ACTH binding to MC2 receptor. CHO cells were transfected with no receptor (Mock) or HA-MC2 receptor and RAMP3, MRAP, or MRAP(18-21A). A, surface expression of HA-MC2 receptor measured by ELISA using anti-HA antibody. B, 125I-ACTH binding. The bar on the far right depicts cells incubated with 1 μm unlabeled ACTH for 20 min before and during the 1-h binding assay. C, competition displacement measured in cells incubated with 125I-ACTH (300,000 cpm/ml, ∼0.1 nm) and unlabeled ACTH at the concentrations shown. *, p < 0.01 versus RAMP3 control.
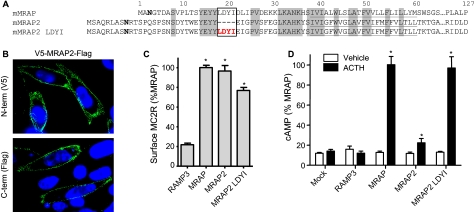
MRAP2—C6orf117 was identified by Metherell et al. (4) as a homolog of MRAP and is now termed MRAP2 in GenBank™. We cloned the gene from cDNA prepared from mouse adrenal glands (mMRAP2) and tagged it with a V5 epitope on the NH2 terminus and 3× FLAG epitope on the COOH terminus. The DNA sequence was identical to that predicted from the mouse genome. Alignment of the mouse MRAP and MRAP2 protein sequences (Fig. 6A) shows that the two proteins share significant homology in regions required for MRAP function (transmembrane domain and region 31-37). Strikingly, MRAP2 lacks amino acids LDYI in the position equivalent to 18-21 in MRAP (Fig. 6A). As a result, we predicted that cells expressing MRAP2 and MC2 receptor would share the phenotype of MRAP(18-21A), with receptor on the plasma membrane but unresponsive to ACTH. We analyzed the orientation of V5-MRAP2-3Flag on live CHO cells by immunofluorescence and found that, as with MRAP, both ends of the molecule were detectable on the extracellular side of the membrane (Fig. 6B). This was expected because the NH2-terminal region proximal to the transmembrane domain of MRAP2 was homologous to the 31-37 region of MRAP. mMRAP2 facilitated MC2 receptor trafficking to the plasma membrane as effectively as MRAP (Fig. 6C), yet cells expressing MC2 receptor and mMRAP2 displayed only a small cAMP response to ACTH. Despite the presence of equal MC2 receptor on the surface of cells expressing MRAP and mMRAP2, ACTH induced an 8-fold larger cAMP response in cells expressing MRAP (Fig. 6D). To test whether the apparent defect in receptor signaling with mMRAP2 resulted from the absence of an LDYI motif, we inserted these amino acids in the position of MRAP2 corresponding to where they appear in MRAP (Fig. 6A). When the MC2 receptor was expressed with MRAP2 containing LDYI it was able to traffic to the plasma membrane (Fig. 6C) and respond robustly to ACTH (Fig. 6D).
FIGURE 6.
Structure and function of mMRAP2. A, sequence of mMRAP2 cloned from mouse adrenal gland cDNA. B, surface staining of live CHO cells expressing V5-mMRAP2-Flag with anti-V5 (top) or anti-FLAG (bottom) antibodies is shown in green; nuclei are counterstained in blue. C, CHO cells were transfected with HA-MC2 receptor and RAMP3, MRAP, mMRAP2, or mMRAP2 with LDYI inserted after residue 28 as shown. Surface HA-MC2 receptor was detected by ELISA in nonpermeabilized cells using anti-HA antibody. D, cAMP concentration in CHO cells expressing HA-MC2 receptor and RAMP3 or MRAP constructs shown following incubation with 0.1 mm isobutylmethylxanthine and 100 nm ACTH for 20 min. Data in C and D are expressed as percent of the value obtained with full-length MRAP. *, p < 0.05 versus RAMP3 (C) or vehicle (D).
