Abstract
The cytoplasmic heme-binding protein PhuS, encoded within the Fur-regulated Pseudomonas heme utilization (phu) operon, has previously been shown to traffic heme to the iron-regulated heme oxygenase (HO). We further investigate the role of PhuS in heme trafficking to HO on disruption of the phuS and hemO genes in a Pseudomonas aeruginosa siderophore-deficient and wild-type background. Previous studies have shown that deletion of hemO prevents the cells from utilizing heme as the sole source of iron. However, disruption of phuS alone resulted in a slow growth phenotype, consistent with its role as a heme-trafficking protein to HO. Furthermore, in contrast to the hemO and hemO/phuS deletion strains, the phuS knockout prematurely produced pyocyanin in the presence of heme. Western blot analysis of PhuS protein levels in the wild-type strain showed that Fur-regulation of the phu operon could be derepressed in the presence of heme. In addition the premature onset of pyocyanin production requires both heme and a functional HO. Suppression of the phenotype on increasing the external heme concentration suggested that the decreased heme-flux through HO results in premature production of pyocyanin. The premature production of pyocyanin was not due to lower intracellular iron levels as a result of decreased heme flux through HO. However, transcriptional analysis of the phuS mutants indicates that the cells are sensing iron deprivation. The present data suggest that PhuS has a dual function in trafficking heme to HO, and in directly or indirectly sensing and maintaining iron and heme homeostasis.
Iron is essential for the growth, survival, and virulence of most bacterial pathogens, with only a few exceptions (1–3). However, within the human body, bacteria encounter an extremely low iron milieu, because the majority of iron is sequestered in iron and heme proteins, such as transferrin and hemoglobin, respectively (4). Bacterial pathogens have therefore evolved multiple mechanisms to obtain iron from the iron- and heme-containing proteins of the host. The opportunistic pathogen Pseudomonas aeruginosa, which is responsible for severe nosocomial infections in immunocompromised patients (5, 6), secretes an array of high affinity iron binding siderophores (pyoverdin and pyochelin) and in addition can directly utilize the host iron- and heme-containing proteins (7, 8).
The P. aeruginosa genome encodes two heme acquisition systems: the has (heme assimilation system) and the phu (Pseudomonas heme utilization) operons. The has locus encodes the hemophore HasA, the hemophore receptor HasR, and a potential ABC transporter HasDEF for export of HasA (8, 9). The phu locus consists of six open reading frames encoding the outer membrane heme receptor PhuR, the periplasmic ABC (ATP binding cassette) transport system PhuTUV, PhuW whose function has not been determined, and the cytoplasmic heme-binding protein PhuS, which is essential for optimal heme utilization (8). The cytoplasmic protein PhuS has been shown in vitro to transfer heme to the iron-regulated heme oxygenase, HO2 encoded by the hemO (also referred to as PigA in the literature) (10, 11). The expression of the phu and has operons is regulated by the ferric uptake regulator (Fur) protein, which has both negative and positive regulatory effects on the expression of iron-regulated genes (8, 12, 13). Under iron-replete conditions, Fe2+-bound Fur represses gene expression by directly binding to the Fur box in the promoter regions of iron starvation-inducible genes (including the iron- and heme-uptake genes) and activating gene expression indirectly through a pair of small regulatory RNAs, PrrF1 and PrrF2 (Pseudomonas regulatory RNA involving iron (Fe)) (13–15). The regulation of iron and heme uptake by Fur is essential in maintaining the iron homeostasis, because free iron can catalyze formation of hydroxyl radicals via the Fenton reaction (1, 14). Additionally a recent proteomic analysis has shown that the ability to use hemoglobin as an iron source is quorum sensing (QS) regulated in P. aeruginosa (16).
QS is a cell density-dependent cell-to-cell communication mechanism, which allows bacteria to sense their environment and coordinate expression of various genes within a bacterial population (17–19). The QS network in P. aeruginosa is highly complex and consists of two interlinked N-acyl homoserine lactone-dependent regulatory pathways, which are further modulated by the Pseudomonas quinolone signal (PQS) (20, 21). Furthermore, it has become increasingly evident that there exists a complex relationship between iron, QS, and virulence (22–26). A number of separate studies have shown that iron concentrations independent of cell density modulate expression of genes that are known to be QS-regulated (22). A recent study has shown the iron-regulated small regulatory RNAs PrrF1 and PrrF2 are linked to QS at the level of regulation of anthranilate (a biosynthetic precursor of PQS) metabolism providing further evidence of the physiological link between iron and QS (27). Furthermore it has been proposed that pyocyanin, the terminal QS signal, itself may play a role in iron uptake and in extracellular electron shuttling in biofilm formation (28–30).
In the present report we further address the role of PhuS in heme trafficking to HO. The phuS mutants in P. aeruginosa in the siderophore-deficient IA614 and wild-type MPAO1 strain were observed to prematurely produce pyocyanin in the presence of heme during transition to stationary phase. As PhuS plays a role in iron metabolism via heme trafficking, and pyocyanin has been suggested to play a direct role in iron acquisition (28, 31, 32), it was hypothesized that the premature pyocyanin phenotype is an indication of an imbalance in iron homeostasis on disruption of PhuS. To further investigate the cause of premature pyocyanin production in the phuS mutants and its relevance to iron homeostasis, we undertook a biochemical and global transcriptional profiling approach. The findings presented herein provide important insight into the critical role of heme in maintaining P. aeruginosa iron homeostasis.
EXPERIMENTAL PROCEDURES
Bacterial Strains and Cultures—The P. aeruginosa strains used in this study are listed in Table 1. Luria-Bertani (LB) medium was routinely used for culture and maintenance of all strains. Tetracycline at 60 μg/ml concentration was used for maintenance and culture of the MPAO1 phuS transposon mutant strain. The location of the transposon insertion in the phuS mutant was confirmed by PCR with primers: PhuS1065R(5′-TCAGAGCGCCTTGAAGGAT-3′) and LacZ-148 (5′-GGGTAACGCCAGGGTTTTCC-3′). Analysis of P. aeruginosa growth in rich medium was carried out in LB medium, which has a heme content of ∼5 μm based on the pyridine hemochrome assay (33). Overnight cultures (15 ml) of the P. aeruginosa strains grown in LB medium were spun down, and the bacterial pellets were resuspended in 5 ml of fresh LB medium followed by measurement of the optical density at 600 nm (A600). The cultures were then used to inoculate LB medium (20 ml) in 250-ml baffled Erlenmeyer flasks to a final A600 of 0.05. The flasks were incubated at 37 °C with shaking at 220 rpm, and the A600 was measured every 30 min for a period of 6–8 h as indicated.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Description of strain or plasmid | Source |
|---|---|---|
| E. coli | ||
| S17.1 | E. coli shuttle vector. 294(recA, pro res mod+) Tpr, Smr, (pRP4-2 Tc::Mu-Km::Tn7) | (61) |
| P. aeruginosa | ||
| MPAO1 | Wild-type P. aeruginosa PAO1 | Pseudomonas Genome Center, Seattle, WA |
| 7520 | phuS mutant containing ISlacZ/hah transposon insertion, derived from MAPO1 | (as above) |
| CDC-5 | pvd-2 derivative of PAO1 strain lacking pyoverdin production | (35) |
| IA614 | Pyochelin-deficient derivative of CDC-5 strain, obtained by ethylmethanesulfonate mutagenesis | (35) |
| IR1648 | Chromosomal knockout of iron-regulated heme oxygenase gene, hemO derived from IA614 | (46) |
| IA614-ΔphuS | Chromosomal knockout of phuS derived from IA614 | This study |
| IR1648-ΔphuS | Chromosomal knockout of phuS derived from IR1648. This mutant lacks both pigA and phuS along with the siderophores pyochelin and pyoverdin | This study |
| Plasmids | ||
| pEX18p | AmpR; allelic replacement vector | (34) |
| pFlp2 | AmpR; source of Flp recombinase | (34) |
| pPS856 | Source of GentR-GFP, GentR-conferring fragment flanked by FRT sites | (34) |
| pFTC1 | Source of TetR-GFP, TetR-conferring fragment flanked by FRT sites | H. Schweizer |
| pEX18p-ΔphuS::Gm | AmpR; allelic replacement vector containing 1.8-kb fragment of in-frame phuS deletion containing the GentR antibiotic marker | This study |
| pEX18p-ΔphuS::tet | AmpR; allelic replacement vector containing 1.8-kb fragment of in-frame phuS deletion containing the TetR antibiotic marker | This study |
Succinate minimal medium supplemented with 5% glycerol (SM) was used to assess growth in nutrient-defined medium. The medium was made iron-replete with either 10 μm iron chloride (FeCl3), 10 μm human hemoglobin, or both. Iron-restricted medium contained 2,2′-dipyridyl (500 μm) where indicated. Overnight cultures (15 ml) were supplemented with iron chloride and/or hemoglobin. The overnight cultures were then used to inoculate 250-ml baffled Erlenmeyer flasks containing the same medium to a final A600 of 0.1. The flasks were incubated at 37 °C with shaking at 220 rpm, and the A600 was measured every 1 h over a period of 7 h.
