Abstract
The authors’ objective was to analyze the impact of respiratory impairment on the risk of physical functional limitations among adults with chronic obstructive pulmonary disease (COPD). They hypothesized that greater pulmonary function decrement would result in a broad array of physical functional limitations involving organ systems remote from the lung, a key step in the pathway leading to overall disability. The authors used baseline data from the Function, Living, Outcomes, and Work (FLOW) study, a prospective cohort study of adults with COPD recruited from northern California in 2005–2007. They studied the impact of pulmonary function impairment on the risk of functional limitations using validated measures: lower extremity function (Short Physical Performance Battery), submaximal exercise performance (6-Minute Walk Test), standing balance (Functional Reach Test), skeletal muscle strength (manual muscle testing with dynamometry), and self-reported functional limitation (standardized item battery). Multiple variable analysis was used to control for confounding by age, sex, race, height, educational attainment, and cigarette smoking. Greater pulmonary function impairment, as evidenced by lower forced expiratory volume in 1 second (FEV1), was associated with poorer Short Physical Performance Battery scores and less distance walked during the 6-Minute Walk Test. Lower forced expiratory volume in 1 second was also associated with weaker muscle strength and with a greater risk of self-reported functional limitation (p < 0.05). In conclusion, pulmonary function impairment is associated with multiple manifestations of physical functional limitation among COPD patients. Longitudinal follow-up can delineate the impact of these functional limitations on the prospective risk of disability, guiding preventive strategies that could attenuate the disablement process.
Keywords: pulmonary disease, chronic obstructive
Chronic obstructive pulmonary disease (COPD) is a common condition, affecting 5–10 percent of the US population (1, 2). During the past two decades, death from COPD has continued to increase, especially among women (1). Disability from the disease is substantial and is expected to increase in the United States and worldwide (3). Despite these trends, efforts to treat COPD have been disappointing. The only medical therapies that clearly reduce disease progression and mortality are smoking cessation and supplemental oxygen (4, 5). Although bronchodilators and inhaled corticosteroids are frequently prescribed, they do not clearly attenuate the deterioration of pulmonary function (5-9). Because currently available treatments have minimal impact on disease progression, a strategy to prevent disability would have important clinical and public health consequences.
Pulmonary function measurement is the most important indicator of respiratory impairment in COPD (10, 11), yet it is a surprisingly weak predictor of disability in terms of daily activity restriction (12-14). To account for this apparent paradox, we have developed a model of disablement in COPD based on the earlier work of Verbrugge and Jette (15). In this model, the impact of respiratory impairment on disability is mediated by its effect on specific functional limitations, which are decrements in basic physical actions such as mobility or strength. Consistent with this model, we theorize that specific functional limitations, including decreased lower extremity function, skeletal muscle weakness, decreased mobility, and reduced exercise performance, will result in disability in daily activities in patients with COPD.
To test the first pathway of this theoretical model, we analyzed the impact of respiratory impairment on the risk of physical functional limitations in the Function, Living, Outcomes, and Work (FLOW) cohort study of COPD. We hypothesized that greater pulmonary function decrement would result in a broad array of physical functional limitations involving organ systems remote from the lung. This analysis is the first step toward evaluating our model of the disablement process in COPD.
MATERIALS AND METHODS
Overview
The FLOW study of COPD is an ongoing prospective cohort study of adult members of an integrated health-care delivery system with a physician’s diagnosis of COPD. The long-term goal is to determine what factors are responsible for the development of disability in COPD. At baseline assessment, we conducted structured telephone interviews that ascertained COPD status, health status, self-reported functional limitations, and sociodemographic characteristics. Subjects then underwent a research clinic visit that included spirometry and other physical assessments. Using these baseline data, we evaluated the cross-sectional association between pulmonary function impairment and the risk of functional limitations among adults with COPD. The baseline structured telephone interviews were conducted between January 27, 2005, and February 3, 2007. The research clinic visit, which included spirometry, was conducted between February 3, 2005, and March 31, 2007. The study was approved by both the University of California, San Francisco, Committee on Human Research and the Kaiser Foundation Research Institute’s institutional review board, and all participants provided written, informed consent.
Subject recruitment
We studied adult members of the Kaiser Permanente Medical Care Program (KPMCP), the nation’s largest nonprofit managed care organization. In northern California, the KPMCP provides the full spectrum of primary-to-tertiary care to approximately 3.1 million members. In northern California, KPMCP’s share of the regional population ranges from 25 to 30 percent (16). The demographic characteristics of KPMCP members are similar to those of the overall northern California population, except for the extremes of income distribution (17).
We identified all adult KPMCP members who were recently treated for COPD using a previously described approach. The age range was restricted to 40–65 years, because a key study outcome includes work disability (18). Using KPMCP computerized databases, we identified all subjects who met each of two criteria: one based on health-care utilization and the second based on medication prescribing. The health-care utilization criterion was one or more ambulatory visits, emergency department visits, or hospitalizations with a principal International Classification of Diseases, Ninth Revision, diagnosis code for COPD (chronic bronchitis (code 491), emphysema (code 492), or COPD (code 496)) during a recent 12-month time period. The medication criterion was two or more prescriptions for a COPD-related medication during a 12-month window beginning 6 months before the index utilization date and ending 6 months after the index date. The criterion medications included inhaled anticholinergic medications, inhaled beta agonists, inhaled corticosteroids, and theophylline. On the basis of medical record review, we demonstrated that this algorithm is a valid method for identifying adults with COPD (18). To facilitate attendance at the research clinic, we restricted the sample to persons living within a 30-mile geographic radius of the research clinic where the study examinations took place. (One mile 1.6093 km.)
Persons identified by the algorithm who were no longer KPMCP members or who had moved away were considered ineligible for study. The primary care physicians for all patients were contacted and given the opportunity to decline contact of any identified patients under their care. Potential subjects were then contacted by a letter describing the study and given an opportunity to decline participation. Those not declining were then contacted by telephone to arrange an interview. At the end of the interview, subjects were invited to participate in the research clinic visit. Persons who were found at the time of interview to have severe communication difficulties attributable to advanced dementia or aphasia were excluded.