DISCUSSION
GPCR accessory proteins (RAMPs, RTPs, REEP, and MRAP) are small, single membrane-spanning proteins. Most of these are inserted in the membrane in either Nexo/Ccyt (RAMPs) or Ncyt/Cexo (RTPs and REEP) orientations. MRAP differs from other accessory proteins in having an extremely unusual dual topology (6). In this report, we have identified features responsible for the unique antiparallel, homodimeric structure of MRAP. MRAP could be inserted in both orientations co-translationally due to an “indecisiveness” regarding orientation, or a fraction of MRAP could reverse its orientation after synthesis. There is precedent for this with transmembrane domain 3 of aquaporin 1, which is synthesized in one direction and flips following synthesis of the remainder of the protein (15). The bimolecular fluorescence complementation approach allowed us to show unequivocally that the dual topology of MRAP is achieved in the ER at the earliest stages of protein synthesis and persists when the protein is at the plasma membrane. Our results do not rule out the possibility that MRAP starts synthesis in one orientation and then partially reverses direction, but they do show that any reorientation must occur quickly. Given the rigid structure of GFP and its derivatives like YFP, it is somewhat surprising that the MRAP-YFP fragments were inserted in the membrane in both directions and functional, because a highly structured region makes inversion across the membrane less likely (16). We have also found that MRAP fused to mOrange (17), a 27-kDa fluorescent protein, has dual topology and is able to support MC2 receptor trafficking (data not shown).
The orientation of hydrophobic segments in the membrane is governed by a number of properties. Most important is the “positive inside” rule, which recognizes that the presence of multiple positively charged amino acids within 15 residues of the hydrophobic segment orients a region to the cytoplasmic face of the membrane (18, 19). For this reason, we investigated the role of the NH2-terminal lysine-rich region on the aminoterminal side of the transmembrane domain of MRAP. Deletion of the region from amino acids 31 to 37 (LKANKHS), which contains two positive charges, was sufficient to disrupt the normal dual orientation of MRAP, because MRAPΔ31-37 was on the plasma membrane in an exclusively Nexo/Ccyt topology and did not form stable dimers. The fact that MRAPΔ31-37 failed to facilitate MC2 receptor trafficking to the plasma membrane strongly suggests that dual topology and perhaps also formation of antiparallel homodimers are required for the role in folding and trafficking of MRAP, although we cannot rule out the possibility that amino acids 31-37 are required for MRAP function through another mechanism.
Insertion of amino acids 29-37 of MRAP (KKLKANKHS) in the NH2 terminus of RAMP3 adjacent to the transmembrane domain forced the originally Nexo/CcytRAMP3 to be inserted in two opposite orientations. This indicates that this short sequence is not only necessary but can be sufficient to transfer dual topology. Based on the positive-inside rule, insertion of lysines on the NH2-terminal side of a single transmembrane protein is expected to increase the probability of an Ncyt/Cexo orientation. In fact, protein topology can be completely, as shown for the Escherichia coli leader peptidase (23), or partially, as shown for asialoglycoprotein (24), inverted by the modification of charge distribution around the membrane anchor domain. The extent to which RAMP3 topology was reversed was unexpected, however. TMHMM, a topology prediction program (25), assigns an Nexo/Ccyt probability of 0.99 to RAMP3 without its signal peptide. This orientation is still strongly favored, with a predicted Nexo/Ccyt probability of 0.85, following addition of the 9-amino acid sequence from MRAP to the NH2-terminal side of the hydrophobic region. Given the importance of the transmembrane segment in establishing topology, it was somewhat surprising that substitution of the RAMP3 transmembrane domain for the hydrophobic region of MRAP had no effect on dual topology. The fact that the chimeric protein was unable to facilitate MC2 receptor trafficking establishes that the transmembrane domain of MRAP is required for function.
