Abstract
Previously, we demonstrated that mitochondria from denervated muscle exhibited dramatically higher Amplex Red dependent fluorescence (thought to be highly specific for hydrogen peroxide) compared with control muscle mitochondria. We now demonstrate that catalase only partially inhibits the Amplex Red signal in mitochondria from denervated muscle. In contrast, ebselen (a glutathione peroxidase mimetic and inhibitor of fatty acid hydroperoxides) significantly inhibits the Amplex Red signal. This suggests that the majority of the Amplex Red signal in mitochondria from denervated muscle is not derived from hydrogen peroxide. Because Amplex Red cannot react with substrates in the lipid environment, we hypothesize that lipid hydroperoxides formed within the mitochondrial lipid bilayer are released as fatty acid hydroperoxides and react with the Amplex Red probe. We also suggest that the release of fatty acid hydroperoxides from denervated muscle mitochondria may be an important determinant of muscle atrophy. In support of this, muscle atrophy and the Amplex Red signal are inhibited in caloric restricted mice and in transgenic mice that overexpress the lipid hydroperoxide-detoxifying enzyme glutathione peroxidase 4. Finally, we propose that cytosolic phospholipase A2 may be a potential source of these hydroperoxides.
A progressive loss of muscle mass leading to a decline in both strength and function is a normal consequence of biological aging (1, 2). Although several mechanisms have been implicated in age-related muscle atrophy (2–5), the loss of motor neurons or innervation may be one of the most important factors responsible for muscle atrophy observed during aging and in neurodegenerative diseases like amyotrophic lateral sclerosis (ALS)3 (6–8). The sciatic nerve transection model of skeletal muscle denervation leads to rapid decline in muscle mass and has been extensively used to investigate the mechanisms of muscle atrophy following the loss of innervation (9–11). Recent studies using this denervation model in rodents point to a role of mitochondrial oxidative stress in the mechanism of muscle atrophy (11, 12).
Studies from our laboratory and others point to oxidative stress and mitochondrial dysfunction as key players in the mechanisms underlying loss of muscle mass during aging and in neurodegenerative diseases, which are characterized by the loss of muscle mass (12–17). We recently reported a significant elevation in mitochondrial production of reactive oxygen species (ROS) using the Amplex Red probe in various mouse models that exhibit muscle atrophy associated with loss of innervation aging, copper-zinc superoxide dismutase knockout (Sod1–/–) mice, and the G93A Sod1 mutant mouse model of ALS (13). In addition, we demonstrated that ROS were significantly elevated in muscle mitochondria isolated from mice 7 days after surgical sciatic nerve transection (13). ROS production was positively correlated with the extent of muscle atrophy, indicating that mitochondrial oxidative stress may have a major role in muscle atrophy associated with loss of innervation. Reports from other laboratories have also demonstrated that mitochondrial ROS production is significantly elevated in atrophied muscles from aging rats and in rats that underwent denervation surgery (11, 18).
In the present study, we investigated the nature of the radical species released from isolated mitochondria following denervation by sciatic nerve transection. We propose that the majority of ROS production from muscle mitochondria post-denervation surgery may be due to fatty acid hydroperoxides rather than hydrogen peroxide/superoxide. We also hypothesize that the release of fatty acid hydroperoxides from denervated muscle mitochondria may be mediated by calcium-dependent cytosolic phospholipase A2 (cPLA2). Finally, our data suggest that fatty acid hydroperoxides may be of pathophysiological relevance because interventions that minimize oxidative stress in general (caloric restriction) as well as lipid hydroperoxides specifically (glutathione peroxidase 4 (Gpx4)) inhibited denervation-induced muscle atrophy.
EXPERIMENTAL PROCEDURES
Experimental Animals—All of the denervation experiments in this study were performed in 3–9-month-old C57BL/6 female mice. The mice were maintained under specific pathogen-free conditions, housed 3–4/cage, maintained in a 12:12 (light:dark) cycle at 22 ± 2 °C and 50 ± 10% relative humidity. The mice were fed either ad libitum (AL) or calorie-restricted (CR, 40% fewer calories than AL) diets. Sod1–/– and G93A mice are described in an earlier publication (12). To harvest skeletal muscle, the mice were euthanized using a CO2 chamber followed by cervical dislocation. All of the procedures were approved by the Institutional Animal Care and Use Committee at the University of Texas Health Science Center at San Antonio and the Audie L. Murphy Veterans Hospital.
Denervation Surgery—Surgical sciatic nerve transection was performed using constant flow isoflurane inhalation anesthesia. In each hindlimb (at the level of femur), a small incision was made, and the sciatic nerve was isolated. In the left leg, the sciatic nerve was severed and a 5-mm section of nerve was removed. The ends of the nerve were folded back and closed with reabsorbable sutures to prevent nerve regrowth. The skin incisions were closed with wound clips. The contralateral limb served as the intra-animal control. All of the experiments were performed in muscle isolated 7 days post-denervation. The gastrocnemius, soleus, and tibialis (the muscles innervated by the sciatic nerve) were collected and used for analysis (12).
Chemicals—All of the chemicals were obtained from Sigma-Aldrich unless specified otherwise.
Isolation of Skeletal Muscle Mitochondria—Mitochondria were purified from lower hind limb skeletal muscle according to Chappell and Perry, as described previously (19). Briefly, hind limb skeletal muscle was excised, weighed, bathed in 150 mm KCl, and placed in Chappell-Perry buffer with the protease nagarse. The minced skeletal muscle was homogenized, and the homogenate was centrifuged for 10 min at 600 × g; supernatant was passed through cheesecloth and centrifuged at 14,000 × g for 10 min. The mitochondrial pellet was washed in modified Chappell-Perry buffer with 0.5% bovine serum albumin and centrifuged at 7,000 × g for 10 min. The pellet was further washed twice in modified Chappell-Perry buffer without bovine serum albumin centrifuging each time at 3,500 × g for 10 min. Isolated mitochondria were used immediately. Protein concentration was measured with the Bradford method.
Mitochondrial H2O2 Release—Mitochondrial H2O2 production was measured by the Amplex Red-horseradish peroxidase method (Molecular Probes, Eugene, OR). Horseradish peroxidase (2 units/ml) catalyzes the H2O2-dependent oxidation of nonfluorescent Amplex Red (80 μm) to fluorescent resorufin red (20). Fluorescence was followed at an excitation wavelength of 545 nm and an emission wavelength of 590 nm using a Fluoroskan Ascent type 374 multi-well plate reader (Labsystems, Helsinki, Finland). The slope of the increase in fluorescence is converted to the rate of H2O2 production with a standard curve (21). All of the assays were performed at 37 °C, in black 96-well plates in the presence or absence of respiratory substrates and inhibitors. The reaction buffer consisted of 125 mm KCl, 10 mm HEPES, 5 mm MgCl2, 2 mm K2HPO4, pH 7.44. For the H2O2 and hydroperoxide inhibition experiments, catalase (∼4 units) and ebselen (10 μm), respectively, were added to the reaction mix prior to the addition of substrates/inhibitors.
Measurements of Superoxide Production—We measured superoxide production by three methods: EPR, the chemiluminescent probe MCLA (2-methyl-6-(p-methoxyphenyl)-3,7-dihydroimidazo[1,2-α]-pyrazin-3-one), and mitochondrial aconitase activity.