Construction of an In-frame Deletion of PhuS in P. aeruginosa IA614 and IR1648—Construction of the in-frame deletions utilized a four-primer PCR method. The EcoRI-KpnI and KpnI-PstI gene fragments were obtained from MPAO1 genomic DNA using the GeneAmp High Fidelity PCR kit. Primer 1 (5′-GATAGAATTCGAGGCTGCGGTCGGCGATC-3′) located upstream in the phuT gene and primer 2 (5′-GATAGGTACCAACCTGCACCTGAA-3′), located at the 3′-end of the phuS gene, generated a 900-bp fragment encoding EcoRI and KpnI restriction sites. Similarly, primer 3 (5′-GATAGGTACCGTCCTGCCAGGCGCGGT-3′), located at the start of the phuS gene and primer 4 (5′-GATACTGCAGGCTTGCCGAGCAGGCTGT-3′), located within the phuR gene generated a 970-bp fragment introducing KpnI and PstI restriction sites at the 5′- and 3′-ends, respectively. The fragments were cloned in tandem into pEX18Ap insert was confirmed by restriction digest and DNA sequencing to ensure that no point mutations had occurred during PCR (34). The gentamycin antibiotic marker (Gm), flanked by FRT sites, was cloned into the KpnI site within the phuS deletion creating pEX18Ap-ΔphuS::Gm. The final construct was transformed into Escherichia coli S17.1 cells for mating (35).
Unmarked deletions of the phuS gene were constructed in the siderophore-deficient P. aeruginosa IA614 strain according to a modification of Hoang et al. (34). For the mating, E. coli S17.1 was grown with gentamycin in LB overnight at 37 °C, and the P. aeruginosa strains at 42 °C overnight. The single crossover was selected on Pseudomonas Isolation Agar (PIA) with 100 μg of gentamycin and 250 μg of carbenicillin. PCR confirmed the presence of both the wild-type and the deletion copy of phuS::Gm. Positive colonies were then plated on PIA containing 200 μg/ml gentamicin plus 5% sucrose to select for loss of the wild-type gene. Deletion of the integrated antibiotic marker with Flp recombinase was carried out by conjugally transferring pFlp2 in E. coli S17.1 into the IA614-ΔphuS deletion strain at 37 °C (34). Single colonies were screened for gentamycin sensitivity (GentS) on PIA plates containing 200 μg/ml gentamicin. Loss of plasmid was tested by screening for on PIA-sucrose and patching onto PIA carbenicillin (250 μg/ml) plates at 37 °C overnight. Colonies that were SucR-CarbS were streaked out on PIA alone and PIA-carbenicillin (200 μg/ml) and gentamycin (200 μg/ml) to confirm sensitivity to both antibiotics. Unmarked mutants were verified by PCR. The ΔphuS::tet pEX18Ap plasmid was constructed to create the ΔphuS knockout in strain IR1648 as described above.
Pyocyanin Purification and Quantification—Pyocyanin was purified and quantified as described previously (36). Briefly, supernatant from 1 ml of bacterial culture was extracted with 1 ml of chloroform. The chloroform phase containing pyocyanin was then extracted into 1 ml of 0.2 n HCl, yielding a pink solution indicating the presence of pyocyanin. The UV-visible spectrum was recorded in 0.2 n HCl. The pyocyanin concentration expressed as micrograms/ml of culture supernatant, was determined by multiplying the A520 by 17.072, as described previously (36). Reversed-phase high-performance liquid chromatography was performed on a 3.0- × 250-mm C18 column (Atlantis™ dC18 5 μm) with modification of a previously described procedure (37). Briefly the elution was carried out at 0.2 ml/min with the following stepwise gradient of acetonitrile/water/trifluoroacetic acid (10:90:0.01) for 0–5 min (10:90:0.1 to 70:30:0.1) for 5–40 min and (70:30:0.1 to 50:50:0.1) for 40–45 min. The elution of pyocyanin was monitored by UV-visible absorbance at 278 nm. The major peak was collected and analyzed on a Finnigan LCQ classic ion-trap Mass Spectrometer (Finnigan MAT, San Jose, CA) equipped with an electrospray ionization source for obtaining the m/z. The MS instrument was operated in a positive-ion mode with Finnigan Xcalibur software 1.1. The spectra were obtained in full scan mode (150–500 m/z).
Attenuation of Pyocyanin Production—Overnight cultures of the phuS mutants were grown in LB medium from a single colony and used to inoculate 20 ml of LB medium in 250-ml baffled Erlenmeyer flasks to a final A600 of 0.05. The medium was supplemented with 0–100 μm human hemoglobin. The flasks were incubated at 37 °C with shaking at 220 rpm, and pyocyanin concentrations were determined following 24 h of growth and correction for optical density differences. Pyocyanin concentrations (micrograms/ml) were expressed as means ± S.E. and were compared by analysis of variance and t-tests for independent variables using SigmaPlot 10.0 (SPSS Inc., Chicago, IL). The pyocyanin increases were also monitored every 30 min over a period of 6 h for the phuS mutant cultured in LB medium ± 100 μm hemoglobin.
Spectroscopic Determination of Intracellular Iron Levels—The intracellular iron content of the P. aeruginosa strains was determined by modification of previously described procedure (38). Briefly, the bacterial cultures were grown with shaking at 37 °C in LB medium, starting from an A600 of 0.1. At an A600 of 1.5 and 3.0, 10-ml samples from each strain were harvested at 10,000 × g rpm for 15 min, and the supernatant was discarded. The pelleted cells were washed once with fresh LB medium and again pelleted. The pellets were then dried overnight at 80 °C in glass tubes after which they were dissolved in concentrated nitric acid, and the iron content was measured by atomic absorption spectroscopy. For each of the strains the measurements were carried out in triplicate. Control experiments to determine the background levels of iron in the nitric acid were also performed. All glassware and plastic ware were acid-washed before use. The iron concentrations (picograms of iron/μg of protein) were expressed as means ± S.E. and were compared by analysis of variance and t-tests for independent variables using SigmaPlot 10.0.
Western Blot Analysis—The determination of PhuS and/or HO expression levels in nutrient-defined succinate medium was determined by Western blot analysis. Cultures were grown overnight in the following nutrient-defined conditions: succinate (iron-restricted), containing either 100 μm FeCl3, or 25 μm hemoglobin, or both. The overnight cultures were used to inoculate 20 ml of the same nutrient-defined medium to an A600 of 0.1. The medium was supplemented with higher concentrations of FeCl3 and hemoglobin than in the previous growth studies to ensure optimal growth at 6 h. Aliquots of the wild-type MPAO1 strain (1 ml) were pelleted every hour over a 7-h growth period, lysed in 100 μl of Bugbuster® (Novagen, EMD Biosciences, Inc.) and pelleted at 10,000 × g at 4 °C to obtain the soluble protein fraction. The total protein content was measured by Bradford assay (39) using Bio-Rad reagent (Bio-Rad Laboratories Inc.), and 20 μg of total protein was loaded and separated on 4–15% Tris-HCl SDS-PAGE gels (Bio-Rad). Proteins were electrophoretically transferred to Immobilon-P transfer membrane (Millipore Corp., Bedford, MA) as described previously (40). Membranes were blocked with Tris-buffered saline containing 5% skim milk and probed with a 1:500 dilution of primary anti-PhuS polyclonal antibody in Tris-buffered saline containing 0.1% Tween 20. The membrane was then probed with the secondary antibody goat-anti-rabbit immunoglobulin G conjugated with horseradish peroxidase (KPL, Inc., Gaithersburg, MD) at a dilution of 1:10,000 in Tris-buffered saline-containing 0.1% Tween 20, and the protein was visualized by enhanced chemiluminescence using the Super-Signal chemiluminescence kit (Pierce).