Type 2 familial glucocorticoid deficiency shows an autosomal recessive pattern of inheritance (1, 2). To date, all of the disease-causing mutations identified in MRAP would produce no protein or a severely truncated MRAP molecule (1, 26). Parents of affected individuals with one normal and one nonfunctional copy of MRAP appear to have normal adrenal function. Because MRAP forms a homodimer, it is possible that dominant negative forms of MRAP will be discovered where mutant MRAP inactivates dimers with one mutant and one normal partner. This could lead to dominant inheritance patterns of some forms of type 2 familial glucocorticoid deficiency. We did not detect dominant negative effects of MRAPΔ31-37, MRAPΔ10-20, or MRAP(18-21A) when we co-expressed them with normal MRAP in a heterologous expression system, but because all of these proteins were overexpressed it is possible that these MRAP mutants exert dominant effects in a normal adrenal cell.
Unexpectedly, several MRAP mutants that allowed MC2 receptor trafficking to the cell surface did not support ACTH-induced cAMP production, in other words they were able to facilitate MC2 receptor trafficking to the plasma membrane but the surface receptor was not able to signal in response to its ligand. Residues 18-21 (LDYI) were critical for the second function of MRAP, allowing ACTH binding by the MC2 receptor. Available data do not allow us to conclude whether MRAP has any additional effects on receptor-G protein coupling or other signaling pathways. When residues 18-21 of MRAP were mutated to alanines, the MC2 receptor was expressed at high levels and localized appropriately on the plasma membrane, but it did not bind 125I-ACTH at all. MRAP, possibly through amino acids 18-21, may favor an ACTH binding conformation, in a way comparable with the manner in which RAMP1 provides binding sites for calcitonin gene-related peptide. In the case of RAMPs, the large extracellular domain is necessary and often sufficient for function, because this region can be fused to an unrelated transmembrane protein with partial retention of activity (27). Because MRAP displays dual topology, we do not know whether the critical regions we have identified are important on the cytoplasmic or exoplasmic face of the membrane.
Mutational analysis allowed us to dissect two functions of MRAP, the facilitation of MC2 receptor expression at the plasma membrane, and an essential and independent role in MC2 receptor binding and, as a result, receptor signaling. This finding can explain the results reported by Roy et al. (5) where MC2 receptor was detected at the plasma membrane of HEK293 cells in the absence of transfected MRAP but was insensitive to ACTH. GPCRs are subject to quality control mechanisms that lead to their degradation in the ER if they are improperly folded (20, 28, 29). MRAP may act as a chaperone in the ER, facilitating correct MC2 receptor folding and allowing subsequent glycosylation and trafficking.
Establishing a role for MRAP in MC2 receptor agonist binding makes MRAP functionally parallel to RAMPs, where deletion of small regions in the extracellular NH2 terminus of RAMPs have consequences similar to substitution of Ala for residues 18-21 in MRAP. Deletion of amino acids 91-103 of RAMP1 (21), 86-92 of RAMP2, and 59-65 of RAMP3 (10) gives rise to proteins capable of facilitating receptor trafficking to the plasma membrane, but the properly localized receptor is unable to bind ligand or signal. There is no obvious similarity between the essential sequences in RAMPs and MRAP.
Characterization of the actions of mouse MRAP2, a naturally occurring MRAP homolog lacking the critical LDYI domain, confirmed the importance of these four amino acids in MC2 receptor-mediated cAMP responses. mMRAP2 was capable of supporting only a weak ACTH response in a heterologous expression system where MRAP promoted strong cAMP responses to ACTH. Chan et al. (22) identified a human MRAP2 in adrenal gland and brain. Human MRAP2 also lacks the LDYI motif, but human MRAP2 is reported to allow MC2 receptor cAMP signaling. The difference between our results and the initial findings with human MRAP may be due to a species difference, or it may be a quantitative difference. If MRAP2 is a less effective accessory protein than MRAP in humans, it could explain why MRAP2 fails to rescue ACTH function in patients with familial glucocorticoid deficiency type 2. Future studies will address a number of fascinating questions such as whether MRAP2 alters the activity of MRAP and whether the balance of MRAPs plays a physiological role in regulating ACTH responsiveness.