EPR—Extramitochondrial superoxide release was measured by EPR using the spin trap, 5-diisopropoxyphosphoryl)-5-methyl-1-pyrroline-N-oxide (DIPPMPO, Alexis Biochemicals). DIPPMPO forms an adduct with superoxide, resulting in the generation of DIPPMPO-OOH, which decays to the DIPPMPO-OH adduct by the action of glutathione peroxidases (22). EPR measurements were performed using an X-band MS200 spectrometer (Magnetech, Berlin). Mitochondria (1 mg/ml) were incubated at 37 °C with/without G/M and DIPPMPO (50 mm) for 30 min in 125 mm KCl, 10 mm MOPS, 2 mm diethylene triamine pentaacetic acid, 5 mm MgCl2, 2 mm K2HPO4, pH 7.44. Although it did not affect the DIPPMPO-OH signal, low levels of catalase (10 units/ml) were also added, because it prevented the appearance of small additional and unidentified spectrum peaks after extended incubation. For measurements, 40 μl of sample was transferred to 50-μl capillary tubes and measured at room temperature with the following settings: receiver gain, 5 × 105; microwave power, 20 milliwatt; microwave frequency, 9.55 GHz; modulation amplitude, 2G; scan time, 40 s; and scan width, 100 G, with an accumulation of 10 scans.
MCLA—The second method of superoxide measurement utilized the probe MCLA (Molecular Probes, Eugene, OR). MCLA reversibly reacts with superoxide, forming an adduct whose irreversible decay generates light (∼465 nm) (23). The apparent rate constant of this reaction is ∼105 m–1 s–1. Light emission was detected and quantified using a Fluoroskan-FL Ascent type 374 microplate luminometer (Labsystems) with opaque (white) 96-well plates. The photomultiplier was set to default with an integration time of 1000 ms. The MCLA signal was quantified as an integral of 20 s of continuous measurement and expressed as relative luminescence units/mg of mitochondrial protein. The reaction was conducted in 100 μl of reaction buffer containing ∼0.5 mg/ml mitochondrial protein. The reaction buffer contained 125 mm KCl, 10 mm HEPES, 5 mm MgCl2, 2 mm K2HPO4, and 5 μm MCLA. Xanthine/xanthine oxidase system was used as a positive control.
Aconitase Activity—Production of superoxide was also measured indirectly by the assay of aconitase activity. Aconitase catalyzes the reversible isomerization of citrate to isocitrate. In most tissues, aconitase is usually present in both the mitochondrial matrix and the cytoplasm. However, in skeletal muscle, only mitochondrial matrix aconitase is present. Aconitase activity was assayed (in Triton-X-100-treated samples) by measuring NADP+ reduction by citrate in the presence of isocitrate dehydrogenase using a fluorometric method (excitation at 355 nm and emission at 460 nm). Skeletal muscle homogenates (∼1.0 mg of protein/ml) were aliquoted in 96-well plates (100 μl of pH 7.44, 125 mm KCl, 10 mm HEPES, 5 mm MgCl2, 2 mm K2HPO4) and incubated at 30 °C up to 40 min. Incubation was stopped, and aconitase activity measurements were begun by the addition of 1 volume (100 μl) of 50 mm Tris, 0.6 mm MnCl2, 60 mm citrate, 0.2% Triton X-100, 100 μm NADP+, and 1 unit of isocitrate dehydrogenase (Sigma). Fluorometric measurements were then started immediately (Fluoroskan-FL Ascent type 374 microplate reader). The “blank” to measure aconitase-independent NADP+ reduction consisted of the same buffer minus the isocitrate dehydrogenase. The slope of the increase in NADPH fluorescence was taken as the amount of aconitase activity.
Effect of Inhibitors on ROS Production—For the cPLA2 and calcium-independent phospholipase A2 (iPLA2) inhibitor studies, 20 μm arachidonyl trifluoromethyl ketone (AACOCF3) or bromoenol lactone were added to the 600 × g muscle homogenate during the mitochondrial isolation process.
Effect of Hydroperoxides on Amplex Red Signal—Fatty acid hydroperoxide, 15(S)-hydroperoxy-(E,Z,Z,Z)-5,8,11,13-eicosatetraenoic acid (15HpETE), tert-butyl hydroperoxide (t-BHP) or H2O2 (0–2500 pmol) were incubated with Amplex Red in the absence of mitochondria and substrates/inhibitors, and end point measurements were made.
Western Blot—Skeletal muscle homogenates were prepared in radioimmune precipitation assay buffer (50 mm Tris-HCl buffer with 150 mm NaCl, 1% Nonidet P-40, 0.25% sodium deoxycholate and 1× protease inhibitor). Equivalent amounts of protein (120 μg) for each sample were resolved in 4–20% Tris-HCl SDS-PAGE gels in triplicate. After electrophoresis, the proteins were transferred to polyvinylidene difluoride membrane. The membranes were incubated in Tris-buffered saline, pH 7.4, with 0.05% Tween 20 (TBS-T) containing 10% nonfat milk for 1 h at room temperature. The blots were reacted with mouse cPLA2 (1:100) and actin (1:2000; Santa Cruz Biotechnology, Inc) antibodies at 4 °C overnight. After washing with TBS-T, they were incubated with either goat anti-rabbit IgG horseradish peroxidase or goat anti-mouse IgG-horseradish peroxidase (1:1000; Sigma) for 2 h at room temperature. The blots were washed four or five times with TBS-T, and the bands were visualized using chemiluminescent detection reagents from Amersham Biosciences.
Measurement of Hydroperoxides by Xylenol-Orange Reagent—Hydroperoxides were measured using the xylenol orange oxidation protocol detailed by Hermes-Lima et al. (24). In an acidic environment, Fe2+ oxidizes to Fe3+ with reduction of hydroperoxides. Fe3+ reacts with xylenol orange forming a purple complex absorbance, which is recorded at 580 nm. In brief, 250 μl of FeSO4 (1 mm), 100 μl of 0.25 m H2SO4, 200 μl of xylenol orange (1 mm), and 400 μl of distilled H2O were pipetted in that sequence into a cuvette. The reaction was commenced with the addition of 50 μl of mitochondrial supernatant. After 3 h of incubation, the absorbance was measured spectrophotometrically at 580 nm. A standard curve was prepared with t-BHP to quantify the amount of hydroperoxides released.
RESULTS
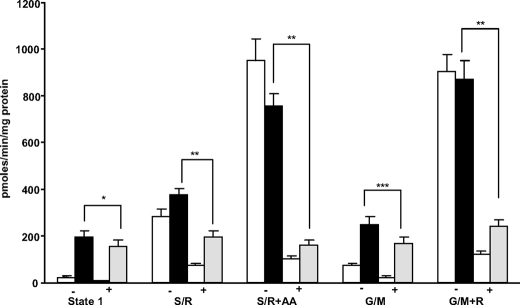
Catalase Only Partially Inhibits the Amplex Red Signal—We performed a series of experiments to determine the nature of radical species released by muscle mitochondria following denervation. We previously reported that 7 days post-denervation, State 1 ROS production measured by Amplex Red is 20–30-fold higher in mitochondria isolated from denervated muscle compared with mitochondria isolated from the contralateral intact muscle (19). We measured mitochondrial ROS production in the presence/absence of catalase to determine whether the signal in response to Amplex Red can be attributed entirely to H2O2 or something in addition to H2O2. As shown in Fig. 1, catalase inhibited the Amplex Red signal ∼65, ∼71, and ∼75% in control mitochondria when respiring in the absence of respiratory substrates (State 1), and the presence of glutamate/malate and succinate/rotenone. Catalase inhibited the Amplex Red signal in mitochondria isolated from denervated muscle by ∼20% (State 1), by ∼33% (glutamate/malate), and by ∼48% in mitochondria respiring on succinate/rotenone. In contrast, catalase inhibited the Amplex Red signal by ∼75–80% in the presence of respiratory inhibitors, antimycin A, and rotenone, conditions that dramatically increase the production of superoxide and H2O2 by the mitochondrial electron transport chain. These results suggested that H2O2 only marginally contributes to the Amplex Red signal, post-denervation surgery.