A total of 5,800 subjects were initially identified by use of the computerized algorithm. Of these, 298 died before they could be recruited into the study. Another 1,011 did not meet study inclusion criteria or were excluded at the time of interview contact as noted above. Among the 2,181 non-respondents, 464 declined by prepaid postcard, 934 declined by telephone, and 783 were not reachable by telephone despite numerous attempts (>10 attempts). The completion rate for structured telephone interviews was 2,310 out of a remaining eligible group of 4,491 (51 percent). This is comparable to our earlier cohort study of adult asthma conducted at KPMCP and compares favorably for other survey-based epidemiologic studies conducted in the United States (19, 20). Among the 2,310 respondents, 112 were not eligible for the clinic visit (eight were subsequently deceased, 10 were no longer Kaiser members, 85 were physically unable to attend, and nine moved out of the area). Of the 2,198 eligible subjects, 1,216 completed the research clinic visit (55 percent of those interviewed and eligible). An additional 10 subjects were excluded because they did not meet the Global Initiative for Chronic Obstructive Lung Disease (GOLD) criteria for COPD after interviews and spirometry were performed (11). Four additional subjects were excluded from this analysis because they could not perform spirometry because of previous tracheostomy placement. Ultimately, there were 1,202 subjects with COPD who completed both interviews and research clinic visits. Of the 982 subjects who were eligible for but did not complete clinic visits, the reported reasons were distance too far (n = 406), scheduling too difficult/inadequate time for clinic visit (n = 388), or other reasons (n = 188).
Demographic information was available for interviewed subjects from their structured telephone interviews and non-interviewed subjects from Kaiser computerized databases. Compared with subjects who were eligible but not interviewed, interviewed subjects were slightly older (by 0.7 years on average), more likely to be female (59 vs. 51 percent), and more likely to be White (69 vs. 56 percent). In terms of race/ethnicity, the two largest minority subgroups were slightly overrepresented among those who completed interviews (Black/African American: 14 percent vs. 11 percent; Hispanic: 9 percent vs. 4 percent). Most of the differences in race were driven by limitations inherent in the Kaiser computerized databases: The prevalence of unknown race was much higher among those who did not complete interviews (17 percent vs. 0.3 percent).
Compared with subjects who completed interviews but not the clinic visit, clinic visit attendees were similar in age (mean difference: 0.3 years), gender (58 percent vs. 55 percent female), and race/ethnicity (67 vs. 61 percent White). We were highly successful recruiting persons of Black or Hispanic background for the research clinic visit (17 percent completed vs. 11 percent not completed and 9 percent vs. 5 percent, respectively).
Structured telephone interviews
Each subject underwent a 30- to 40-minute structured telephone interview that used customized computer-assisted telephone interview software. Interviews ascertained socio-demographic characteristics. Cigarette smoking was measured by questions developed for the National Health Interview Survey (21). As in previous studies, we defined educational attainment as high school or less, some college, or college/graduate degree (22). Race/ethnicity was categorized as previously described (22).
Assessment of pulmonary function impairment
Respiratory impairment was the main predictor variable for this analysis. To assess respiratory impairment, we conducted spirometry according to American Thoracic Society guidelines (23, 24). Because of cost and logistic considerations, we selected a portable flow-based spirometer that could be reliably deployed in a research setting. We used the EasyOne Frontline spirometer (NDD Medical Technologies, Andover, Massachusetts), which is known for its reliability, accuracy, and durability (25, 26). The EasyOne spirometer has been used by two large-scale, multicenter, international epidemiologic studies of COPD: the Burden of Obstructive Lung Disease (BOLD) Study and the Latin American Project for the Investigation of Obstructive Lung Disease (Platino) Study (26, 27). These two studies combined have successfully used the EasyOne spirometer to measure pulmonary function in approximately 15,000 subjects on six continents. Consequently, the EasyOne spirometer has become the most widely used and reliable portable spirometer for epidemiologic studies of obstructive lung disease. Although no calibration is recommended by the company, we checked the calibration of the device daily using a 3-liter syringe (Hans-Rudolph, Kansas City, Missouri). During the study period, the device was always within a range of ±1 percent.
To calculate the percent predicted pulmonary function values, we used predictive equations derived from the Third National Health and Nutrition Examination Survey (28). On the basis of forced expiratory volume in 1 second (FEV1), the FEV1/forced vital capacity ratio, and respiratory symptoms, COPD severity was staged according to National Heart, Lung, and Blood Institute/World Health Organization GOLD criteria (stages 0-IV) (11, 29). Because research clinic examinations were conducted by trained nonmedical personnel, we did not administer bronchodilators for study purposes. However, 90 percent of subjects had taken their own short-acting bronchodilator within 4 hours of spirometry or had taken a long-acting bronchodilator earlier in the same day.
Assessment of functional limitations at research clinic visit
We assessed functional limitations, which are decrements in basic physical actions, using a multifaceted evaluation that combined a survey-based measure (self-reported functional limitation as described below) and physical assessment. Submaximal exercise performance was measured by the 6-Minute Walk Test, which was developed by Guyatt and has been widely used in studies of COPD (30, 31). We measured submaximal rather than maximal exercise performance (cardiopulmonary exercise testing), because most daily activities and work tasks are likely to require sustained submaximal exertion, rather than high peak exercise levels. We used a standardized flat, straight course of 30 m in accordance with American Thoracic Society guidelines (32). Subjects who routinely used home oxygen or who had a resting oxygen saturation of <90 percent were supplied with supplemental oxygen by nasal cannula during the test. Every 2 minutes, the technician used standardized phrases to encourage effort, as recommended by the American Thoracic Society guidelines. The primary outcome was the total distance walked in 6 minutes.
Lower extremity function was measured by use of the validated Short Physical Performance Battery (33-35). This battery includes three performance measures, each scored from 0 to 4 points. The standing balance test asks subjects to maintain their feet in a side-by-side, semitandem stand (heel of one foot next to the big toe of the other foot) or tandem stand (heel of one foot directly in front of the other foot) for 10 seconds. The maximum score of 4 is assigned for maintaining the tandem stand for 10 seconds; a low score of 1 is assigned for side-by-side standing for 10 seconds, with inability to hold a semitandem position for 10 seconds. A test of walking speed requires subjects to walk 4 m at their normal pace. Participants are assigned a score from 1 to 4 on the basis of the quartile of length of time needed to complete the test. The chair-stand test, which reflects lower extremity extensor muscle strength, measures the time required for the subject to stand up and sit down from a chair five times with arms folded across the chest. The chair height is standardized for all subjects. Scores from 1 to 4 are assigned by quartile of length of time to complete the task. A summary performance score integrates the three performance measures, ranging from 0 to 12. Previous work indicates that the battery has excellent interobserver reliability, test-retest reliability, and predictive validity (33-35).