RNA Extraction, Labeling, and Hybridization—Overnight cultures of the P. aeruginosa strains were grown from a single colony in 15 ml of RNase-free LB medium at 37 °C for 16–18 h. Aliquots from the samples were spun down, the bacterial pellets were resuspended in 5 ml of fresh RNase-free LB medium, and the A600 was measured. The resuspended cells were then used to inoculate triplicate 20-ml cultures in RNase-free LB medium in 250-ml baffled Erlenmeyer flasks to a final A600 of 0.1.
Aliquots (1 ml) of the bacterial cultures were removed at an A600 of 1.5 and 3.0 for the 7520 phuS::Tn mutant and MPAO1 strains. For the IA614, IR1648, IA614-ΔphuS, and IR1648-ΔphuS strains, samples were removed at an A600 of 1.5. RNAprotect Bacteria Reagent (Qiagen) was added to the samples to stabilize the RNA prior to extraction. Total RNA was then extracted from all strains using the RNeasy Mini kit (Qiagen). Contaminating DNA was removed with an on-column RNase-free DNase I treatment (Qiagen) followed by an off-column DNase I treatment (0.1 unit/μg of RNA) according to the manufacturer's recommendations (Qiagen). The RNA was concentrated to a final volume of 15 μl using the RNeasy MinElute kit (Qiagen), and the RNA was quantified by measurement of the absorbance at 260 nm. The quality of the purified RNA was determined on a 2100 Bioanalyzer (Agilent Technologies, Inc., Santa Clara, CA) at the University of Maryland, Baltimore, School of Medicine, Biopolymer/Genomics Core Facility.
Microarray Analysis—Synthesis of cDNA, target hybridization, staining, scanning, and extraction of the hybridization intensity data were performed at the University of Maryland, Baltimore, School of Medicine, Biopolymer/Genomics Core Facility using an Affymetrix GeneChip® system (GeneChip® hybridization oven 640, fluidics workstation 450, Scanner, and Operating Software, GCOS version 1.4).
The determination of normalized gene expression signals by statistical analysis was performed at the Microarray Core Facility, Johns Hopkins University, School of Medicine. The quality of the microarray experiment was assessed with affyPLM and Affy, two Bioconductor packages for statistical analysis of microarray data. To estimate the normalized gene expression signals, data analysis was conducted on the probe signal values in the Chips' CEL file at the Affymetrix probe pair (perfect match probe and mismatch probe) level, using the statistical algorithm RMA (Robust Multi-array expression measure) with Affy (41). This probe level data processing includes a quantile normalization method (42) to reduce the obscuring variation between microarrays, which might be introduced during the processes of sample preparation, manufacture, fluorescence labeling, hybridization, and/or scanning. With the signal estimates, principal component analysis was performed in R to assess sample variability. With the signal data in a log-transformed format, differential gene expression between controls and mutant strains was assessed by statistical linear model analysis using the bioconductor package limma, in which an empirical Bayes method is used to moderate the standard errors of the estimated log-fold changes of gene expression, and results in more stable inference and improved power, especially for experiments with small numbers of microarrays (43). The moderated t-statistic p values derived from the linear model analysis above were further adjusted by Benjamini and Hochbergs' method to estimate false discovery rate (FDR). The FDR was used to obtain the list of differentially expressed genes. All Bioconductor packages are available at www.bioconductor.org, and all computation was performed under the R environment (www.r-project.org) (44), which is a highly extensible language and environment for statistical computing and graphics and provides a wide variety of statistical (linear and nonlinear modeling, classic statistical tests, time-series analysis, classification, clustering, etc.) and graphical techniques. The annotations were obtained from the Pseudomonas Genome project at www.pseudomonas.com (45). Confirmation of the transcriptional analysis was carried out by reverse transcription-PCR analysis of a select set of genes (supplemental Table S1). Changes in transcript levels by reverse transcription-PCR compared well with those obtained by microarray analysis (supplemental Table S2).
RESULTS
PhuS Is Required for Efficient Heme Utilization in P. aeruginosa—It has previously been shown in vitro that PhuS functions to traffic heme to HO. The in vivo role of PhuS in trafficking heme to HO was examined in a series of bacterial knockout strains in which the phuS and hemO genes were deleted individually, and as a double hemO/phuS knockout. The knockouts were constructed in the IA614 siderophore-deficient strain to study the effects on heme utilization in the absence of high affinity iron-uptake systems that are able to scavenge trace amounts of iron.
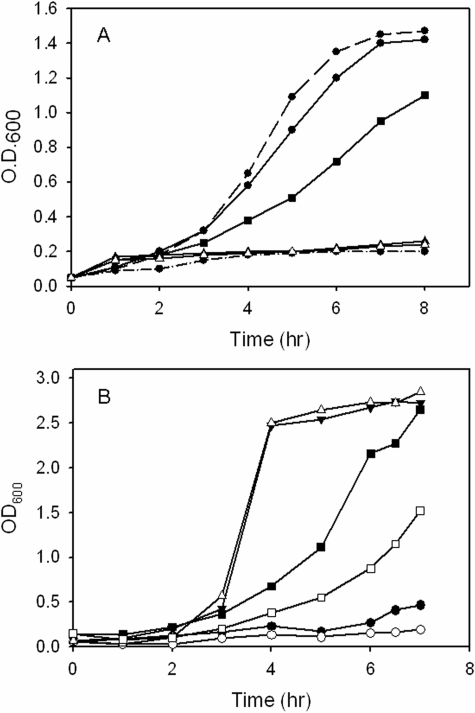
The siderophore-deficient IA614 strain when supplemented with 10 μm FeCl3 is capable of growth to an optical density of 1.4 (Fig. 1A). All of the mutants grew at the same rate as the parent IA614 strain to optical densities of 1.2–1.4 (data not shown). As expected the parent IA614 strain when supplemented with heme as the sole iron source grew to a similar optical density as the iron-supplemented strains (Fig. 1A). However, in contrast the phuS mutant had a slower growth profile and reached a lower optical density. As previously shown the hemO mutant was unable to grow when given heme as the sole source of iron (46). Similarly, in the absence of a functional heme oxygenase to release iron from the porphyrin macrocycle, the hemO/phuS mutant is also unable to grow (Fig. 1A). These data taken together are consistent with our previous in vitro studies, which have characterized PhuS as a heme-trafficking protein to the iron-regulated HO for degradation and release of iron (10, 11, 47). Interestingly, the phuS mutant in contrast to the parent strain or the mutants carrying the hemO gene deletion began to produce a blue-green pigment (supplemental Fig. S1A).
FIGURE 1.
Growth phenotypes of the P. aeruginosa heme utilization mutant strains in the wild-type MPAO1 and siderophore-deficient strains. A, P. aeruginosa IA614 siderophore-deficient mutants. Isogenic IA614 siderophore-deficient strain (•) in iron-restricted medium (500 μm dipyridyl) (dashed-dotted line); supplemented with 10 μm FeCl3 (dashed line); supplemented with 10 μm hemoglobin (solid line); IA614-ΔphuS deletion strain (▪) supplemented with 10 μm hemoglobin; IR1648 (hemO mutant) (▵) supplemented with 10 μm hemoglobin; IR1648-ΔphuS (hemO/ΔphuS double mutant) (▴) supplemented with hemoglobin. The phuS and hemO mutants supplemented with FeCl3 all had similar growth curves as the isogenic IA614 strain and for clarity are not shown. B, P. aeruginosa MPAO1 and 7520 phuS::Tn in nutrient-defined succinate media. MPAO1 (•) and 7520 phuS::Tn (phuS transposon mutant) (○) in iron-restricted medium (500 μm dipyridyl); MPAO1 (▾) and 7520 phuS::Tn (▵) supplemented with 10 μm FeCl3; MPAO1 (▪) and 7520 phuS::Tn (□) supplemented with 10 μm hemoglobin.
The growth profile and phenotype of the phuS transposon mutant and the wild-type MPAO1 strains were also analyzed to determine if the pigment production is related to the compromised ability of the IA614 strain to acquire iron via siderophore mechanisms. As observed for the IA614 strain the growth rate of the phuS transposon mutant was similar to that of the parent MPAO1 strain in nutrient-rich LB medium (data not shown). However, in iron-restricted conditions or when hemoglobin is the sole iron source, the growth of the phuS mutant was much slower when compared with the parent MPAO1 strain (Fig. 1B). Furthermore, as for the phuS mutant in the siderophore-deficient strain, the phuS transposon mutant also began to secrete the blue-green pigment on entering stationary phase (supplemental Fig. S1A).