FIGURE 1.
CAT partially inhibits the Amplex Red signal in mitochondria from denervated muscle. The Amplex Red assay was performed in the absence/presence of catalase and/or respiratory substrates (glutamate/malate (G/M), 5 mm; succinate (S), 10 mm) and inhibitors (rotenone (R), antimycin A (AA), 0.5 μm). Catalase significantly inhibited but did not completely remove the Amplex Red signal in mitochondria isolated from denervated muscle in the absence (State 1) and in the presence of respiratory substrates (glutamate/malate and succinate/rotenone). Statistical significance was based on the difference in Amplex Red signal in the absence (–) and presence (+) of catalase in mitochondria isolated from denervated muscle within each experimental condition (*, p < 0.001; **, p < 0.05; ***, p < 0.01) by one-way ANOVA with Newman Keul's multiple comparison test. The results are the means ± S.E. of 13–14 experiments (control, white bars; denervated (–catalase), black bars; denervated (+catalase), gray bars).
No Difference in Superoxide Production between Mitochondria Isolated from Control and Denervated Muscle—In our assay system, the Amplex Red signal represents both H2O2 and superoxide anion. In the absence of a superoxide trapping agent, either exogenous SOD or spontaneous dismutation readily converts all of the superoxide that is released from the mitochondria to H2O2 to be detected by the Amplex Red probe. To determine the relative contribution of superoxide generation to the ROS produced in response to denervation, we measured superoxide radical production in mitochondria from control and denervated muscle by three independent methods.
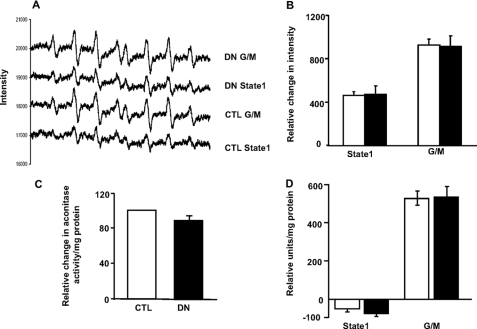
First, we measured superoxide release from mitochondria directly using EPR spectroscopy. EPR is the only technique that can directly detect superoxide radical, but because it occurs in such low abundance, it is only detectable using spin traps. We used the spin trap, DIPPMPO to measure extramitochondrial superoxide release in absence/presence of respiratory substrate in muscle mitochondria. As shown in Fig. 2A, superoxide signal was detectable under State 1 conditions and increased considerably in the presence of complex I-linked substrate, glutamate/malate. However, as Fig. 2B indicates, there was no difference between the superoxide signal in mitochondria isolated from the control and denervated muscle. The addition of exogenous SOD completely removed the superoxide signal (data not shown). We also used aconitase activity inhibition assay as an indirect measure of superoxide released into the mitochondrial matrix in homogenates from control and denervated muscle (Fig. 2C). Our assay did not show any difference in aconitase activity between homogenates from control and denervated muscle. The data suggests that superoxide production by the electron transport chain may not be the primary source of the signal detected by Amplex Red in mitochondria isolated from denervated muscle. To further assess the species of ROS released post-denervation, we used MCLA, a chemiluminescent probe that is highly selective for superoxide and is widely used for its detection in mitochondria (25). Fig. 2D shows superoxide production under State 1 conditions and in the presence of respiratory substrate glutamate/malate. The assay was unable to detect superoxide in mitochondria from denervated muscle in the absence of respiratory substrates, but a significant increase in the MCLA signal was observed with glutamate/malate. However, there was no difference in the MCLA signal between mitochondria isolated from control and denervated muscle. The results from these three independent measurements indicated that electron transport chaingenerated superoxide does not contribute to the increase in Amplex Red signal measured in mitochondria from denervated muscle.
FIGURE 2.
A and B, the superoxide signal was unaltered in mitochondria from control and denervated muscle by EPR. A shows the EPR traces of DIPPMPO-measured superoxide release from skeletal muscle mitochondria in the absence (State 1) and presence of respiratory substrate, glutamate/malate (G/M) (5 mm). Each trace shows the average of four independent animals, with 10 scans performed for each. The quantified data are presented (n = 4–5, mean ± S.E.) in Fig. 2B (control (CTL), white bar; denervated (DN), black bars). C, aconitase activity does not change with denervation. Muscle homogenate (0.5 mg of protein/ml) was incubated with buffers (with or without isocitrate dehydrogenase), and aconitase activity was measured at 355-nm excitation and 460-nm emission. The difference in fluorescence reading in the presence/absence of isocitrate dehydrogenase was taken as a measure of aconitase activity. The results are expressed as the means ± S.E. of 4–5 experiments (control, white bar; denervated, black bar). D, no difference in superoxide release in mitochondria from control and denervated muscles by the MCLA probe. Mitochondria isolated from control and denervated muscles were incubated in the presence of MCLA, and fluorescence was measured at ∼465 nm. The MCLA signal was undetectable in mitochondria isolated from control and denervated muscle in the absence of respiratory substrates (State 1). No difference in the MCLA signal was detected in the presence of respiratory substrate, glutamate/malate. The results are expressed as the means ± S.E. of nine experiments (control, white bars; denervated, black bars).
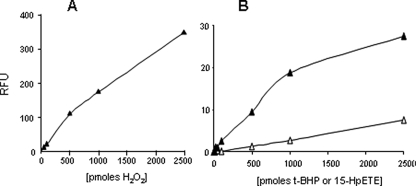
Amplex Red Reacts with Other Hydroperoxides in Addition to H2O2— Our results using the Amplex Red probe in the presence/absence of catalase and the assays of superoxide production suggested the release of species other than H2O2 and/or superoxide from mitochondria isolated from denervated muscle. This led us to consider the possibility that the Amplex Red probe may not be specific for H2O2. To determine whether the –OOH moiety in other molecules may react with Amplex Red, we performed the Amplex Red assay with H2O2 and other molecules that have –OOH in their structure, hydroperoxides, t-BHP, and 15HpETE. We performed the assays in the absence of mitochondria to measure the specificity of the Amplex Red probe in vitro. As we anticipated, H2O2 gives a strong signal with the Amplex Red probe (Fig. 3A). Interestingly, 15HpETE and t-BHP also reacted with the Amplex Red probe, albeit with dose-response intensities lower than that with H2O2 (Fig. 3B). These data suggest that Amplex Red can also react with organic hydroperoxides/fatty acid hydroperoxides in addition to H2O2.
FIGURE 3.
Amplex Red probe reacts with organic/fatty acid hydroperoxides besides hydrogen peroxide. Fig. 3 shows the dose response (0–2,500 pmol) when hydrogen peroxide (A) and the hydroperoxides, t-BHP (open triangle) and 15HpETE (closed triangle) were incubated with the Amplex Red probe (B), and end point measurements were made. Both 15HpETE and t-BHP increased the Amplex Red signal in a dose-dependent manner but at levels much lower than with H2O2.