In addition to measuring the balance component of the Short Physical Performance Battery, we also measured balance with the Functional Reach Test. This test measures how far a subject can reach forward beyond arm’s length while maintaining a fixed base of support in the standing position, without losing balance (36). The Functional Reach Test has excellent test-retest reliability and validity (36-39).
Isometric skeletal muscle strength was evaluated following standard manual muscle-testing procedures (40). A hand-held dynamometer was used to improve the objectivity of the force estimates (MicroFet2 dynamometer; Saemmons Preston, Bolingbrook, Illinois) (40). The examiners were trained in manual muscle testing by the same experienced physical therapist. Each of the examiners practiced testing control subjects until there was agreement between the raters 90 percent of the time within 5 pounds of force. (One pound = 0.4536 kg.)
Knee extensor (quadriceps), hip extensor, and hip abductor (i.e., gluteus medius) strengths were measured because these muscles are considered critical for standing and walking. In addition, previous work has also suggested the importance of quadriceps weakness as a predictor of reduced maximal exercise performance in COPD (41-44). In the upper extremity, grip and elbow flexion strength were measured because these muscles are important for performing many daily activities.
Isometric knee extensor strength was measured with the subject in the sitting position with the knee over the side of the table (knee and hip flexed at 90 degrees), hands on the table, and the examiner placing one hand under the thigh to cushion against the pressure of the table and help isolate knee extension (40, 45). Resistance was applied by the examiner in the direction of flexion with the dynamometer placed on the anterior aspect of the tibia with its lower edge at the level of the malleoli. Subjects were asked to extend their leg and gradually increase the force applied against the examiner to a maximum over 3–5 seconds. The force was matched by the examiner. Peak force values were recorded for three trials on each side in alternating fashion. The strengths of hip extensors, hip abductors, and elbow flexion were measured in similar fashion by standard manual muscle-testing positions and stabilization techniques (45).
Power grip strength was measured by use of a Jamar Hydraulic Hand Dynamometer (Stoelting, Wood Dale, Illinois) according to the protocol of the American Society of Hand Therapists (46, 47). The width of the dynamometer opening was adjusted to the size of the subject’s hand. In a seated position, each subject kept his or her arm at the side, with the shoulder adducted and neutrally rotated, the elbow flexed at 90 degrees, and the forearm in a neutral position between supination and pronation. The examiner stabilized the elbow, and the subject was asked to squeeze the dynamometer, exerting a maximum grip. Three trials were performed of each hand in alternating fashion. For this analysis, the maximum of three trials on the dominant side was selected.
Assessment of self-reported functional limitation
Self-reported functional limitation was measured by a previously validated approach used by Sternfeld et al. (48). The scale comprises 10 questions that assess the degree of difficulty in multiple domains of basic physical functioning, such as pushing, stooping, kneeling, getting up from a seated position, lifting lighter or heavier objects, standing, sitting, standing from a seated position, walking up stairs, and walking in the neighborhood. Subjects who indicate “a lot of difficulty” with one or more functions or not doing a function because they were unable or they were told by a doctor not to do so are defined by this measure as having a self-reported functional limitation (48).
In addition, two additional questions were derived from the Medical Outcomes Study 36-item short-form health survey (SF-36) scale to measure functional limitation (49). These items ask whether the respondent’s health limits him or her a lot, a little, or not at all in moderate activities (e.g., moving a table, pushing a vacuum cleaner, bowling, or playing golf) or climbing several flights of stairs. Subjects who indicated “a lot” of limitation were defined as having functional limitation on each item.
Statistical analysis
Statistical analysis was conducted with SAS, version 9.1, software (SAS Institute, Inc., Cary, North Carolina) and STATA, version 10, software (StataCorp LP, College Station, Texas). We used linear regression analysis to elucidate the association between pulmonary function impairment (FEV1) and performance on the Short Physical Performance Battery, 6-Minute Walk Test, Functional Reach Test, and skeletal muscle strength tests. To examine potential confounding, we present two sets of analyses that control for covariates: age, sex, and height; age, sex, height, race (White, non-Hispanic vs. other), educational attainment, and smoking status (current or past vs. never). Income was not included as a covariate, because it may be the outcome of COPD health status; that is, COPD may lead to work disability and diminished income.
We used linear regression diagnostics to evaluate model assumptions. The linearity assumption was evaluated by examining scatterplots of the data; we also compared the fitted regression line with a regression line fitted by the locally weighted regression scatterplot smoother (LOWESS) procedure (50). Residual versus fitted plots and residual versus predicted plots were used to evaluate the functional form and the constant variance assumption. We used normal/normal probability (QQ) plots to evaluate the normality requirement. Boxplots of “dfbeta” statistics (a STATA term) were used to check for outliers. The assumptions were met for all linear models except for the Short Physical Performance Battery score, which we dichotomized into the lowest quartile to correspond to “poor” Short Physical Performance Battery scores and used logistic regression analysis (below).
Logistic regression was used to analyze the relation between FEV1 and the risk of self-reported functional limitations in analogous fashion. In addition, we used logistic regression to evaluate the impact of FEV1 on the risk of poor lower extremity function (defined as the lowest quartile of Short Physical Performance Battery score, as described above). We used the LOWESS procedure to plot the logit of each dependent variable versus FEV1. For the functional limitation measures, the LOWESS plot suggested a quadratic relation between the outcome variable and FEV1, which was confirmed by including a quadratic term for FEV1 in the logistic regression model. The Hosmer-Lemeshow goodness-of-fit test was used to evaluate logistic regression model fit. In all cases, model fit was adequate (p > 0.40).
Tests for interaction were performed to evaluate whether GOLD stage, symptom status (chronic bronchitis symptoms, dyspnea on exertion, both, or neither), or index diagnosis (chronic bronchitis, emphysema, or COPD) modified the association between pulmonary function impairment and functional limitation. There was no evidence of statistical interaction in any case (p > 0.20 for each); therefore, we did not include such interactions in any of the final models presented.
RESULTS
Baseline sociodemographic and clinical characteristics of the FLOW cohort
The mean age was 58 years, and more than half of the subjects were female (57 percent) (table 1). There was substantial diversity of race/ethnicity and socioeconomic status, as indicated by educational attainment and household income. Consistent with causal association between smoking and COPD, the majority (87 percent) reported smoking during their lifetime.
TABLE 1.