The phuS Mutants Exhibit Premature Pyocyanin Production—The phuS mutant in either the wild-type or siderophore-deficient strain upon reaching the transition to stationary phase (A600 of ∼3.0) secretes a blue-green pigment (supplemental Fig. S1A). Interestingly, the pigment was not observed when the hemO (IR1648) or both the phuS and hemO genes (IR1648-ΔphuS) were deleted (supplemental Fig. S1A). The excreted pigment was further identified as pyocyanin by a combination of UV-visible spectroscopy, high-performance liquid chromatography, and electrospray ionization-mass spectrometry (supplemental Fig. S1B).
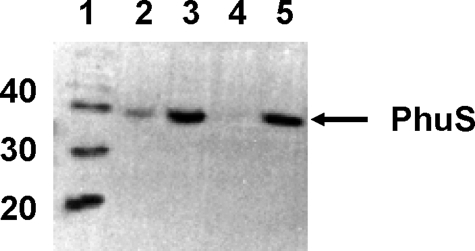
Heme Positively Regulates the Expression of PhuS in P. aeruginosa—The premature pyocyanin phenotype on disruption of phuS in iron-replete LB medium suggests that in the wild-type strain the protein must be expressed. Therefore, the phu operon must be positively regulated in a manner distinct form that of the negative repression by Fur. We hypothesized that heme if present may act as a positive regulator of the phu operon. Because LB medium contains ∼6 μm heme in yeast extract (as calculated by pyridine hemochrome), we postulated that the presence of heme alleviates the iron-regulated Fur suppression of the heme utilization genes. In keeping with this hypothesis we observed a constitutive expression of PhuS in the wild-type PAO1 strain grown in iron-replete LB medium (data not shown). To directly test this hypothesis, the expression of PhuS in the wild-type PAO1 was analyzed in nutrient-defined succinate medium supplemented with either FeCl3 or hemoglobin. As shown in Fig. 2 in iron-restricted succinate medium the PhuS protein is expressed at high levels. On supplementation with 100 μm FeCl3 the expression of PhuS is repressed. In contrast, addition of hemoglobin to the medium induces the expression of PhuS. Furthermore, in the presence of both FeCl3 and hemoglobin the PhuS protein levels are detectable in contrast to the culture with FeCl3 alone. Taken together the data indicate that, even in the presence of iron, heme positively regulates the expression of the heme uptake genes.
FIGURE 2.
Western blot analysis of the wild-type MPAO1 strain. Cells were grown in succinate minimal medium with the indicated iron and heme supplements. Lane 1, molecular mass markers in kilodaltons; lane 2, 100 μm FeCl3 and 25 μm hemoglobin; lane 3, 25 μm hemoglobin; lane 4, 100 μm FeCl3; lane 5, 500 μm 2,2′-dipyridyl. Cells were harvested 6 h post-inoculation, and 20 μg of total protein was loaded in each well and separated by SDS-PAGE.
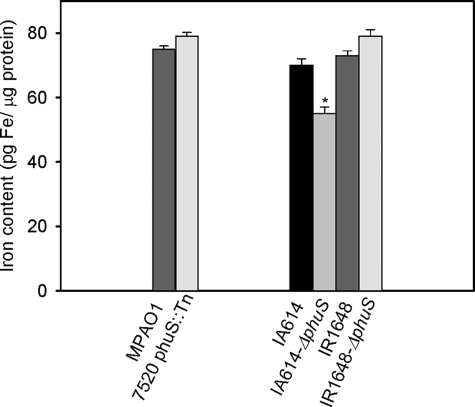
Premature Production of Pyocyanin Is Not Due to Iron Starvation—If the disruption of the phuS gene results in inefficient heme utilization, it might be expected that the intracellular iron levels in the phuS mutants would be lower than the wild-type strains. Interestingly in the MPAO1 strain the intracellular iron levels between the wild-type and phuS mutant were not significantly different (Fig. 3). However, in the siderophore-deficient background the phuS mutant has significantly lower levels of intracellular iron, whereas both the hemO and hemO/phuS knockouts were similar to that of the siderophore-deficient IA614 strain (Fig. 3). On loss of the PhuS protein in the MPAO1 strain it appears that the cells' iron requirements are met by alternate iron-uptake mechanisms. However, the siderophore-deficient strain on loss of PhuS is unable to adequately maintain its iron balance when combined with the inability to efficiently utilize heme. The relationship between heme utilization and iron balance will be discussed in greater detail in the following section.
FIGURE 3.
Iron content in the MPAO1 and IA614 mutant strains. Iron content as measured by atomic absorption spectroscopy. Cells were analyzed for iron content as described under “Experimental Procedures.” Data are means ± S.E. of three experiments. *, p < 0.02 (versus control strain IA614).
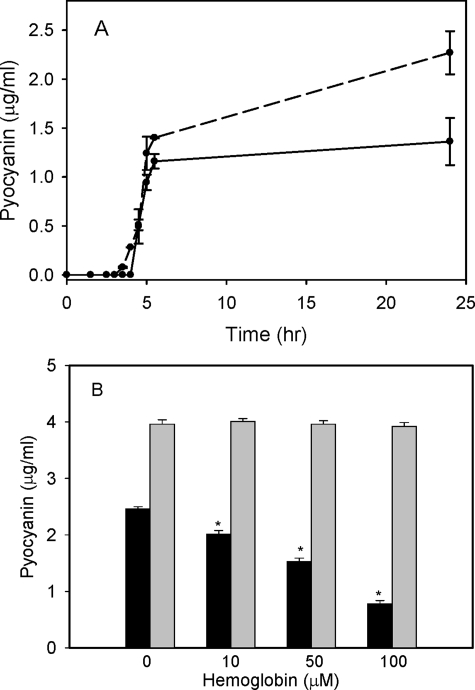
The Premature Production of Pyocyanin Is Directly Linked to the Cells Ability to Utilize Heme as an Iron Source—The premature production of pyocyanin on disruption of phuS in the presence of a functional HO, suggests the pyocyanin phenotype is directly related to inefficient utilization of heme. Therefore to test this hypothesis we attempted to override the inefficiency by increasing the concentration of heme in the extracellular environment. Pyocyanin production was monitored over a period of 24 h following the addition of 100 μm exogenous hemoglobin. As shown in Fig. 4A over a 24-h period the level of pyocyanin was significantly decreased. The decrease in pyocyanin on addition of hemoglobin to the medium was further examined as a function of hemoglobin concentration. The phuS transposon mutant in the wild-type background showed a concentration-dependent suppression of pyocyanin (Fig. 4B). However, the phuS mutant in siderophore-deficient background did not show a concentration-dependent suppression of pyocyanin (Fig. 4B). Although it is clear from the manifestation of the phenotype on mutation of phuS in the wild-type MPAO1 background that iron alone is not responsible for the premature production of pyocyanin, the siderophore-deficient strain produced higher levels of pyocyanin, most likely as a result of the additive effect of the lower intracellular iron levels. These data suggest that, in addition to the previously documented relationship between iron availability and pyocyanin biosynthesis (27, 48), heme utilization also independently influences pyocyanin biosynthesis.
FIGURE 4.
Attenuation of pyocyanin production in the P. aeruginosa MPAO1 and IA614 phuS mutants on supplementing the medium with hemoglobin. A, pyocyanin production over a 24-h growth period in the absence (dashed line) or presence (solid line) of 100 μm hemoglobin. All pyocyanin extractions were carried out on cultures of the wild-type MPAO1 and 7520 phuS::Tn mutant strains at equivalent cell densities. Data are means ± S.E. of three separate experiments. B, pyocyanin production in the 7520 phuS::Tn (black bars) and IA614-ΔphuS (gray bars) mutants as a function of hemoglobin concentration. Extraction and quantification of pyocyanin were carried out as described under “Experimental Procedures.” Data are means ± S.E. of three separate experiments. *, p < 0.001 (versus control value no exogenous hemoglobin).
Iron Homeostasis Is Perturbed in the phuS Mutants—To further investigate the effect of disruption of heme utilization on the premature pyocyanin phenotype a global microarray approach was undertaken. The global transcriptional changes were performed on the wild-type and siderophore-deficient strains at an A600 1.5, prior to the production of pyocyanin. In addition the expression profile of the wild-type and phuS transposon mutant strain were analyzed at an A600 of 3.0, the time point at which the mutant strains begin to produce pyocyanin. The wild-type strain was performed at the later time point to assess the expression profiles of the heme and iron-uptake systems in a non-compromised iron-uptake background.