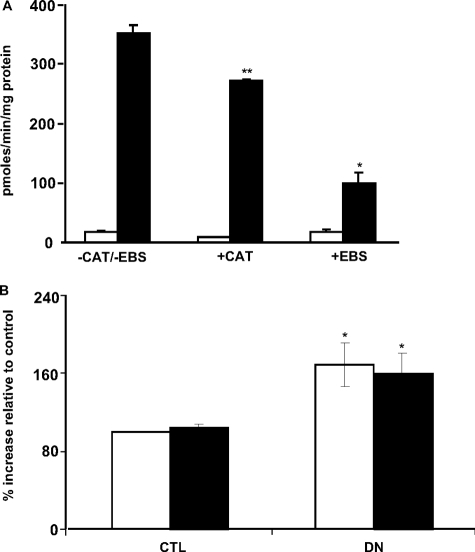
Ebselen Inhibits the Amplex Red Signal in Mitochondria from Denervated Muscle—Ebselen is a synthetic seleno-organic compound that has been widely studied for its antioxidant properties. Ebselen is capable of scavenging H2O2 and hydroperoxides, in particular, membrane-bound phospholipid and cholesterylester hydroperoxides (26, 27). Therefore, we performed the Amplex Red assay in the presence of ebselen in mitochondria from denervated muscle and compared it with the effect of catalase. Although ebselen decreased State 1 Amplex Red signal by ∼70–75% in mitochondria from denervated muscle (Fig. 4A), catalase only decreased it by ∼22–25%. These data further support the idea that mitochondria from denervated muscle may release fatty acid hydroperoxides in addition to H2O2.
FIGURE 4.
A, ebselen inhibits mitochondrial State 1 Amplex Red signal in mitochondria from denervated muscle. Amplex Red signal was measured in mitochondria from control (CTL) and denervated (DN) muscle in the presence of 10 μm ebselen (EBS) or 4 units of catalase. The values are the means ± S.E. for three experiments. Statistical significance was based on the difference in Amplex Red signal in mitochondria from denervated muscle between –CAT/–EBS and +EBS (*, p < 0.0001) and between –CAT/–EBS and +CAT (**, p < 0.05) by Student's t test. (control, white bars; denervated, black bars). B, denervation induces release of hydroperoxides from muscle mitochondria. Mitochondria were isolated from control and denervated muscles, and release of hydroperoxides was measured with xylenol-orange dye under acidic conditions in the presence/absence of catalase. The values are the means ± S.E. for three experiments. Statistical significance was based on the difference in absorbance in mitochondria from control and denervated muscle (with or without catalase) (*, p < 0.05) by one-way ANOVA with Newman Keul's multiple comparison test (–catalase, white bars; +catalase, black bars).
Increased Release of Hydroperoxides from Mitochondria Isolated from Denervated Muscle—To confirm the release of fatty acid hydroperoxides by mitochondria isolated from denervated muscle, we utilized the xylenol-orange oxidation method. Because xylenol-orange can also react with H2O2 (but with much less reactivity than other hydroperoxides), we performed the assay in the presence/absence of catalase. In the absence of catalase, mitochondria from denervated muscle released ∼66% more hydroperoxides compared with control mitochondria (Fig. 4B). The presence of catalase minimally decreased the hydroperoxide signal (∼ 6%), suggesting that fatty acid hydroperoxides are the main radical species released from muscle mitochondria in response to denervation.
AACOCF3 Decreases the Amplex Red Signal in Mitochondria from Denervated Muscle—We next asked whether the Amplex Red signal in mitochondria from denervated muscle might be a consequence of activated PLA2 causing the release of fatty acid hydroperoxides. To address this possibility, we tested the effect of AACOCF3, a highly selective cPLA2 inhibitor, and bromoenol lactone, a iPLA2 inhibitor on the Amplex Red signal in mitochondria isolated from denervated muscle. PLA2 catalyzes the first step in the inflammatory pathway leading to production of pro-inflammatory prostaglandins and leukotrienes. The addition of AACOCF3 in the homogenate decreased the Amplex Red signal in mitochondria from denervated muscle to control values in the absence of substrates and when respiring with glutamate/malate and succinate/rotenone. However, in the presence of complex I and III inhibitors rotenone and antimycin A, where there is a dramatic increase in superoxide/H2O2 generation, AACOCF3 only marginally decreased the Amplex Red signal. Thus, the effect of AACOCF3 is specific in that it does not inhibit mitochondrial H2O2 production (Fig. 5A). Bromoenol lactone had no effect on the Amplex Red signal (data not shown). These results indicate that cPLA2 is a key mediator of the high State 1 Amplex Red signal that occurs in response to denervation, most likely by preventing the release of fatty acid hydroperoxides from lipid hydroperoxides in the bilayer. We also tested the effect of AACOCF3 in additional mouse models of muscle atrophy associated with the loss of innervation, namely, Sod1–/– and G93A ALS mutant mice (28, 29). We previously reported that mitochondrial ROS production is significantly elevated in these mice (12). As shown in Fig. 5B, the addition of AACOCF3 to the muscle homogenate decreased mitochondrial State 1 Amplex Red signal to control levels in Sod1–/– mice, and we found a similar effect in mitochondria isolated from G93A mice (data not shown). These data suggest that the elevation of cPLA2 is potentially a common phenomenon in mice that exhibit muscle atrophy associated with a loss of innervation.
FIGURE 5.
A, cPLA2 inhibitor inhibits the Amplex Red signal in mitochondria from denervated muscle. The Amplex Red assay was performed in the absence/presence of the cPLA2 inhibitor, AACOCF3 (20 μm) with substrates (glutamate/malate (G/M), 5 mm; succinate (S), 10 mm) and inhibitors (rotenone (R), antimycin A (AA), 0.5 μm). AACOCF3 inhibited the Amplex Red signal in mitochondria from denervated muscle to control values in the absence (State 1) and in the presence of respiratory substrates (glutamate/malate and succinate/rotenone). The values are the means ± S.E. for six or seven experiments. Statistical significance was based on the difference in Amplex Red signal in the absence (–) and presence (+) of AACOCF3 within each experimental condition (*, p < 0.001; **, p < 0.01) by one-way ANOVA with Newman Keul's multiple comparison test. (control, white bars; denervated (–AACOCF3), black bars; denervated (+AACOCF3), gray bars). B, cPLA2 inhibitor inhibits the Amplex Red signal in mitochondria from Sod1–/– mice. The Amplex Red assay was performed in the presence/absence of AACOCF3 (20μm) in 12-month-old Sod1–/– mice. AACOCF3 inhibited the Amplex Red signal (State 1) to control values. The values are the means ± S.E. for three or four experiments. Statistical significance was based on the difference in Amplex Red signal in the absence (–) and presence (+) of AACOCF3 in Sod1–/– (*, p < 0.001) by one-way ANOVA with Newman Keul's multiple comparison test (wild type, white bars; Sod1–/–, black bars).
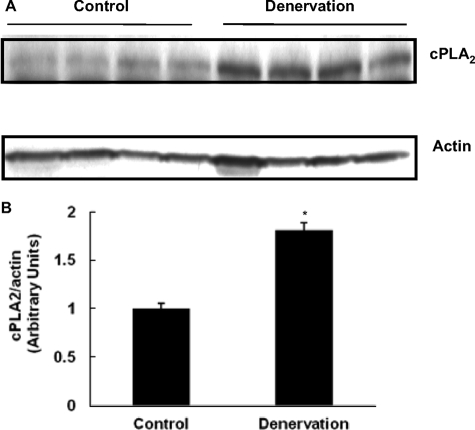
cPLA2 Protein Levels Are Higher in Muscle Following Denervation—To determine whether cPLA2 is up-regulated in muscle following denervation, we measured cPLA2 protein in homogenates from control and denervated muscles using an antibody specific for cPLA2. As shown in Fig. 6A, cPLA2 protein levels are increased ∼2-fold in muscle homogenates 7 days post-denervation (Fig. 6B). cPLA2 protein levels are also elevated in Sod1–/– and G93A mice (data not shown).