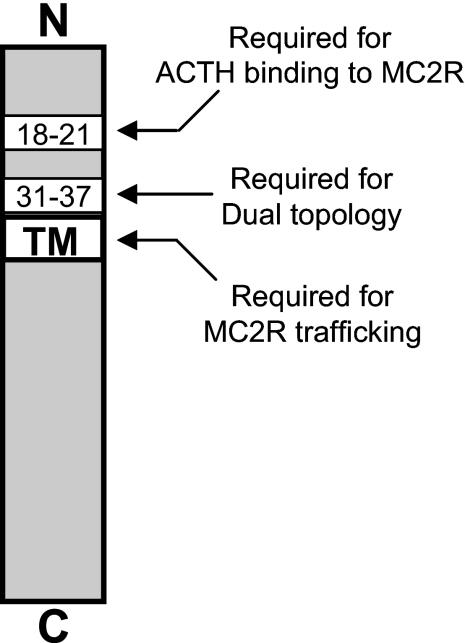
We conclude that the unique antiparallel homodimeric structure of MRAP is achieved in the ER during or soon after MRAP synthesis. A positively charged region proximal to the transmembrane domain confers dual topology, and dual topology appears to be required for MRAP function. The ability of MRAP to promote MC2 receptor trafficking requires the transmembrane domain of MRAP and portions of the amino-terminal domain, whereas a four-amino acid region in the NH2 terminus of MRAP that is not required for MC2 receptor trafficking is essential for signal transduction. This is shown schematically in Fig. 7. The results uncover distinct regions of MRAP critical for dual topology, MC2 receptor maturation and trafficking, and ligand binding. In principle, identification of molecules with high affinity for the 18-21 region of MRAP could lead to drugs capable of inhibiting MC2 receptor signaling that could be used in some forms of hypercortisolism such as Cushing disease.
FIGURE 7.
Schematic representation of essential MRAP regions. Shown are MRAP regions that appear to be required for dual topology and for two functions: facilitating MC2 receptor trafficking to the plasma membrane, and facilitating MC2 receptor binding.
Supplementary Material
This work was supported, in whole or in part, by National Institutes of Health Grant DK19974 (to P. M. H.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1-S4.
Footnotes
The abbreviations used are: ACTH, adrenocorticotropic hormone; ER, endoplasmic reticulum; GPCR, G protein-coupled receptor; MC2, melanocortin-2; Ncyt/Cexo and Nexo/Ccyt, cytoplasmic NH2 terminus and exoplasmic COOH terminus and vice versa, respectively; MRAP, MC2-receptor accessory protein; RAMP, receptor activity modifying protein; HA, hemagglutinin; CHO, Chinese hamster ovary; ELISA, enzyme-linked immunosorbent assay; PBS, phosphate-buffered saline; YFP, yellow fluorescent protein.
References
- 1.Chan, L. F., Clark, A. J., and Metherell, L. A. (2008) Horm. Res. 69 75-82 [DOI] [PubMed] [Google Scholar]
- 2.Cooray, S. N., Chan, L., Metherell, L., Storr, H., and Clark, A. J. (2008) Endocr. Dev. 13 99-116 [DOI] [PubMed] [Google Scholar]
- 3.Metherell, L. A., Chan, L. F., and Clark, A. J. (2006) Best Pract. Res. Clin. Endocrinol. Metab. 20 547-560 [DOI] [PubMed] [Google Scholar]
- 4.Metherell, L. A., Chapple, J. P., Cooray, S., David, A., Becker, C., Ruschendorf, F., Naville, D., Begeot, M., Khoo, B., Nurnberg, P., Huebner, A., Cheetham, M. E., and Clark, A. J. (2005) Nat. Genet. 37 166-170 [DOI] [PubMed] [Google Scholar]
- 5.Roy, S., Rached, M., and Gallo-Payet, N. (2007) Mol. Endocrinol. 21 1656-1669 [DOI] [PubMed] [Google Scholar]
- 6.Sebag, J. A., and Hinkle, P. M. (2007) Proc. Natl. Acad. Sci. U. S. A. 104 20244-20249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cooray, S. N., Almiro Do Vale, I., Leung, K. Y., Webb, T. R., Chapple, J. P., Egertova, M., Cheetham, M. E., Elphick, M. R., and Clark, A. J. (2008) Endocrinology 149 1935-1941 [DOI] [PubMed] [Google Scholar]