Baseline characteristics of the FLOW* cohort (n = 1,202), California, 2005-2007
| Characteristic | Mean (SD*) | |
|---|---|---|
| Age (years) | 58.2 (6.2) |
|
| No. | % | |
| Sex (female) | 691 | 57 |
| Race/ethnicity | ||
| White, non-Hispanic | 810 | 67 |
| Black/African American | 206 | 17 |
| Asian/Pacific Islander | 35 | 3 |
| Hispanic | 111 | 9 |
| Other | 40 | 3 |
| Smoking history | ||
| Never smoked | 162 | 13 |
| Current smoker | 393 | 33 |
| Former smoker | 644 | 54 |
| Educational attainment | ||
| High school or less | 352 | 29 |
| Some college | 524 | 44 |
| College or graduate degree | 326 | 27 |
| Household income ($) | ||
| Low (<20,000) | 129 | 11 |
| Intermediate (20,000–80,000) | 699 | 58 |
| High (>80,000) | 276 | 23 |
| Declined to report | 98 | 8 |
FLOW, Function, Living, Outcomes, and Work
SD, standard deviation
Table 2 shows pulmonary function and related measurements. The mean FEV1 was 1.79 liters, and the majority of subjects were GOLD stage 1 or greater. A substantial minority reported home oxygen use (10 percent), consistent with advanced disease. Most subjects had exertional dyspnea either with or without concomitant chronic bronchitis symptoms. The most common index diagnosis within the broader COPD spectrum was COPD itself, followed by chronic bronchitis and emphysema.
TABLE 2.
Pulmonary function and stage of chronic obstructive pulmonary disease in the FLOW* cohort, California, 2005-2007
| Measure | Mean (SD*) | |
|---|---|---|
| FEV1* (liters) | 1.79 (0.78) | |
| FEV1 (% predicted) | 62 (23) | |
| FEV1/FVC* ratio | 0.61 (0.15) |
|
| No. | % | |
| GOLD* stage | ||
| 0 | 374 | 31 |
| 1 | 87 | 7 |
| 2 | 381 | 32 |
| 3 | 248 | 21 |
| 4 | 112 | 9.3 |
| Home oxygen use | 118 | 10 |
| Symptom status | ||
| Chronic bronchitis† | 75 | 6.2 |
| Dyspnea on exertion‡ | 504 | 42 |
| Both | 452 | 38 |
| Neither | 171 | 14 |
| Index diagnosis code (ICD-9*)§ | ||
| Chronic bronchitis (491.xx) | 249 | 21 |
| Emphysema (492.xx) | 65 | 5 |
| COPD* (496.xx) | 845 | 70 |
| Multiple diagnoses | 43 | 4 |
FLOW, Function, Living, Outcomes, and Work
SD, standard deviation; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; GOLD, Global Initiative for Chronic Obstructive Lung Disease; ICD-9, International Classification of Diseases, Ninth Revision; COPD, chronic obstructive pulmonary disease.
Sputum production for ≥3 months for at least 2 years.
From the Medical Research Council dyspnea scale.
The index diagnosis was the initial utilization identified for cohort selection by Kaiser Permanente Medical Care Program computerized databases.
Association between pulmonary function impairment and functional limitation
As shown in table 3 and figure 1, greater pulmonary function impairment, as evidenced by lower FEV1, was associated with a greater risk of poor lower extremity function (per 1-liter decrement in FEV1: odds ratio 1.5, 95 percent confidence interval (CI): 1.2, 1.9). A LOWESS plot of the log odds (logit) of poor lower extremity function versus FEV1 revealed a linear relation over most of the range of FEV1 (below approximately 4.5 liters). Lower FEV1 was also associated with less distance walked during the 6-Minute Walk Test (per 1-liter decrement in FEV1: mean score decrease = −160 feet, 95 percent CI: −128, −191) (1 foot = 0.3048 m) (table 3; figure 1). There was no clear statistical relation between pulmonary function impairment and the Functional Reach Test of standing balance. In addition, there was no statistical association between FEV1 and the tandem stand test of balance that comprises one component of the Short Physical Performance Battery (p = 0.82).
TABLE 3.
Impact of pulmonary function impairment on functional limitation (lower extremity functioning, exercise performance, and balance), California, 2005-2007
| Functional limitation | Test | Odds ratio or mean change in score* |
95% confidence interval |
p value | Odds ratio or mean change in score† |
95% confidence interval |
p value |
|---|---|---|---|---|---|---|---|
| Lower extremity function‡ |
Short Physical Performance Battery total score (lowest quartile) |
1.6§ | 1.3, 2.0 | <0.0001 | 1.5§ | 1.2, 1.9 | 0.0003 |
| Submaximal exercise performance¶ |
6-Minute Walk Test (feet#) | -172 | -141, -204 | <0.0001 | -160 | -128, -191 | <0.0001 |
| Standing balance¶ | Functional Reach Test (cm) | -0.61 | 1.32, -0.10 | 0.09 | -0.50 | 1.21, -0.22 | 0.17 |
Per 1-liter decrease in forced expiratory volume in 1 second (FEV1) controlling for age, sex, and height.
Per 1-liter decrease in FEV1 controlling for age, sex, height, race, educational attainment, and smoking.
Because the requirement for linear regression was not met for the Short Physical Performance Battery (SPPB) score, we dichotomized it into the lowest quartile (“poor SPPB score”) as the dependent variable. We used logistic analysis, and the odds ratio is the risk of poor SPPB score for each 1-liter decrement in FEV1.
Odds ratio.
Results from linear regression are the mean change in the dependent variable for each 1-liter decrement in FEV1. There were 18 subjects who could not perform the 6-Minute Walk Test and 35 who could not perform the Functional Reach Test. The chair stand and gait speed subscores were statistically related to FEV1 (p<0.05), whereas the tandem stand balance subscore was not (p = 0.78).
One foot = 0.3048 m.
FIGURE 1.