Consistent with the premature pyocyanin production the genes involved in pyocyanin biosynthesis, including phzABCDEFG operons 1 and 2, phzS and phzM (49) were significantly up-regulated at A600 of 3.0 in the phuS transposon mutant (Table 2). Similarly, genes previously reported to be specifically regulated by pyocyanin namely, the root nodule cell division (RND) efflux pump MexGHI-OmpD (29), were also up-regulated (Table 2). Additionally at the earlier time point genes involved in PQS biosynthesis (a positive signal for pyocyanin biosynthesis), the phnAB and pqsABCDE operons were up-regulated in the phuS mutants in both the wild-type and siderophore-deficient strains (Table 2). The transcriptional data agree with the increased excretion of PQS and other HAQs compared with the wild-type strain as determined by high-performance liquid chromatography with a known PQS standard (supplemental Fig. S2) and confirmed by electrospray ionization-mass spectrometry analysis. In contrast the HO-deficient (IR1648 or IR1648-ΔphuS) strains did not show significant up-regulation of the QS networks, consistent with the hypothesis that both heme and a functional HO are required for premature pyocyanin production (Table 2).
TABLE 2.
Differential transcription of the PQS and pyocyanin biosynthesis and efflux genes
O.D., optical density at 600 nm.
|
Open reading frame
|
Gene name
|
Product name
|
IA614aversus
|
MPAO1bversus
|
|||
|---|---|---|---|---|---|---|---|
| IA614-ΔphuS | IR1648 (hemO) | IR1648-(hemO/ΔphuS) | 7520 phuS::Tn | 7520 phuS::Tn | |||
| O.D. 1.5 | O.D. 1.5 | O.D. 3.0 | |||||
| PQS biosynthesis genes | |||||||
| PA0996 | pqsA | Probable coenzyme A ligase | 4.2 | NSc | NS | 6.4 | 10.2 |
| PA0997 | pqsB | Homologous to β-keto-acyl-acyl carrier protein synthase | 7.8 | NS | 1.2 | 12.1 | 5.3 |
| PA0998 | pqsC | Homologous to β-keto-acyl-acyl carrier protein synthase | 10.0 | NS | NS | 16.2 | 5.3 |
| PA0999 | pqsD | 3-Oxoacyl-[acyl-carrier-protein] synthase III | 6.5 | NS | NS | 10.4 | 5.2 |
| PA1000 | pqsE | Quinolone signal response protein | 3.0 | NS | NS | 6.0 | 7.0 |
| PA1001 | phnA | Anthranilate synthase component I | 3.4 | NS | NS | 5.8 | 10.0 |
| PA1002 | phnB | Anthranilate synthase component II | 1.7 | NS | NS | 3.0 | 5.4 |
| PA1003 | pqsR | Transcriptional regulator MvfR | 1.2 | NS | NS | NS | 2.0 |
| Phenazine biosynthesis genes | |||||||
| PA1898 | qscR; phzR | Quorum-sensing control repressor | NS | NS | NS | NS | 2.7 |
| PA1901 | phzC2 | Phenazine biosynthesis protein PhzC | NS | NS | 1.2 | 1.20 | 15.4 |
| PA1902 | phzD2 | Phenazine biosynthesis protein PhzD | NS | -1.20 | NS | 1.30 | 13.9 |
| PA1903 | phzE2 | Phenazine biosynthesis protein PhzE | NS | NS | NS | 1.32 | 18.2 |
| PA1904 | phzF2 | Probable phenazine biosynthesis protein | NS | NS | NS | NS | 17.9 |
| PA1905 | phzG2 | Probable pyridoxamine 5′-phosphate oxidase | 1.1 | NS | NS | 1.5 | 23.0 |
| PA4209 | phzM | Probable phenazine-specific methyltransferase | NS | NS | NS | 1.3 | 7.8 |
| PA4210 | phzA1 | Probable phenazine biosynthesis protein | NS | NS | NS | NS | 40.32 |
| PA4211 | phzB1 | Probable phenazine biosynthesis protein | 1.3 | NS | NS | 1.8 | 34.0 |
| PA4217 | phzS | Flavin-containing monooxygenase | NS | -1.2 | -1.1 | 1.5 | 12.7 |
| Pyocyanin regulated efflux pumps | |||||||
| PA4205 | mexG | Hypothetical protein | 2.6 | NS | NS | NS | 81.9 |
| PA4206 | mexH | Probable RND efflux membrane fusion | 2.5 | NS | NS | NS | 26.6 |
| PA4207 | mexI | Probable RND efflux transporter | 1.8 | NS | NS | NS | 12.1 |
| PA4208 | opmD | Probable outer membrane protein precursor | 1.7 | NS | NS | NS | 5.8 |
Ratio of expression of the IA614-ΔphuS, IR1648, and IR1648-ΔphuS deletion strains compared to IA614 from three separate microarray experiments. Changes were considered significant at or below a 0.05 FDR as described under “Experimental Procedures.”
Ratio of expression of the 7520 phuS::Tn strain compared to MPA01 from three separate microarray experiments. Changes were considered significant at or below a 0.05 FDR as described under “Experimental Procedures.”
NS, not significant.
Not surprisingly the lower intracellular levels of iron on the loss of PhuS in the siderophore-deficient background correlated with the up-regulation of genes involved in iron uptake and storage (Tables 3 and 4). However, interestingly, in the wild-type strain a similar up-regulation of the heme and iron-uptake systems is observed, including genes involved in siderophore-mediated iron acquisition, the extra cytoplasmic function σ-factor pvdS, the PvdS-regulated pyoverdin biosynthesis genes (pvd), and the ferripyoverdin receptor fpvA (8, 50) (Table 3). Furthermore the accessory iron-uptake gene tonB, the ferrous iron-uptake receptor (feoA), and the heme-uptake system (phuR and phuTUV) were also up-regulated (Table 4). In addition the up-regulation of genes such as fumC, sodA, ferredoxin bfd and fpr, which encode proteins that do not require iron for their function and can substitute for essential iron-containing proteins under iron-starvation conditions further confirm an iron starvation response (15). Therefore, despite the fact that the intracellular iron levels in the transposon mutant are not different from the wild-type strain, the phuS transposon mutant is sensing iron deprivation (Tables 3 and 4). At the earlier time point in both the phuS transposon mutant and the siderophore-deficient phuS strain, genes involved in heme uptake (phuRTV) are also up-regulated (Table 4).
TABLE 3.
Differential transcription of the pyoverdin and pyochelin biosynthesis and uptake genes
O.D., optical density at 600 nm.
|
Open reading frame
|
Gene name
|
Product name
|
IA614aversus
|
MPAO1bversus
|
|||
|---|---|---|---|---|---|---|---|
| IA614-ΔphuS | IR1648 (hemO) | IR1648-ΔphuS | 7520 phuS::Tn | 7520 phuS::Tn | |||
| O.D. 1.5 | O.D. 1.5 | O.D. 3.0 | |||||
| Pyoverdin biosynthesis and uptake system | |||||||
| PA2383 | Probable transcriptional regulator | -1.2 | NSc | -1.2 | NS | 2.3 | |
| PA2384 | Hypothetical protein | 1.2 | NS | NS | -1.3 | 11.2 | |
| PA2385 | pvdQ | PvdQ | NS | NS | NS | NS | 2.4 |
| PA2386 | pvdA | l-ornithine N5-oxygenase | NS | NS | 1.30 | -1.4 | NS |
| PA2392 | pvdP | PvdP | NS | NS | NS | NS | 2.3 |
| PA2393 | Probable dipeptidase precursor | NS | NS | NS | NS | 4.17 | |
| PA2394 | pvdN | PvdN | NS | -1.1 | 1.3 | NS | 2.9 |
| PA2396 | pvdF | Pyoverdine synthetase F | NS | NS | NS | NS | 3.2 |
| PA2397 | pvdE | Pyoverdine biosynthesis protein PvdE | NS | NS | NS | NS | 2.8 |
| PA2398 | fpvA | Ferripyoverdine receptor | -1.2 | NS | NS | -2.9 | 2.4 |
| PA2399 | pvdD | Pyoverdine synthetase D | NS | NS | 1.4 | NS | 2.4 |
| PA2413 | pvdH | l-2,4-diaminobutyrate:2-ketoglutarate 4-aminotransferase | NS | NS | 1.3 | NS | 3.8 |
| PA2425 | pvdG | PvdG | NS | NS | NS | NS | 2.6 |
| PA2426 | pvdS | Sigma factor PvdS | 1.1 | NS | NS | -1.3 | 13.5 |
| Pyochelin biosynthesis and uptake genes | |||||||
| PA4218 | fptX | Probable transporter | NS | NS | 1.4 | 7.4 | 1.4 |
| PA4219 | fptC; yfpB | Hypothetical protein | NS | NS | 1.3 | 3.3 | NS |
| PA4220 | fptB | Hypothetical protein | NS | NS | 1.5 | 13.4 | 2.0 |
| PA4221 | fptA | Fe(III)-pyochelin outer membrane receptor precursor | NS | NS | 1.3 | 4.7 | NS |
| PA4222 | pchI | Probable ATP-binding component of ABC transporter | NS | NS | 1.2 | 4.1 | NS |
| PA4223 | pchH | Probable ATP-binding component of ABC transporter | NS | NS | 1.2 | 6.2 | NS |
| PA4224 | pchG | Pyochelin biosynthetic protein PchG | NS | NS | NS | 5.5 | NS |
| PA4225 | pchF | Pyochelin synthetase | NS | NS | 1.2 | 5.1 | NS |
| PA4226 | pchE | Dihydroaeruginoic acid synthetase | NS | NS | NS | 4.7 | NS |
| PA4227 | pchR | Transcriptional regulator PchR | -1.1 | NS | NS | NS | 4.0 |
| PA4228 | pchD | Pyochelin biosynthesis protein PchD | NS | -1.2 | 1.2 | 2.6 | NS |
| PA4229 | pchC | Pyochelin biosynthetic protein PchC | NS | NS | 1.4 | 2.7 | NS |
| PA4230 | pchB | Salicylate biosynthesis protein PchB | NS | NS | 1.2 | 6.5 | NS |
| PA4231 | pchA | Salicylate biosynthesis isochorismate synthase | NS | NS | 1.3 | 4.5 | NS |
Ratio of expression of the IA614-ΔphuS, IR1648, and IR1648-ΔphuS deletion strains compared to IA614 from three separate microarray experiments. Changes were considered significant at or below a 0.05 FDR as described under “Experimental Procedures.”