FIGURE 6.
cPLA2 protein expression is elevated in denervated muscle. Control and denervated muscles were homogenized in radioimmune precipitation assay buffer with Triton X-100 and 1× protease inhibitor. Equivalent amounts of protein (120 μg) were resolved by gel electrophoresis and transferred to polyvinylidene difluoride membrane, and cPLA2 was identified by using mouse monoclonal antibody with actin as a loading control. *, p < 0.001 versus control by unpaired t test.
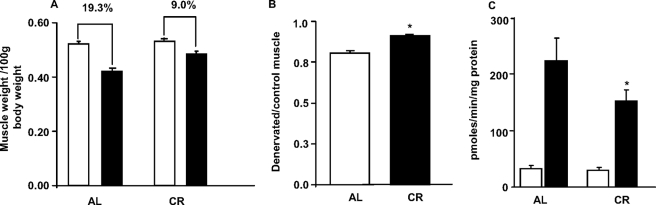
Inhibition of Muscle Atrophy and Decrease in Amplex Red Signal in CR Denervated Mice—Because caloric restriction can prevent oxidative stress in general and can specifically decrease fatty acid hydroperoxides (30), we next performed sciatic nerve transection in 40% CR mice (fed 60% of food consumed by ad libitum fed mice) and measured muscle mass and the Amplex Red signal. As shown in Fig. 7A, denervation-induced loss of gastrocnemius muscle was decreased in CR mice (∼9.0%) compared with AL mice (∼19.3%). Denervated muscle mass/control muscle mass ratio was significantly higher in CR mice (Fig. 7B). We also observed a ∼30% decrease in Amplex Red signal in mitochondria isolated from denervated muscle of CR mice compared with mitochondria from denervated muscle of AL mice, under State 1 conditions (Fig. 7C). We propose that caloric restriction may decrease denervation-induced muscle atrophy by inhibiting the mitochondrial release of fatty acid hydroperoxides.
FIGURE 7.
Inhibition of muscle atrophy and decrease in Amplex Red signal in CR mice in response to denervation. The mice were sacrificed, the tissues were collected, and Amplex Red signal was measured 7 days after surgery in mitochondria from control and denervated muscle from AL and CR mice (State 1). A, loss of gastrocnemius muscle mass/100g body weight in AL and CR mice in response to denervation. B, denervated/control muscle ratio in AL and CR mice (*, p < 0.000001) by Student's t test. C, Amplex Red signal in muscle mitochondria from AL and CR mice in response to denervation surgery. Statistical significance was based on the difference in Amplex Red signal in mitochondria from AL and CR denervated muscle (*, p < 0.05) by one-way ANOVA with Newman Keul's multiple comparison test. The values are expressed as the means ± S.E. of n = 15–16 mice (muscle mass) and n = 8 (ROS). A and C, control, white bars; denervated, black bars. B, AL, white bar; CR, black bar.
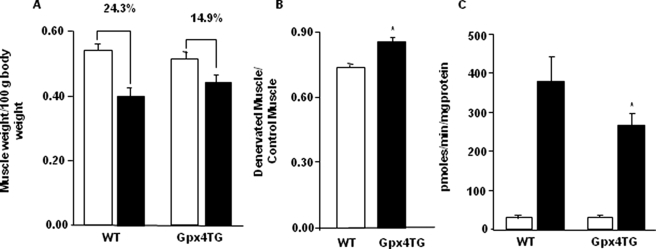
Inhibition of Muscle Atrophy and Decrease in Amplex Red Signal in Gpx4-Tg Mice after Denervation.—To further address the role of fatty phy, we performed sciatic nerve transection in GPx4-Tg mice that overexpress phospholipid hydroperoxide glutathione peroxidase (Gpx4), an enzyme that detoxifies lipid hydroperoxides, converting them to lipid hydroxides. The enzyme Gpx4 is present in all cell types and is associated with membranes, including mitochondrial membranes. As shown in Fig. 8A, denervation-induced loss of gastrocnemius muscle mass was decreased in Gpx4-Tg mice (∼14.9%) compared with wild type mice (∼24.3%). When the data were expressed as denervated muscle mass/control muscle mass, the ratio was significantly higher in Gpx4-Tg mice (Fig. 8B). These data suggest that fatty acid hydroperoxides, in part, may contribute to denervation-induced loss of muscle mass.
FIGURE 8.
Inhibition of muscle atrophy and decrease in Amplex Red signal in Gpx4-Tg mice in response to denervation. Denervation was induced by surgical transection of the sciatic nerve at the level of femur. The contralateral limb served as a control. The mice were sacrificed, tissues were collected, and Amplex Red signal was measured 7 days after surgery in mitochondria isolated from control and denervated muscle from WT and Gpx4-Tg mice (State 1). A, loss of gastrocnemius muscle mass/100 g of body weight in WT and Gpx4Tg mice in response to denervation. B, denervated/control muscle ratio in WT and Gpx4-Tg mice (*, p = 0.0001) by Student's t test. C, Amplex Red signal in mitochondria from WT and Gpx4-Tg mice in response to denervation surgery. Statistical significance was based on the difference in Amplex Red signal in mitochondria from WT and Gpx4-Tg denervated muscle (*, p < 0.05) by one-way ANOVA with Newman Keul's multiple comparison test. The values are expressed as the means ± S.E. of n = 8 mice. A and C, control, white bars; denervated, black bars. B, WT, white bar; Gpx4-Tg, black bar.
We speculated that if the Amplex Red signal in mitochondria isolated from denervated muscle comes partly from fatty acid hydroperoxides, conversion of hydroperoxides to hydroxides in Gpx4-Tg mice would inhibit the Amplex Red signal. Our data showed a ∼30% decline in Amplex Red signal in Gpx4-Tg mitochondria from denervated muscle compared with mitochondria from control muscle, under State 1 conditions (Fig. 8C). This result further indicates that in addition to H2O2, fatty acid hydroperoxides may contribute to the Amplex Red signal in mitochondria isolated from denervated muscle.
No Inhibition of Muscle Atrophy in CAT Transgenic (CAT-Tg) Mice despite a Decrease in Amplex Red Signal—Our results to this point suggested that mitochondrial fatty acid hydroperoxides may be involved in denervation-induced muscle atrophy. To confirm our findings, we performed sciatic nerve transection in mice that overexpress manganese superoxide dismutase (Sod2-Tg) or copper-zinc superoxide dismutase (Sod1-Tg), antioxidant enzymes that convert superoxide anion to H2O2 and in mice that overexpress catalase (CAT-Tg). As shown in Table 1, there was no difference in Amplex Red signal between WT and Sod2-Tg and Sod1-Tg mice, 7 days post-denervation. In contrast, Amplex Red signal was decreased ∼22% (State 1) and ∼25% (glutamate/malate) in mitochondria isolated from muscle of denervated CAT-Tg mice. However, denervation-dependent muscle atrophy was not attenuated in any of these transgenic mouse models (data not shown). This experiment suggests that mitochondrial release of fatty acid hydroperoxides rather than H2O2 and superoxide may be an important determinant of denervation-induced muscle atrophy.
TABLE 1.