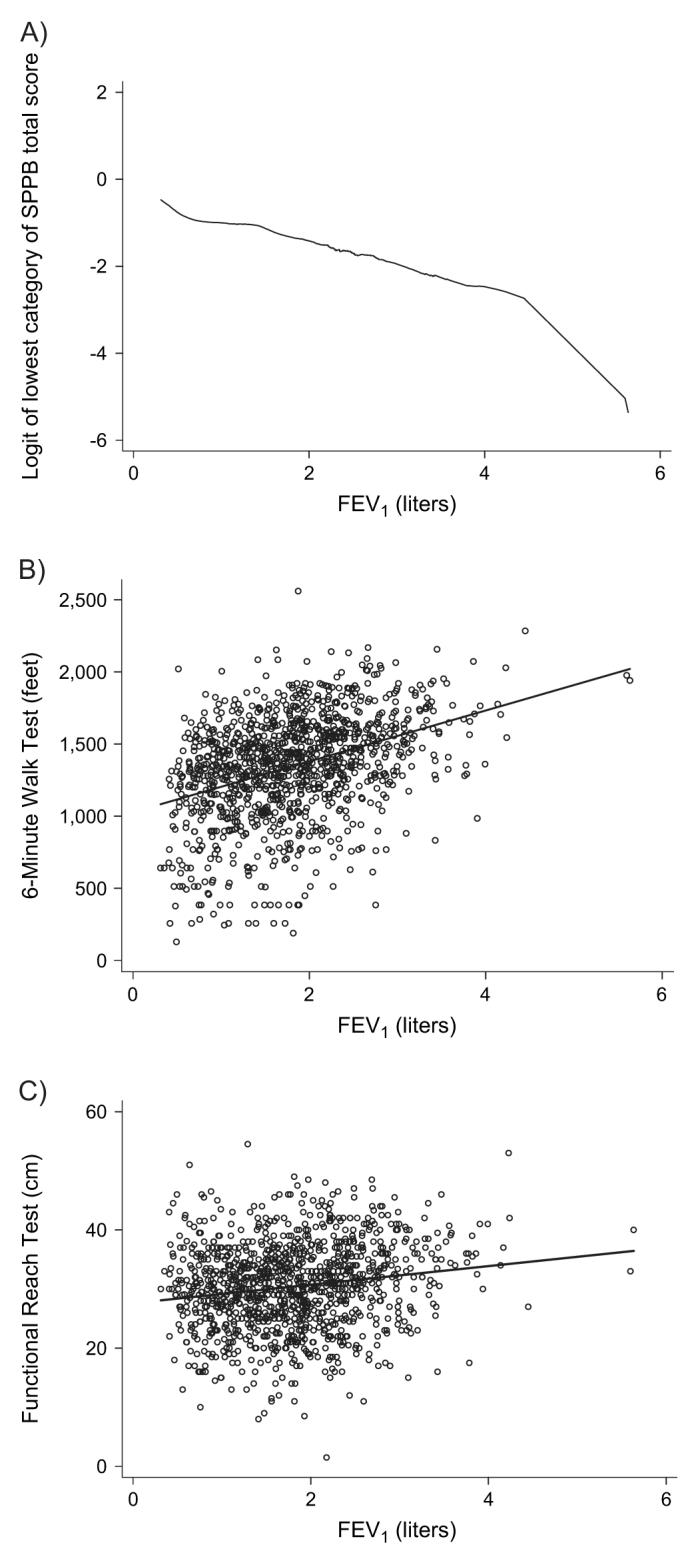
Impact of pulmonary function impairment on functional limitation (lower extremity functioning, exercise performance, and balance), California, 2005-2007. The figure shows scatterplots and the fitted regression lines. Part A shows the relation between the log odds (logit) of poor lower extremity function (i.e., the lowest quartile of the Short Physical Performance Battery (SPPB) score) and forced expiratory volume in 1 second (FEV1). Part B depicts the association between distance walked in 6 minutes (6-Minute Walk Test) and FEV1. Part C displays the association between the Functional Reach Test of standing balance and FEV1. One foot = 0.3048 m.
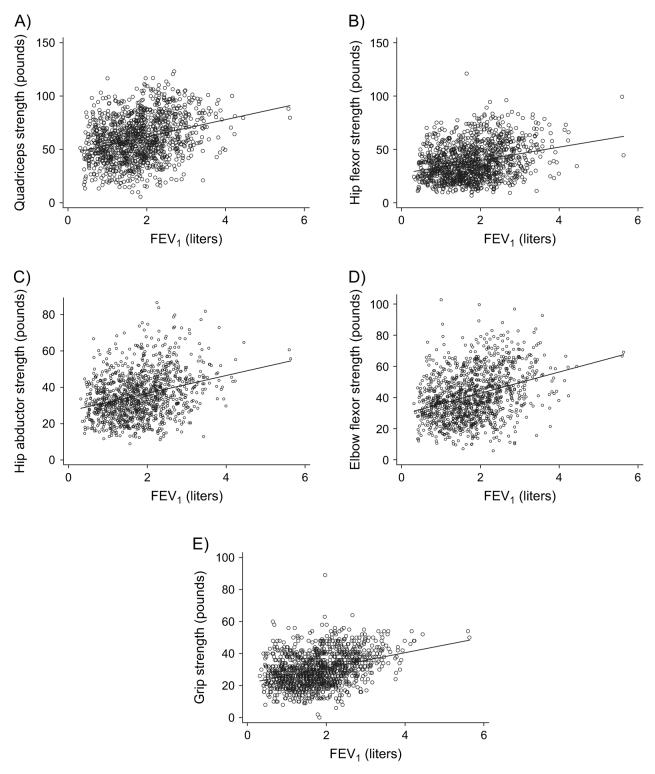
Greater pulmonary function impairment was related to weaker skeletal muscle strength in all muscle groups tested (table 4; figure 2). Both upper and lower extremity strength were affected. Poorer pulmonary function was also associated with a greater risk of self-reported functional limitation, moderate activity limitation, and limitation in stair climbing (table 5). Because the model included a quadratic term for FEV1, there is no single odds ratio for FEV1. Figure 3 shows the curvilinear increase in the logit of each self-reported functional limitation outcome as FEV1 decreases.
TABLE 4.
Impact of pulmonary function impairment on functional limitation (skeletal muscle strength), California, 2005-2007
| Region | Muscle group* |
Mean change in score (pounds†)‡ |
95% confidence interval |
p value | Mean change in score (pounds)§ |
95% confidence interval |
p value |
|---|---|---|---|---|---|---|---|
| Lower extremity | Quadriceps | -3.6 | -2.0, -5.1 | 0.0001 | -3.4 | -1.9, -5.0 | <0.0001 |
| Hip flexors | -2.2 | -0.92, -3.4 | 0.0007 | -2.2 | -0.9, -3.4 | 0.0008 | |
| Hip abductors | -2.1 | -1.1, -3.1 | <0.0001 | -1.9 | -0.9, -3.0 | <0.0001 | |
| Upper extremity | Elbow flexors | -1.8 | -0.8, -2.9 | 0.0008 | -1.9 | -0.8, -2.9 | 0.0007 |
| Grip | -0.72 | -0.14, -1.30 | 0.015 | -0.75 | -0.16, -1.34 | 0.012 |
A total of 20 subjects could not perform quadriceps or elbow flexor testing, 19 could not perform hip flexor testing, 32 could not perform hip abductor testing, and 10 could not perform grip strength.