Ratio of expression of the 7520 phuS::Tn strain compared to MPA01 from three separate microarray experiments. Changes were considered significant at or below a 0.05 FDR as described under “Experimental Procedures.”
NS, not significant.
TABLE 4.
Differential transcription of iron-regulated genes involved in iron acquisition and metabolism
O.D., optical density at 600 nm.
|
Open reading frame
|
Gene name
|
Product name
|
IA614aversus
|
MPAO1bversus
|
|||||
|---|---|---|---|---|---|---|---|---|---|
| IA614-ΔphuS | IR1648 (hemO) | IR1648-ΔphuS | 7520 phuS::Tn | 7520 phuS::Tn | |||||
| O.D. 1.5 | O.D. 1.5 | O.D. 3.0 | |||||||
| PA0470 | fiuA | Ferrichrome receptor FiuA | -1.2 | -1.2 | NSc | NS | NS | ||
| PA0471 | fiuR | Probable transmembrane sensor | 1.2 | NS | 1.2 | -1.8 | 1.7 | ||
| PA0472 | fiuI | Probable sigma-70 factor, ECF subfamily | 1.2 | NS | 1.3 | -1.9 | 2.5 | ||
| PA0672 | hemO | Heme oxygenase | NS | NS | 1.2 | -1.6 | 2.4 | ||
| PA0693 | exbB2 | Transport protein ExbB2 | 4.8 | NS | NS | NS | 1.8 | ||
| PA0694 | exbD2 | Transport protein ExbD | 2.59 | NS | NS | NS | NS | ||
| PA0929 | pirR | Two-component response regulator | 1.2 | -1.2 | NS | -2.6 | 2.1 | ||
| PA1302 | hxuC | Probable heme utilization protein precursor | NS | NS | 1.3 | NS | NS | ||
| PA1365 | Probable siderophore receptor | NS | NS | NS | NS | -1.3 | |||
| PA2686 | pfeR | Two-component response regulator PfeR | NS | NS | NS | NS | 1.3 | ||
| PA3397 | fpr | Ferredoxin-NADP+ reductase | NS | NS | -1.1 | NS | 2.8 | ||
| PA3410 | Probable sigma-70 factor, ECF subfamily | NS | NS | 1.2 | -1.3 | 2.7 | |||
| PA3530 | bfd | Conserved hypothetical protein | 1.1 | NS | 1.2 | -1.3 | 23.5 | ||
| PA3531 | bfrB | Bacterioferritin | -1.3 | 1.6 | -1.1 | 1.5 | -6.5 | ||
| PA3812 | iscA | Probable iron-binding protein IscA | NS | NS | NS | NS | 1.6 | ||
| PA3814 | iscS | l-cysteine desulfurase | NS | NS | NS | -1.3 | 3.0 | ||
| PA3899 | Probable sigma-70 factor, ECF subfamily | NS | NS | 1.2 | -1.3 | 1.6 | |||
| PA3901 | fecA | Fe(III) dicitrate transport protein FecA | -1.5 | NS | -1.2 | -1.5 | -2.5 | ||
| PA4359 | feoA | Conserved hypothetical protein | 1.9 | NS | -1.2 | -2.2 | 8.7 | ||
| PA4513 | piuB | Probable oxidoreductase | NS | NS | 1.13 | -1.1 | NS | ||
| PA4514 | piuA | Probable outer membrane iron-receptor | -1.4 | NS | 1.4 | -2.0 | NS | ||
| PA4515 | piuC | Conserved hypothetical protein | 1.2 | -1.3 | 1.2 | -3.4 | 4.4 | ||
| PA4675 | optH; iutA | Probable TonB-dependent receptor | -2.0 | NS | NS | -2.2 | -2.4 | ||
| PA4687 | hitA | Ferric iron-binding periplasmic protein HitA | NS | -1.4 | -1.3 | -2.4 | NS | ||
| PA4688 | hitB | Iron (III)-transport system permease HitB | -1.2 | NS | -1.1 | -1.8 | NS | ||
| PA4706 | phuV | ABC transporter (ATPase) | 1.2 | NS | 1.5 | 8.6 | 3.4 | ||
| PA4708 | phuT | Heme-transport protein, PhuT | 1.8 | NS | 1.9 | 13.2 | 10.8 | ||
| PA4709 | phuS | Probable hemin degrading factor | NS | NS | NS | NS | 1.8 | ||
| PA4710 | phuR | Heme/hemoglobin uptake outer membrane receptor PhuR precursor | 2.4 | NS | 2.5 | 1.7 | 2.3 | ||
| PA4896 | Probable sigma-70 factor, ECF subfamily | NS | -1.2 | NS | -1.4 | 2.1 | |||
| PA5217 | Probable component of ABC iron transporter | 1.2 | NS | 1.2 | -3.4 | NS | |||
| PA5531 | tonB | TonB protein | 1.3 | -1.3 | 1.2 | -3.4 | 4.2 | ||
Ratio of expression of the IA614-ΔphuS, IR1648, and IR1648-ΔphuS deletion strains compared to IA614 from three separate microarray experiments. Changes were considered significant at or below a 0.05 FDR as described under “Experimental Procedures.”
Ratio of expression of the 7520 phuS::Tn strain compared to MPA01 from three separate microarray experiments. Changes were considered significant at or below a 0.05 FDR as described under “Experimental Procedures.”
NS, not significant.
Perhaps more interestingly in the hemO/phuS mutant the expression profile for the iron-uptake systems is very similar to that of the phuS mutant alone, despite the fact that it does not display a pyocyanin phenotype (Table 4). Therefore the microarray analysis indicates that deletion of the phuS gene despite the intracellular iron levels disrupts the ability to sense the iron homeostasis and is distinct from the premature pyocyanin phenotype. The current data suggest that the PhuS protein may have a dual function in intracellular heme trafficking to HO, and in sensing and maintaining the balance between heme and iron uptake.
DISCUSSION
The slow growth phenotype observed for the phuS mutants in the presence of heme as the sole source of iron confirmed that the cytoplasmic heme-binding protein, PhuS, is required for efficient heme utilization. This is consistent with previous in vitro reports that PhuS functions to traffic heme to the iron regulated HO (10, 11, 47). The observation of the premature production of pyocyanin in the phuS mutants and the confirmation that heme can override suppression of the phu operon by Fur, indicates that there is a separate and distinct heme-dependent regulation of the phu operon. Therefore, whereas Fur acts as a global negative regulator of the iron and heme-uptake systems, heme can independently and selectively up-regulate the heme uptake system via an as yet unidentified mechanism. Regulation of heme utilization by heme itself has previously been reported in a number of bacteria, including Bordetella sp. and Coryne-bacterium diphtheriae (51–53). The ability of the organism to respond to the environmental iron source and differentially express the iron and heme uptake systems has relevance not only for survival (54) but also for the ability to establish infection. It has previously been shown in Staphylococcus aureus that, during the early stages of infection, the cell preferentially utilizes heme as an iron source and switches to iron-containing proteins such as transferrin much later in the disease progression (55).