Inhibition of mitochondrial ROS production in CAT Tg mice
|
Control
|
Denervation
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Wild type | SOD1 Tg | SOD2 Tg | CAT Tg | Wild type | SOD1 Tg | SOD2 Tg | CAT Tg | |
| State 1 | 9.76 ± 2.19 | 6.13 ± 1.14 | 10.96 ± 1.56 | 8.56 ± 1.24 | 307.50 ± 8.30 | 291.92 ± 64.48 | 311.47 ± 50.46 | 239.81 ± 5.76a |
| G/M | 30.43 ± 8.46 | 16.47 ± 4.93 | 38.45 ± 7.46 | 41.05 ± 13.04 | 323.50 ± 13.21 | 298.67 ± 64.13 | 356.74 ± 4.88 | 243.40 ± 13.00a |
p < 0.001 versus wild type denervation by one-way ANOVA with Newman Keul's multiple comparison test. The values are expressed as the means ± S.E. of n = 3-6 mice. The units are pmol/min/mg of protein.
DISCUSSION
The mitochondrial electron transport chain is believed to be a major source of cellular oxidative stress (31, 32). Previous studies have reported that mitochondrial ROS production (measured as H2O2) is significantly elevated in the atrophied muscles (11, 12, 18). However, the present study suggests that atrophied skeletal muscle mitochondria in fact releases significantly high levels of fatty acid hydroperoxides, in addition to H2O2 and/or superoxide. The study further suggests that augmenting the defenses against lipid hydroperoxides, but not H2O2 and superoxide, may attenuate denervation-induced muscle atrophy.
The Amplex Red signal we detect in isolated mitochondria is due to both H2O2 and superoxide converted to H2O2 caused by spontaneous dismutation (by Manganese SOD in the matrix and by CuZnSOD in the intermembrane space) and the addition of exogenous SOD to the assay buffer. In the present study, the Amplex Red assay executed in the absence/presence of catalase showed that H2O2 is not the major active oxygen species released by mitochondria from denervation-induced muscle atrophy. Interestingly, superoxide radical generation was not elevated in mitochondria from denervated muscle despite a 30-fold higher Amplex Red signal. We found that the Amplex Red probe can react with organic hydroperoxides (t-BHP and 15HpETE) in addition to H2O2 in the absence of mitochondria. We noted that Belikova et al. (33) used the Amplex Red probe to measure alkyl hydroperoxides in intact cells and mitochondria using a high pressure liquid chromatography-based assay. These authors found that Amplex Red would not react with lipid hydroperoxides in the bilayer but did so readily once the lipid hydroperoxides had been hydrolyzed to fatty acid/alkyl hydroperoxides by PLA2. Ebselen, a glutathione peroxidase mimetic, inhibited the Amplex Red signal in mitochondria from denervated muscle. Ebselen differs from other antioxidants in that it is a weak free radical scavenger but very effective in scavenging fatty acid hydroperoxides (26, 34). The xylenol orange assay also confirmed the fact that mitochondria from denervated muscle release fatty acid hydroperoxides.
We mentioned above that the Amplex Red probe cannot react with lipid hydroperoxides but only with fatty acid hydroperoxides after cleavage by PLA2. Therefore, we looked at PLA2 as a potential source of these hydroperoxides in our mouse model. PLA2 belongs to a superfamily of enzymes that catalyze the hydrolysis of ester bonds on membrane phospholipids to generate free fatty acids and lysophospholipids. Mammalian PLAs are classified into three main categories; whereas calcium-dependent cPLA2 and calcium-independent iPLA2 are present intracellularly and have preference for AA at sn-2 position of membrane phospholipids, sPLA2 is extracellular in distribution and lacks specificity for arachidonic acid over other fatty acids at the sn-2 position. Based on protein expression and inhibitor studies, we propose that cPLA2 may release fatty acid hydroperoxides from membranes following denervation. In contrast, the iPLA2 inhibitor bromoenol lactone did not inhibit the Amplex Red signal (data not shown). This is an important finding considering that two of the major iPLA2 isoforms (iPLA2β and iPLA2γ) are located within the mitochondria (35, 36).
cPLA2 exists in different isoforms: α, β, and γ. Several studies have demonstrated the involvement of cPLA2 in diverse pathological processes including neurodegenerative diseases. cPLA2α–/– mice are resistant to ischemia-reperfusion induced oxidative stress (37–39). cPLA2α–/– mice are resistant to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine toxicity (40). Increased cPLA2 immunoreactive astrocytes are detected in Alzheimer disease patients in regions that a have higher deposition of β-amyloid (41), and β-amyloid stimulates cPLA2 activity in cortical neurons thereby inhibiting neuronal apoptosis (42). Cytosolic PLA2 protein expression is also elevated in the spinal cord of the G93A mouse model of ALS (43). Finally, cPLA2α–/– mice are known to have increased striated muscle growth (44). This study is the first to suggest a correlation between cPLA2 and muscle atrophy associated with loss of innervation. Our future studies would be designed to identify the role of different cPLA2 isoforms in muscle atrophy using specific inhibitors and knock-out mouse models.
Among the different forms of Gpx present in mammalian systems, Gpx4 is unique in that it uses phospholipid hydroperoxides as substrates, thereby protecting biomembranes against oxidative damage (45, 46). Overexpression of Gpx4 in cell lines has been extensively used to exhibit the protective effects of Gpx4 against hydroperoxides (47, 48). Cells that overexpress Gpx4 in mitochondria are more resistant to cell death against extracellular oxidative stressors such as t-BHP, 15HpETE, and H2O2 compared with cells that overexpress Gpx4 in nonmitochondrial compartments (49). These findings suggest that mitochondrial Gpx4 plays an important role in protecting cells from hydroperoxide induced oxidative stress. Studies in GPx4-Tg mice also show the protective effect of Gpx4 against oxidative stress (50). We addressed the pathophysiological relevance of lipid hydroperoxides in muscle atrophy by comparing mouse models that overexpress the major antioxidant enzymes. Increasing protection specifically against lipid hydroperoxides (Gpx4-Tg) significantly decreased sciatic nerve transection-induced muscle atrophy. In contrast, transgenic mice that protect against superoxide (Sod1-Tg and Sod2-Tg) or H2O2 (CAT-Tg) did not afford any protection.
Based on the existing data, it is not clear whether it is the presence of lipid hydroperoxides in mitochondrial membranes or the cleavage of fatty acid hydroperoxides from membranes that is a critical factor in denervation-induced muscle atrophy. We noted that Gpx4 heterozygous knockout mice (50% reduction of Gpx4 expression) have higher losses of gastrocnemius muscle mass and higher Amplex Red signal compared with wild type mice, 14 days post denervation surgery (data not shown). These data together with the data from Gpx4-Tg mice suggest the possibility that mitochondrial generation of fatty acid hydroperoxides could be an important determinant of muscle atrophy associated with the loss of innervation.
The detailed mechanism involved in the protective effect of GPx4 on muscle atrophy is beyond the scope of this study, but the inhibition of mitochondrial apoptotic pathway could be one of the likely mechanisms (51). Innervation is critical for growth and maintenance of muscle fibers, and loss of innervation is known to cause muscle atrophy (52). Previous studies have indicated that mitochondrial ROS and apoptosis play important roles in muscle atrophy during aging and surgical denervation (11, 12, 18). Increases in pro-apoptotic factors, cytochrome c, Smac/DIABLO, and apoptosis-inducing factor and an increase in the Bax/Bcl-2 ratio have been reported in denervated muscles (11, 18, 53). We have previously shown that GPx4-Tg mice inhibit apoptosis (by inhibiting cytochrome c and caspase-3) in the presence of stressors like diquat, t-BHP, and H2O2 (50, 54). Thus, we propose that the decrease in mitochondrial apoptosis in Gpx4-Tg mice may prevent denervation-induced muscle atrophy.