One pound = 453.5924 g.
Per 1-liter decrease in forced expiratory volume in 1 second (FEV1) controlling for age, sex, and height.
Per 1-liter decrease in FEV1 controlling for age, sex, height, race, educational attainment, and smoking.
FIGURE 2.
Impact of pulmonary function impairment on functional limitation (skeletal muscle strength), California, 2005-2007. The figure depicts scatterplots of the relation with the fitted regression line. Part A shows the relation between quadriceps strength and forced expiratory volume in 1 second (FEV1). The remaining parts depict the association between the following muscle groups and FEV1: part B, hip flexor strength; part C, hip abductor strength; part D, elbow flexor strength; and part E, grip strength. One pound = 453.5924 g.
TABLE 5.
Impact of pulmonary function impairment on self-reported functional limitation, California, 2005-2007*
| Functional limitation measure |
β-coefficient per 1-liter decrease in FEV1 (SE†) |
p value |
β-coefficient per 1-liter decrease in FEV12 (quadratic term) (SE) |
p value |
|---|---|---|---|---|
| Self-reported functional limitation‡ |
-1.40 (0.30) | <0.0001 | 0.17 (0.075) | 0.020 |
| Limitation in moderate activities§ |
-1.28 (0.32) | <0.0001 | 0.15 (0.08) | 0.056 |
| Limitation in climbing several flights of stairs |
-1.87 (0.31) | <0.0001 | 0.23 (0.071) | 0.001 |
Multiple variable logistic regression analysis controlling for age, sex, race, height, smoking, and education. The model includes a quadratic term for 1-liter decrease in forced expiratory volume in 1 second (FEV1) to account for the curvilinear relation between FEV1 and the log odds of functional limitation (refer to figure 3).
SE, standard error.
“Unable to do” or “severe limitation” in a battery of basic physical activities (refer to Materials and Methods).
Health limits “a lot” in performing moderate activities, such as moving a table, pushing a vacuum cleaner, bowling, or playing golf.
FIGURE 3.
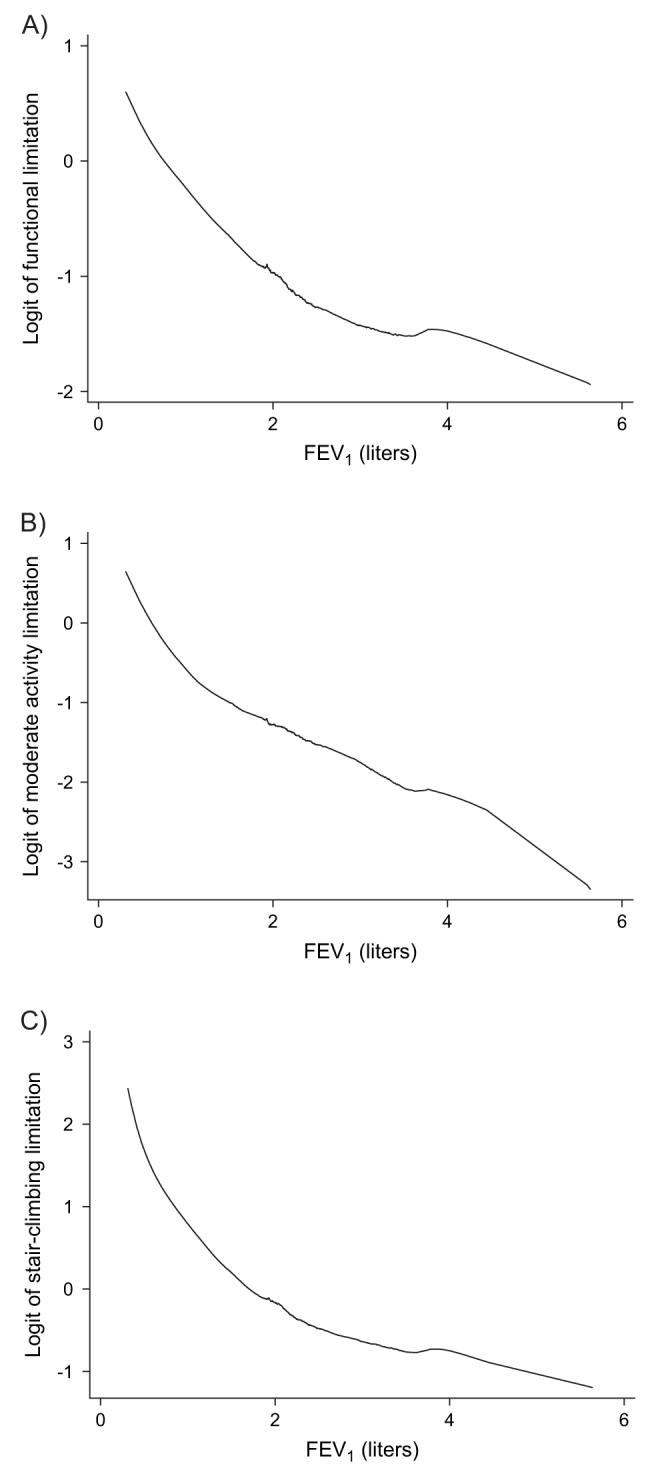
Impact of pulmonary function impairment on self-reported functional limitation, California, 2005-2007. The figure shows the association between the log odds (logit) of each self-reported functional limitation and forced expiratory volume in 1 second (FEV1) fitted by the LOWESS procedure. Part A shows self-reported functional limitation (“unable to do” or “severe limitation” in a battery of basic physical activities (refer to Materials and Methods)). Part B shows limitation of moderate activities (health limits “a lot” in performing moderate activities, such as moving a table, pushing a vacuum cleaner, bowling, or playing golf). Part C depicts limitation in climbing several flights of stairs. In each case, the relation was curvilinear, and a quadratic term was statistically significant in the logistic regression analysis. LOWESS, locally weighted regression scatterplot smoother.