The premature pyocyanin phenotype observed with the phuS mutants but not evident in either the hemO-deficient (IR1648) or the hemO/phuS double mutant (IR1648-ΔphuS) strains confirmed that, in addition to heme in the external environment, a functional HO is also required. This led us to hypothesize that the pyocyanin phenotype in the absence of PhuS is a result of decreased flow of heme to HO. This is further supported with the concentration-dependent suppression of the phenotype by hemoglobin in the phuS transposon mutant. Therefore, an inability to efficiently utilize heme signals the cell to acquire iron via alternative mechanisms. Addition of exogenous hemoglobin did not suppress the premature production of pyocyanin in the siderophore-deficient strain, presumably as a result of the increased iron restriction and lower intracellular iron levels. It has previously been reported that pyocyanin production is influenced not only by cell density and QS but also by several environmental factors, including iron (48, 56–58). Therefore, in the siderophore-deficient strain the cumulative effect of both inefficient heme and iron utilization appear to have an additive effect. Furthermore, it has been previously reported that pyocyanin itself can participate in iron uptake due to its ability to reduce Fe3+-transferrin and ferric iron oxides to release the Fe2+ ion (28, 30). Interestingly the premature production of pyocyanin in the phuS mutants indicates that heme utilization may be coupled to iron acquisition via transferrin, as suggested by the up-regulation of the ferrous iron uptake protein (feoA) required for active transport of the Fe2+ ion. This also may be physiologically relevant during infection where both heme and transferrin are likely sources of iron.
It is intriguing to speculate that the distinct regulation of heme utilization versus that of iron is linked to the flux of heme through HO. In such a model the by-product of heme degradation by the iron-regulated HO, δ-biliverdin, acts as a feedback regulator in maintaining the up-regulation of the heme uptake system. The premature production of pyocyanin may be a result of decreased production of δ-biliverdin, which maintains the up-regulation of the pathway but provides lower levels of available iron. In contrast deletion of HO shuts down the heme flux such that the cell switches to alternate non-heme iron sources. In effect HO acts as the valve for controlling heme utilization via a metabolic feedback loop. We are currently testing this hypothesis by complementation of the phuS mutant in the siderophore-deficient strain with either the wild-type HO, which oxidatively cleaves heme at the δ-meso-carbon to yield δ-biliverdin, or a mutant HO that yields α-biliverdin. If our hypothesis is correct, the complementation with the wild-type HO will induce the premature pyocyanin phenotype in the absence of PhuS. In contrast complementation with the α-biliverdin-producing HO should have no effect on pyocyanin production. It has previously been reported that the P. aeruginosa genome encodes a second heme oxygenase BphO, in an operon containing a bacterial phytochrome, BphP (59). The BpHO is an α-selective heme oxygenase that does not accept heme from PhuS (11). Furthermore, it has been shown that δ-biliverdin, in contrast to α-biliverdin, the product of BphO oxidative cleavage, does not bind to the downstream acceptor phytochrome, BphP (60). These data further suggest that the unique regioselectivity of the differentially expressed heme oxygenases in P. aeruginosa is required for their distinct functions in phytochrome signaling and iron metabolism, respectively.
Interestingly, despite the fact that the premature production of pyocyanin is not observed in either the hemO or hemO/phuS mutant strains, it is evident from the microarray analysis that disruption of phuS with or without a functional HO causes a disruption in iron homeostasis. As can be seen in the transcriptional changes of the phuS mutants several iron-uptake systems are up-regulated despite the iron-replete conditions, suggesting that PhuS in addition to facilitating heme utilization also acts to directly or indirectly sense the intracellular heme/iron levels. In a separate study we have recently shown that the PhuS homolog ShuS of S. dysenteriae binds DNA in a heme-dependent manner (60). Recent studies with the apo- and holo-PhuS have shown similar results whereby the apo-PhuS binds to DNA, and the holo-PhuS has no DNA binding ability.3 We are actively pursuing the physiological relevance of this observation in light of the current studies.
Therefore, we hypothesize that the disruption of iron homeostasis is a function of the cells' inability to sense the intracellular heme levels, whereas, the premature production of pyocyanin is solely related to the flux of heme through heme oxygenase. This is further supported by the disruption of iron homeostasis in the hemO/phuS mutant (IR1648-ΔphuS), which does not display the pyocyanin phenotype as the heme flux or valve is shut off. However, because PhuS also acts as an intracellular sensor of heme and/or iron, the up-regulation of the iron- and heme-uptake systems is similar to that of the phuS mutants. In contrast as would be expected there is no increase in the transcriptional levels of the heme- and iron-uptake systems in the hemO mutant strain (IR1648), because the presence of PhuS allows the cell to sense the intracellular heme/iron levels and the absence of HO shuts down heme flux maintaining iron homeostasis.
In summary the PhuS protein plays a pivotal role in maintaining the iron homeostasis of the cell and facilitating heme utilization. Furthermore this central role in cellular metabolism suggests that disruption of PhuS may be an effective mechanism in reducing virulence.
Supplementary Material
Acknowledgments
We thank Dr. Susanne Haüssler, Helmholtz Center for Infection Research, Inhoffenstrasse, Braunschweig, Germany, for providing the PQS standard and Dr. Valeria Cullotta, The Johns Hopkins School of Public Health, Baltimore, MD, for use of her atomic absorption spectrometer.
This work was supported, in whole or in part, by National Institutes of Health Grant AI-55912 (to A. W.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The on-line version of this article (available at http://www.jbc.org) contains supplemental Table S1, Figs. S1 and S2, and references.
Footnotes
The abbreviations used are: HO, heme oxygenase; QS, quinolone signal; PQS, Pseudomonas quinolone signal; Phu, Pseudomonas heme utilization; ABC, ATP binding cassette; Fur, ferric uptake regulator; PIA, Pseudomonas isolation agar; FDR, false discovery rate; RND, root nodule cell division.