- 8.Gantz, I., and Fong, T. M. (2003) Am. J. Physiol. 284 E468-E474 [DOI] [PubMed] [Google Scholar]
- 9.Saito, H., Kubota, M., Roberts, R. W., Chi, Q., and Matsunami, H. (2004) Cell 119 679-691 [DOI] [PubMed] [Google Scholar]
- 10.Parameswaran, N., and Spielman, W. S. (2006) Trends Biochem. Sci. 31 631-638 [DOI] [PubMed] [Google Scholar]
- 11.Sexton, P. M., Morfis, M., Tilakaratne, N., Hay, D. L., Udawela, M., Christopoulos, G., and Christopoulos, A. (2006) Ann. N. Y. Acad. Sci. 1070 90-104 [DOI] [PubMed] [Google Scholar]
- 12.Bouschet, T., Martin, S., and Henley, J. M. (2005) J. Cell Sci. 118 4709-4720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hynes, T. R., Tang, L., Mervine, S. M., Sabo, J. L., Yost, E. A., Devreotes, P. N., and Berlot, C. H. (2004) J. Biol. Chem. 279 30279-30286 [DOI] [PubMed] [Google Scholar]
- 14.Kerppola, T. K. (2006) Nat. Rev. Mol. Cell Biol. 7 449-456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lu, Y., Turnbull, I. R., Bragin, A., Carveth, K., Verkman, A. S., and Skach, W. R. (2000) Mol. Biol. Cell 11 2973-2985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Denzer, A. J., Nabholz, C. E., and Spiess, M. (1995) EMBO J. 14 6311-6317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shaner, N. C., Campbell, R. E., Steinbach, P. A., Giepmans, B. N., Palmer, A. E., and Tsien, R. Y. (2004) Nat. Biotechnol. 22 1567-1572 [DOI] [PubMed] [Google Scholar]
- 18.von Heijne, G. (1986) EMBO J. 5 3021-3027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hartmann, E., Rapoport, T. A., and Lodish, H. F. (1989) Proc. Natl. Acad. Sci. U. S. A. 86 5786-5790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gurevich, V. V., and Gurevich, E. V. (2008) Trends Neurosci. 31 74-81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuwasako, K., Kitamura, K., Nagoshi, Y., Cao, Y. N., and Eto, T. (2003) J. Biol. Chem. 278 22623-22630 [DOI] [PubMed] [Google Scholar]
- 22.Chan, L. F., Metherell, L. A., Webb, T. R., Elphick, M. R., Chapple, J. P., and Clark, A. J. L. (2008) Abstracts of the Endocrine Society Annual Meeting, San Francisco, CA
- 23.von Heijne, G. (1989) Nature 341 456-458 [DOI] [PubMed] [Google Scholar]
- 24.Beltzer, J. P., Fiedler, K., Fuhrer, C., Geffen, I., Handschin, C., Wessels, H. P., and Spiess, M. (1991) J. Biol. Chem. 266 973-978 [PubMed] [Google Scholar]
- 25.Viklund, H., and Elofsson, A. (2004) Protein Sci. 13 1908-1917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Modan-Moses, D., Ben-Zeev, B., Hoffmann, C., Falik-Zaccai, T. C., Bental, Y. A., Pinhas-Hamiel, O., and Anikster, Y. (2006) J. Clin. Endocrinol. Metab. 91 3713-3717 [DOI] [PubMed] [Google Scholar]
- 27.Kuwasako, K., Shimekake, Y., Masuda, M., Nakahara, K., Yoshida, T., Kitaura, M., Kitamura, K., Eto, T., and Sakata, T. (2000) J. Biol. Chem. 275 29602-29609 [DOI] [PubMed] [Google Scholar]
- 28.Bernier, V., Bichet, D. G., and Bouvier, M. (2004) Curr. Opin. Pharmacol. 4 528-533 [DOI] [PubMed] [Google Scholar]
- 29.Conn, P. M., Ulloa-Aguirre, A., Ito, J., and Janovick, J. A. (2007) Pharmacol. Rev. 59 225-250 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.