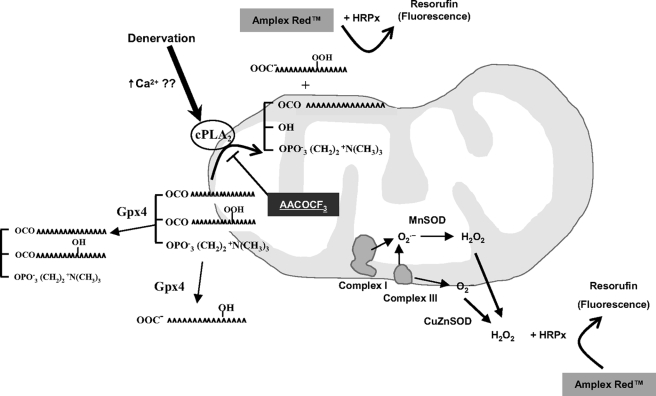
In conclusion, the dramatic increase in Amplex Red signal in denervation associated muscle atrophy may be attributed to fatty acid hydroperoxides rather than H2O2/superoxide (Fig. 9). We propose that cPLA2 could be a likely source of hydroperoxides. These organic hydroperoxides are likely of pathophysiological significance because Gpx4-Tg mice are protected from muscle atrophy. Our future studies would further address the roles of cPLA2 and fatty acid hydroperoxides in muscle atrophy using knock-out mouse models and therapeutic interventions (e.g. vitamin E).
FIGURE 9.
Muscle mitochondria release fatty acid hydroperoxides in response to denervation. Denervation induced by sciatic nerve transection is associated with dramatic release of fatty acid hydroperoxides from skeletal muscle mitochondria. We propose cPLA2 as a source for these hydroperoxides because cPLA2 expression is increased in denervated muscle and the cPLA2 inhibitor; AACOCF3 inhibits the Amplex Red signal in mitochondria from denervated muscle. Gpx4 reduces fatty acid hydroperoxides to hydroxides, thereby decreasing the Amplex Red signal in Gpx4-Tg mice.
Acknowledgments
We thank Corinne Price for editing the manuscript. We also thank Dr. Asish Chaudhuri (Barshop Institute for Longevity and Aging Studies, University of Texas Health Science Center at San Antonio) for many helpful discussions.
This work was supported, in whole or in part, by National Institutes of Health Grant AG20591 (to H. V. R.). This work was also supported by Muscular Dystrophy Association Grant MDA 3879 (to H. V. R.) and a Veterans Affairs merit grant (to H. V. R.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: ALS, amyotrophic lateral sclerosis; cPLA2, cytosolic phospholipase A2; AACOCF3, arachidonyl trifluoromethyl ketone; CAT, catalase; MCLA, 2-methyl-6-(p-methoxyphenyl)-3,7-dihydroimidazo[1,2-α]-pyrazin-3-one; ROS, reactive oxygen species; AL, ad libitum; CR, calorie-restricted; DIPPMPO, 5-diisopropoxyphosphoryl)-5-methyl-1-pyrroline-N-oxide; MOPS, 4-morpholinepropanesulfonic acid; t-BHP, tert-butyl hydroperoxide; 15HpETE, 15(S)-hydroperoxy-(E,Z,Z,Z)-5,8,11,13-eicosatetraenoic acid; ANOVA, analysis of variance; WT, wild type; iPLA2, calcium-independent phospholipase A2.
References
- 1.Karakelides, H., and Nair, K. S. (2005) Curr. Top Dev. Biol. 68 123–148 [DOI] [PubMed] [Google Scholar]
- 2.Greenlund, L. J., and Nair, K. S. (2003) Mech. Ageing Dev. 124 287–299 [DOI] [PubMed] [Google Scholar]
- 3.Doherty, T. J., Vandervoort, A. A., Taylor, A. W., and Brown, W. F. (1993) J. Appl. Physiol. 74 868–874 [DOI] [PubMed] [Google Scholar]
- 4.Thomason, D. B., and Booth, F. W. (1990) J. Appl. Physiol. 68 1–12 [DOI] [PubMed] [Google Scholar]
- 5.Delbono, O. (2003) Aging Cell 2 21–29 [DOI] [PubMed] [Google Scholar]
- 6.Brown, W. F. (1972) J. Neurol. Neurosurg. Psychiatry 35 845–852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown, W. F., and Chan, K. M. (1997) Muscle Nerve. 5 (suppl.) S70–S73 [PubMed] [Google Scholar]
- 8.Brown, R. H., Jr. (1997) Arch. Neurol. 54 1246–1250 [DOI] [PubMed] [Google Scholar]
- 9.Wicks, K. L., and Hood, D. A. (1991) Am. J. Physiol. 260 C841–C850 [DOI] [PubMed] [Google Scholar]
- 10.Eisenberg, H. A., and Hood, D. A. (1994) J. Appl. Physiol. 76 859–866 [DOI] [PubMed] [Google Scholar]
- 11.Adhihetty, P. J., O'Leary, M. F., Chabi, B., Wicks, K. L., and Hood, D. A. (2007) J. Appl. Physiol. 102 1143–1151 [DOI] [PubMed] [Google Scholar]
- 12.Muller, F. L., Song, W., Jang, Y. C., Liu, Y., Sabia, M., Richardson, A., and Van Remmen, H. (2007) Am. J. Physiol. 293 R1159–R1168 [DOI] [PubMed] [Google Scholar]
- 13.Muller, F. L., Song, W., Liu, Y., Chaudhuri, A., Pieke-Dahl, S., Strong, R., Huang, T. T., Epstein, C. J., Roberts, L. J., 2nd, Csete, M., Faulkner, J. A., and Van Remmen, H. (2006) Free Radic. Biol. Med. 40 1993–2004 [DOI] [PubMed] [Google Scholar]
- 14.Fulle, S., Protasi, F., Di Tano, G., Pietrangelo, T., Beltramin, A., Boncompagni, S., Vecchiet, L., and Fano, G. (2004) Exp. Gerontol. 39 17–24 [DOI] [PubMed] [Google Scholar]
- 15.Powers, S. K., Kavazis, A. N., and DeRuisseau, K. C. (2005) Am. J. Physiol. 288 R337–R344 [DOI] [PubMed] [Google Scholar]
- 16.Lass, A., Sohal, B. H., Weindruch, R., Forster, M. J., and Sohal, R. S. (1998) Free Radic. Biol. Med. 25 1089–1097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mahoney, D. J., Kaczor, J. J., Bourgeois, J., Yasuda, N., and Tarnopolsky, M. A. (2006) Muscle Nerve 33 809–816 [DOI] [PubMed] [Google Scholar]
- 18.Chabi, B., Ljubicic, V., Menzies, K. J., Huang, J. H., Saleem, A., and Hood, D. A. (2008) Aging Cell 7 2–12 [DOI] [PubMed] [Google Scholar]
- 19.Mansouri, A., Muller, F. L., Liu, Y., Ng, R., Faulkner, J., Hamilton, M., Richardson, A., Huang, T. T., Epstein, C. J., and Van Remmen, H. (2006) Mech. Ageing Dev. 127 298–306 [DOI] [PubMed] [Google Scholar]
- 20.Zhou, M., Diwu, Z., Panchuk-Voloshina, N., and Haugland, R. P. (1997) Anal. Biochem. 253 162–168 [DOI] [PubMed] [Google Scholar]