A. P. Kaur and A. Wilks, unpublished data.
References
- 1.Andrews, S. C., Robinson, A. K., and Rodriguez-Quinones, F. (2003) FEMS Microbiol. Rev. 27 215–237 [DOI] [PubMed] [Google Scholar]
- 2.Vasil, M. L., and Ochsner, U. A. (1999) Mol. Microbiol. 34 399–413 [DOI] [PubMed] [Google Scholar]
- 3.Ratledge, C., and Dover, L. G. (2000) Annu. Rev. Microbiol. 54 881–941 [DOI] [PubMed] [Google Scholar]
- 4.Otto, B. R., Verweij-van Vught, A. M., and MacLaren, D. M. (1992) Crit. Rev. Microbiol. 18 217–233 [DOI] [PubMed] [Google Scholar]
- 5.Sadikot, R. T., Blackwell, T. S., Christman, J. W., and Prince, A. S. (2005) Am. J. Respir. Crit. Care Med. 171 1209–1223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Van Delden, C., and Iglewski, B. H. (1998) Emerg. Infect. Dis. 4 551–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cox, C. D., and Adams, P. (1985) Infect. Immun. 48 130–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ochsner, U. A., Johnson, Z., and Vasil, M. L. (2000) Microbiology 146 185–198 [DOI] [PubMed] [Google Scholar]
- 9.Letoffe, S., Ghigo, J. M., and Wandersman, C. (1994) Proc. Natl. Acad. Sci. U. S. A. 91 9876–9880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bhakta, M. N., and Wilks, A. (2006) Biochemistry 45 11642–11649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lansky, I. B., Lukat-Rodgers, G. S., Block, D., Rodgers, K. R., Ratliff, M., and Wilks, A. (2006) J. Biol. Chem. 281 13652–13662 [DOI] [PubMed] [Google Scholar]
- 12.Vasil, M. L. (2007) Biometals 20 587–601 [DOI] [PubMed] [Google Scholar]
- 13.Wilderman, P. J., Sowa, N. A., FitzGerald, D. J., FitzGerald, P. C., Gottesman, S., Ochsner, U. A., and Vasil, M. L. (2004) Proc. Natl. Acad. Sci. U. S. A. 101 9792–9797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ochsner, U. A., Vasil, A. I., and Vasil, M. L. (1995) J. Bacteriol. 177 7194–7201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ochsner, U. A., Wilderman, P. J., Vasil, A. I., and Vasil, M. L. (2002) Mol. Microbiol. 45 1277–1287 [DOI] [PubMed] [Google Scholar]
- 16.Arevalo-Ferro, C., Hentzer, M., Reil, G., Gorg, A., Kjelleberg, S., Givskov, M., Riedel, K., and Eberl, L. (2003) Environ. Microbiol. 5 1350–1369 [DOI] [PubMed] [Google Scholar]
- 17.Juhas, M., Wiehlmann, L., Salunkhe, P., Lauber, J., Buer, J., and Tummler, B. (2005) FEMS Microbiol. Lett. 242 287–295 [DOI] [PubMed] [Google Scholar]
- 18.Lazdunski, A. M., Ventre, I., and Sturgis, J. N. (2004) Nat. Rev. Microbiol. 2 581–592 [DOI] [PubMed] [Google Scholar]
- 19.Schuster, M., and Greenberg, E. P. (2006) Int. J. Med. Microbiol. 296 73–81 [DOI] [PubMed] [Google Scholar]
- 20.Pesci, E. C., Milbank, J. B., Pearson, J. P., McKnight, S., Kende, A. S., Greenberg, E. P., and Iglewski, B. H. (1999) Proc. Natl. Acad. Sci. U. S. A. 96 11229–11234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wells, I. C. (1952) J. Biol. Chem. 196 331–340 [PubMed] [Google Scholar]
- 22.Bollinger, N., Hassett, D. J., Iglewski, B. H., Costerton, J. W., and McDermott, T. R. (2001) J. Bacteriol. 183 1990–1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Juhas, M., Wiehlmann, L., Huber, B., Jordan, D., Lauber, J., Salunkhe, P., Limpert, A. S., von Gotz, F., Steinmetz, I., Eberl, L., and Tummler, B. (2004) Microbiology 150 831–841 [DOI] [PubMed] [Google Scholar]
- 24.Cornelis, P., and Aendekerk, S. (2004) Microbiology 150 752–756 [DOI] [PubMed] [Google Scholar]
- 25.Kim, E. J., Wang, W., Deckwer, W. D., and Zeng, A. P. (2005) Microbiology 151 1127–1138 [DOI] [PubMed] [Google Scholar]
- 26.Zheng, P., Sun, J., Geffers, R., and Zeng, A. P. (2007) J. Biotechnol. 132 342–352 [DOI] [PubMed] [Google Scholar]
- 27.Oglesby, A. G., Farrow, J. M., 3rd, Lee, J. H., Tomaras, A. P., Greenberg, E. P., Pesci, E. C., and Vasil, M. L. (2008) J. Biol. Chem. 283 15558–15567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cox, C. D. (1986) Infect. Immun. 52 263–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dietrich, L. E., Price-Whelan, A., Petersen, A., Whiteley, M., and Newman, D. K. (2006) Mol. Microbiol. 61 1308–1321 [DOI] [PubMed] [Google Scholar]
- 30.Hernandez, M. E., and Newman, D. K. (2001) Cell Mol. Life Sci. 58 1562–1571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miller, R. A., Rasmussen, G. T., Cox, C. D., and Britigan, B. E. (1996) Infect. Immun. 64 182–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Britigan, B. E., Railsback, M. A., and Cox, C. D. (1999) Infect. Immun. 67 1207–1212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fuhrop, J. H., and Smith, K. M. (eds). (1975) Porphyrins and Metalloporphyrins, pp. 804–807, Elsevier, Amsterdam
- 34.Hoang, T. T., Karkhoff-Schweizer, R. R., Kutchma, A. J., and Schweizer, H. P. (1998) Gene (Amst.) 212 77–86 [DOI] [PubMed] [Google Scholar]
- 35.Ankenbauer, R. G., and Cox, C. D. (1988) J. Bacteriol. 170 5364–5367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Essar, D. W., Eberly, L., Hadero, A., and Crawford, I. P. (1990) J. Bacteriol. 172 884–900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kerr, J. R., Taylor, G. W., Rutman, A., Hoiby, N., Cole, P. J., and Wilson, R. (1999) J. Clin. Pathol. 52 385–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Outten, C. E., and O'Halloran, T. V. (2001) Science 292 2488–2492 [DOI] [PubMed] [Google Scholar]
- 39.Bradford, M. M. (1976) Anal. Biochem. 72 248–254 [DOI] [PubMed] [Google Scholar]
- 40.Sambrook, J., Fritsch, E. F., and Maniatis, T. (1989) Molecular Cloning: A Laboratory Manual, Cold Spring Harbor Laboratory, Cold Spring Harbor, NY.
- 41.Irizarry, R. A., Bolstad, B. M., Collin, F., Cope, L. M., Hobbs, B., and Speed, T. P. (2003) Nucleic Acids Res. 31 e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bolstad, B. M., Irizarry, R. A., Astrand, M., and Speed, T. P. (2003) Bioinformatics 19 185–193 [DOI] [PubMed] [Google Scholar]
- 43.Smyth, G. K. (2004) Stat. Appl. Genet. Mol. Biol. 3 1–25 [DOI] [PubMed] [Google Scholar]
- 44.Ihaka, R., and Gentleman, R. (1996) J. Comput. Graph. Stat. 5 299–314 [Google Scholar]
- 45.Stover, C. K., Pham, X. Q., Erwin, A. L., Mizoguchi, S. D., Warrener, P., Hickey, M. J., Brinkman, F. S., Hufnagle, W. O., Kowalik, D. J., Lagrou, M., Garber, R. L., Goltry, L., Tolentino, E., Westbrock-Wadman, S., Yuan, Y., Brody, L. L., Coulter, S. N., Folger, K. R., Kas, A., Larbig, K., Lim, R., Smith, K., Spencer, D., Wong, G. K., Wu, Z., Paulsen, I. T., Reizer, J., Saier, M. H., Hancock, R. E., Lory, S., and Olson, M. V. (2000) Nature 406 959–964 [DOI] [PubMed] [Google Scholar]
- 46.Ratliff, M., Zhu, W., Deshmukh, R., Wilks, A., and Stojiljkovic, I. (2001) J. Bacteriol. 183 6394–6403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Block, D. R., Lukat-Rodgers, G. S., Rodgers, K. R., Wilks, A., Bhakta, M. N., and Lansky, I. B. (2007) Biochemistry 46 14391–14402 [DOI] [PubMed] [Google Scholar]
- 48.Jensen, V., Lons, D., Zaoui, C., Bredenbruch, F., Meissner, A., Dieterich, G., Munch, R., and Haussler, S. (2006) J. Bacteriol. 188 8601–8606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mavrodi, D. V., Bonsall, R. F., Delaney, S. M., Soule, M. J., Phillips, G., and Thomashow, L. S. (2001) J. Bacteriol. 183 6454–6465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Palma, M., Worgall, S., and Quadri, L. E. (2003) Arch. Microbiol. 180 374–379 [DOI] [PubMed] [Google Scholar]
- 51.Schmitt, M. P. (1999) J. Bacteriol. 181 5330–5340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bibb, L. A., King, N. D., Kunkle, C. A., and Schmitt, M. P. (2005) Infect. Immun. 73 7406–7412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vanderpool, C. K., and Armstrong, S. K. (2003) J. Bacteriol. 185 909–917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Poole, K., and McKay, G. A. (2003) Front. Biosci. 8 d661–d686 [DOI] [PubMed] [Google Scholar]
- 55.Skaar, E. P., Humayun, M., Bae, T., DeBord, K. L., and Schneewind, O. (2004) Science 305 1626–1628 [DOI] [PubMed] [Google Scholar]
- 56.Price-Whelan, A., Dietrich, L. E., and Newman, D. K. (2006) Nat. Chem. Biol. 2 71–78 [DOI] [PubMed] [Google Scholar]
- 57.Price-Whelan, A., Dietrich, L. E., and Newman, D. K. (2007) J. Bacteriol. 189 6372–6381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.van Rij, E. T., Wesselink, M., Chin, A. W. T. F., Bloemberg, G. V., and Lugtenberg, B. J. (2004) Mol. Plant Microbe Interact. 17 557–566 [DOI] [PubMed] [Google Scholar]
- 59.Wegele, R., Tasler, R., Zeng, Y., Rivera, M., and Frankenberg-Dinkel, N. (2004) J. Biol. Chem. 279 45791–45802 [DOI] [PubMed] [Google Scholar]
- 60.Kaur, A. P., and Wilks, A. (2007) Biochemistry 46 2994–3000 [DOI] [PubMed] [Google Scholar]
- 61.Simon, R., Priefer, U., and Puhler, A. (1983) BioTechnology 1 784–791 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.