Submaximal exercise performance and lower extremity function
To elucidate the contribution of lower extremity function to submaximal exercise performance, we regressed the distance walked in 6 minutes (6-Minute Walk Test) on the Short Physical Performance Battery score and lower extremity muscle strength (hip flexors, hip abductors, and quadriceps strength). After controlling for FEV1 and the other covariates, higher Short Physical Performance Battery scores were related to a greater distance walked in 6 minutes (115 feet per 1-point increment, 95 percent CI: 92, 139 feet). In addition, better strengths of quadriceps and hip flexor muscles were associated with a greater 6-Minute Walk Test distance (2.4 feet per each 1-pound increment of muscle force, 95 percent CI: 1.2, 3.7; and 1.8 feet per pound of muscle force, 95 percent CI: 0.3, 3.3, respectively). There was no relation between hip abductor strength and distance walked.
DISCUSSION
Pulmonary function impairment was related to a broad array of physical functional limitations among patients with COPD, including lower extremity functioning, exercise performance, skeletal muscle strength, and self-reported limitation in basic physical actions. These findings are consistent with the theory that the central pulmonary function impairment of COPD translates into diverse impacts on body systems that ultimately compromise physical functioning.
Although knowledge about functional limitation in COPD has been limited, cohort studies of older, community-living adults without lung disease indicate that limitation of lower extremity function is a key determinant of subsequent disability (34). In a general population sample of 1,122 older adults, lower extremity function was measured using the Short Physical Performance Battery (33). During 4-year follow-up, reduced baseline lower extremity function was associated with a greater risk of developing incident disability in activities of daily living. Decreased lower extremity function was also related to all-cause mortality, with a gradient of risk across the entire functional spectrum (35). Our finding that pulmonary function impairment is related to poorer lower extremity function in COPD may translate into a greater risk of disability during longitudinal follow-up.
Respiratory muscle weakness, especially of the diaphragm, has long been considered to be an important determinant of dyspnea and decreased exercise tolerance in COPD (51). Emerging evidence suggests that peripheral skeletal muscle strength is also reduced in COPD (52-54). Although some studies indicate that lower extremity strength is more affected than upper extremity strength in COPD, other investigators have found significant upper extremity involvement, suggesting a generalized myopathy (41-44, 52, 55-58). Our findings suggest that greater pulmonary function impairment may result in both upper and lower extremity muscle weakness in COPD, consistent with systemic involvement from the disease. Skeletal muscle weakness, in turn, could mediate COPD-related disability. Prospective follow-up will be necessary to determine the impact of this key functional limitation on the disablement process in COPD.
The etiology of peripheral muscle dysfunction in COPD remains unclear. Skeletal muscle metabolism appears to be altered, with reduced oxidative metabolism (59). Specific effects of the disease, such as hypoxemia or acidosis, may impair muscle function (60). Deconditioning from inactivity, malnutrition, oxidative stress, and chronic inflammation are other explanations (52, 61, 62). “Sarcopenia,” or reduced muscle mass, may occur (52). Treatment with corticosteroids may also reduce skeletal and respiratory muscle strength (42, 55).
In our analysis, reduced lower extremity function and muscle strength contributed to decreased exercise performance, an integrative functional limitation that may contribute to the development of COPD-related disability. The negative effect of COPD on exercise performance has been demonstrated for both submaximal and maximal exercise performance (31, 63-66). Submaximal exercise performance, such as the distance walked in 6 minutes, may have a greater impact on the risk of developing COPD-related disability than maximal exercise. This is because daily activities and work usually require sustained lower level exertion, rather than briefer periods of maximum exercise. Our finding that greater pulmonary function impairment is linked with poorer exercise performance among COPD patients may have longer term significance for the disablement process.
Pulmonary rehabilitation programs have become an important management strategy for improving quality of life in patients with COPD (67). Our findings suggest that rehabilitation programs aimed at increasing lower extremity strength, in particular, may improve walking and other activities that involve submaximal exercise capacity. Future longitudinal follow-up of the FLOW cohort should elaborate the impact of functional limitation on the risk of disability, which would also have important implications for pulmonary rehabilitation.
A notable study strength is the large sample of COPD patients who have a broad spectrum of disease severity, ranging from mild to severe. Our study is, to our knowledge, the largest prospective COPD cohort study to systematically evaluate a broad range of functional limitations, such as lower extremity function and skeletal muscle strength. Recruitment from a large health plan should also help to ensure generalizability to patients who are being treated for COPD in clinical practice. Availability of interview data, pulmonary function tests, and extensive physical characterization allows robust conclusions about physical functioning.
Our study is also subject to several limitations. Although the inclusion criteria required health-care utilization for COPD, misclassification of asthma could have occurred. Our COPD definition required concomitant treatment with COPD medications to increase the specificity of the definition. In addition, all patients had a physician diagnosis of COPD and reported having the condition. The observed lifetime smoking prevalence was similar to that in other population-based epidemiologic studies of COPD, supporting the diagnosis of COPD rather than asthma (1, 68). We also previously demonstrated the validity of our approach using medical record review (18). Nonetheless, we cannot exclude the possibility that some subjects, especially those with GOLD stage 0, may have conditions other than COPD. When we excluded GOLD stage 0 from key analyses, however, the results were not substantively affected (data not shown). For the present analysis, we would expect any misclassification to have a conservative effect (i.e., reducing the impact of pulmonary function on functional limitation).
Because our ultimate focus is on disability prevention, we intentionally sampled younger adults with COPD. Therefore, these results may underestimate the impact of pulmonary function impairment among older patients with COPD. In addition, Kaiser Permanente members, because they have health-care access, may also be different from the general population of adults with COPD. Mitigating these limitations, the sociodemographic characteristics of Northern California Kaiser Permanente members are similar to those of the regional population, with some underrepresentation of income extremes (16, 17). Moreover, selection bias could have been introduced by nonparticipation in the study. There were some differences among subjects who did and did not participate in the interviews and clinic visits, but they were modest in scope and not likely to affect the relation between pulmonary function and functional limitation. In addition, when we repeated the main analysis weighted to account for nonparticipation, there were no substantive differences in the results (data not shown). Nonetheless, we acknowledge the potential for selection bias as a limitation of our study.
Our analysis is guided by a specific theoretical model about how pulmonary function impairment leads to subsequent functional limitations and disability. This is based on the earlier work of Verbrugge and Jette (15). We acknowledge, however, that there could be areas in which the causal pathway has not yet been fully elucidated.