- 21.Starkov, A. A., Fiskum, G., Chinopoulos, C., Lorenzo, B. J., Browne, S. E., Patel, M. S., and Beal, M. F. (2004) J. Neurosci. 24 7779–7788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frejaville, C., Karoui, H., Tuccio, B., Le Moigne, F., Culcasi, M., Pietri, S., Lauricella, R., and Tordo, P. (1995) J. Med. Chem. 38 258–265 [DOI] [PubMed] [Google Scholar]
- 23.Nakano, M. (1998) Cell Mol. Neurobiol. 18 565–579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hermes-Lima, M., Willmore, W. G., and Storey, K. B. (1995) Free Radic. Biol. Med. 19 271–280 [DOI] [PubMed] [Google Scholar]
- 25.Muller, F. L., Liu, Y., and Van Remmen, H. (2004) J. Biol. Chem. 279 49064–49073 [DOI] [PubMed] [Google Scholar]
- 26.Maiorino, M., Roveri, A., Coassin, M., and Ursini, F. (1988) Biochem. Pharmacol. 37 2267–2271 [DOI] [PubMed] [Google Scholar]
- 27.Sies, H. (1993) Free Radic. Biol. Med. 14 313–323 [DOI] [PubMed] [Google Scholar]
- 28.Thomas, C. K., and Zijdewind, I. (2006) Muscle Nerve 33 21–41 [DOI] [PubMed] [Google Scholar]
- 29.Flood, D. G., Reaume, A. G., Gruner, J. A., Hoffman, E. K., Hirsch, J. D., Lin, Y. G., Dorfman, K. S., and Scott, R. W. (1999) Am. J. Pathol. 155 663–672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guo, Z., Mitchell-Raymundo, F., Yang, H., Ikeno, Y., Nelson, J., Diaz, V., Richardson, A., and Reddick, R. (2002) Mech. Ageing Dev. 123 1121–1131 [DOI] [PubMed] [Google Scholar]
- 31.Wallace, D. C. (1999) Science 283 1482–1488 [DOI] [PubMed] [Google Scholar]
- 32.Finkel, T., and Holbrook, N. J. (2000) Nature 408 239–247 [DOI] [PubMed] [Google Scholar]
- 33.Belikova, N. A., Jiang, J., Tyurina, Y. Y., Zhao, Q., Epperly, M. W., Greenberger, J., and Kagan, V. E. (2007) Int. J. Radiat. Oncol. Biol. Phys. 69 176–186 [DOI] [PubMed] [Google Scholar]
- 34.Nakamura, Y., Feng, Q., Kumagai, T., Torikai, K., Ohigashi, H., Osawa, T., Noguchi, N., Niki, E., and Uchida, K. (2002) J. Biol. Chem. 277 2687–2694 [DOI] [PubMed] [Google Scholar]
- 35.Mancuso, D. J., Sims, H. F., Han, X., Jenkins, C. M., Guan, S. P., Yang, K., Moon, S. H., Pietka, T., Abumrad, N. A., Schlesinger, P. H., and Gross, R. W. (2007) J. Biol. Chem. 282 34611–34622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Williams, S. D., and Gottlieb, R. A. (2002) Biochem. J. 362 23–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bonventre, J. V., Huang, Z., Taheri, M. R., O'Leary, E., Li, E., Moskowitz, M. A., and Sapirstein, A. (1997) Nature 390 622–625 [DOI] [PubMed] [Google Scholar]
- 38.Engelbrecht, A. M., and Ellis, B. (2007) Prostaglandins Leukot. Essent. Fatty Acids 77 37–43 [DOI] [PubMed] [Google Scholar]
- 39.Gabryel, B., Chalimoniuk, M., Stolecka, A., and Langfort, J. (2007) Cell Biol. Int. 31 958–965 [DOI] [PubMed] [Google Scholar]
- 40.Klivenyi, P., Beal, M. F., Ferrante, R. J., Andreassen, O. A., Wermer, M., Chin, M. R., and Bonventre, J. V. (1998) J. Neurochem. 71 2634–2637 [DOI] [PubMed] [Google Scholar]
- 41.Stephenson, D. T., Lemere, C. A., Selkoe, D. J., and Clemens, J. A. (1996) Neurobiol. Dis. 3 51–63 [DOI] [PubMed] [Google Scholar]
- 42.Kriem, B., Sponne, I., Fifre, A., Malaplate-Armand, C., Lozac'h-Pillot, K., Koziel, V., Yen-Potin, F. T., Bihain, B., Oster, T., Olivier, J. L., and Pillot, T. (2005) FASEB J. 19 85–87 [DOI] [PubMed] [Google Scholar]
- 43.Kiaei, M., Kipiani, K., Petri, S., Choi, D. K., Chen, J., Calingasan, N. Y., and Beal, M. F. (2005) J. Neurochem. 93 403–411 [DOI] [PubMed] [Google Scholar]
- 44.Haq, S., Kilter, H., Michael, A., Tao, J., O'Leary, E., Sun, X. M., Walters, B., Bhattacharya, K., Chen, X., Cui, L., Andreucci, M., Rosenzweig, A., Guerrero, J. L., Patten, R., Liao, R., Molkentin, J., Picard, M., Bonventre, J. V., and Force, T. (2003) Nat. Med. 9 944–951 [DOI] [PubMed] [Google Scholar]
- 45.Girotti, A. W. (1998) J. Lipid Res. 39 1529–1542 [PubMed] [Google Scholar]
- 46.Thomas, J. P., Maiorino, M., Ursini, F., and Girotti, A. W. (1990) J. Biol. Chem. 265 454–461 [PubMed] [Google Scholar]
- 47.Yagi, K., Komura, S., Kojima, H., Sun, Q., Nagata, N., Ohishi, N., and Nishikimi, M. (1996) Biochem. Biophys. Res. Commun. 219 486–491 [DOI] [PubMed] [Google Scholar]
- 48.Brigelius-Flohe, R., Maurer, S., Lotzer, K., Bol, G., Kallionpaa, H., Lehtolainen, P., Viita, H., and Yla-Herttuala, S. (2000) Atherosclerosis 152 307–316 [DOI] [PubMed] [Google Scholar]
- 49.Arai, M., Imai, H., Koumura, T., Yoshida, M., Emoto, K., Umeda, M., Chiba, N., and Nakagawa, Y. (1999) J. Biol. Chem. 274 4924–4933 [DOI] [PubMed] [Google Scholar]
- 50.Ran, Q., Gu, M., Van Remmen, H., Strong, R., Roberts, J. L., and Richardson, A. (2006) J. Neurosci. Res. 84 202–208 [DOI] [PubMed] [Google Scholar]
- 51.Nomura, K., Imai, H., Koumura, T., Arai, M., and Nakagawa, Y. (1999) J. Biol. Chem. 274 29294–29302 [DOI] [PubMed] [Google Scholar]
- 52.Jackman, R. W., and Kandarian, S. C. (2004) Am. J. Physiol. 287 C834–C843 [DOI] [PubMed] [Google Scholar]
- 53.Siu, P. M., and Alway, S. E. (2005) J. Physiol. 565 309–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ran, Q., Liang, H., Gu, M., Qi, W., Walter, C. A., Roberts, L. J., II, Herman, B., Richardson, A., and Van Remmen, H. (2004) J. Biol. Chem. 279 55137–55146 [DOI] [PubMed] [Google Scholar]