We measured muscle strength with a hand-held dynamometer rather than a computerized isokinetic dynamometer, such as the Cybex II (Lumex, Inc., New York, New York). Although isokinetic dynamometers can provide better isolation of muscle groups and more information about muscle dynamics, they are expensive and require specialized training to use. In contrast, hand-held dynamometers are more feasible, in terms of both cost (about $1,000) and ease of use. Hand-held dynamometers provide reliable and valid results, correlating strongly with isokinetic dynamometer results (69-74). In particular, hand-held dynamometers have been used to measure knee extension strength in studies of COPD (41, 55, 57). In epidemiologic studies, hand-held dynamometer measurements of knee extensor strength and grip strength have been associated with disability of mobility tasks, supporting the construct validity of this testing approach (69, 75-77). We minimized measurement variability by having the same experienced physical therapist train the examiners in a standardized fashion and ensuring adequate interrater reliability.
In sum, greater pulmonary function impairment was associated with physical functional limitation among COPD patients. Longitudinal follow-up will determine the impact of these functional limitations on the risk of disability. Preventive strategies may ultimately be designed to target loss of physical function to attenuate the disablement process.
ACKNOWLEDGMENTS
Supported by National Heart, Lung, and Blood Institute grant R01HL077618 from the National Institutes of Health.
Abbreviations
- CI
confidence interval
- COPD
chronic obstructive pulmonary disease
- FEV1
forced expiratory volume in 1 second
- FLOW
Function, Living, Outcomes, and Work
- GOLD
Global Initiative for Chronic Obstructive Lung Disease
- KPMCP
Kaiser Permanente Medical Care Program
- LOWESS
locally weighted regression scatterplot smoother
Footnotes
Conflict of interest: none declared.
REFERENCES
- 1.Mannino DM, Homa DM, Akinbami LJ, et al. Chronic obstructive pulmonary disease surveillance—United States, 1971–2000. MMWR Surveill Summ. 2002;51:1–16. [PubMed] [Google Scholar]
- 2.Halbert RJ, Isonaka S, George D, et al. Interpreting COPD prevalence estimates: what is the true burden of disease? Chest. 2003;123:1684–92. doi: 10.1378/chest.123.5.1684. [DOI] [PubMed] [Google Scholar]
- 3.Murray CJ, Lopez AD. Alternative projections of mortality and disability by cause 1990–2020: Global Burden of Disease Study. Lancet. 1997;349:1498–504. doi: 10.1016/S0140-6736(96)07492-2. [DOI] [PubMed] [Google Scholar]
- 4.Continuous or nocturnal oxygen therapy in hypoxemic chronic obstructive lung disease: a clinical trial. Nocturnal Oxygen Therapy Trial Group. Ann Intern Med 198093391–8. [DOI] [PubMed] [Google Scholar]
- 5.Anthonisen NR, Connett JE, Kiley JP, et al. Effects of smoking intervention and the use of an inhaled anticholinergic bronchodilator on the rate of decline of FEV1. The Lung Health Study. JAMA. 1994;272:1497–505. [PubMed] [Google Scholar]
- 6.Burge PS, Calverley PM, Jones PW, et al. Randomised, double blind, placebo controlled study of fluticasone propionate in patients with moderate to severe chronic obstructive pulmonary disease: the ISOLDE trial. BMJ. 2000;320:1297–303. doi: 10.1136/bmj.320.7245.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Effect of inhaled triamcinolone on the decline in pulmonary function in chronic obstructive pulmonary disease. N Engl J Med. 2000;343:1902–9. doi: 10.1056/NEJM200012283432601. [DOI] [PubMed] [Google Scholar]
- 8.Pauwels RA, Lofdahl CG, Laitinen LA, et al. Long-term treatment with inhaled budesonide in persons with mild chronic obstructive pulmonary disease who continue smoking. European Respiratory Society Study on Chronic Obstructive Pulmonary Disease. N Engl J Med. 1999;340:1948–53. doi: 10.1056/NEJM199906243402503. [DOI] [PubMed] [Google Scholar]
- 9.Vestbo J, Sorensen T, Lange P, et al. Long-term effect of inhaled budesonide in mild and moderate chronic obstructive pulmonary disease: a randomised controlled trial. Lancet. 1999;353:1819–23. doi: 10.1016/s0140-6736(98)10019-3. [DOI] [PubMed] [Google Scholar]
- 10.Mannino DM. COPD: epidemiology, prevalence, morbidity and mortality, and disease heterogeneity. Chest. 2002;121(suppl):121S–6S. doi: 10.1378/chest.121.5_suppl.121s. [DOI] [PubMed] [Google Scholar]
- 11.Pauwels RA, Buist AS, Calverley PM, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) Workshop summary. Am J Respir Crit Care Med. 2001;163:1256–76. doi: 10.1164/ajrccm.163.5.2101039. [DOI] [PubMed] [Google Scholar]
- 12.Bestall JC, Paul EA, Garrod R, et al. Usefulness of the Medical Research Council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax. 1999;54:581–6. doi: 10.1136/thx.54.7.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jette DU, Manago D, Medved E, et al. The disablement process in patients with pulmonary disease. Phys Ther. 1997;77:385–94. doi: 10.1093/ptj/77.4.385. [DOI] [PubMed] [Google Scholar]
- 14.Williams SJ, Bury MR. ‘Breathtaking’: the consequences of chronic respiratory disorder. Int Disabil Stud. 1989;11:114–20. doi: 10.3109/03790798909166409. [DOI] [PubMed] [Google Scholar]
- 15.Verbrugge LM, Jette AM. The disablement process. Soc Sci Med. 1994;38:1–14. doi: 10.1016/0277-9536(94)90294-1. [DOI] [PubMed] [Google Scholar]
- 16.Karter AJ, Ferrara A, Liu JY, et al. Ethnic disparities in diabetic complications in an insured population. JAMA. 2002;287:2519–27. doi: 10.1001/jama.287.19.2519. [DOI] [PubMed] [Google Scholar]
- 17.Krieger N. Overcoming the absence of socioeconomic data in medical records: validation and application of a census-based methodology. Am J Public Health. 1992;82:703–10. doi: 10.2105/ajph.82.5.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sidney S, Sorel M, Quesenberry CP, Jr, et al. COPD and incident cardiovascular disease hospitalizations and mortality: Kaiser Permanente Medical Care Program. Chest. 2005;128:2068–75. doi: 10.1378/chest.128.4.2068. [DOI] [PubMed] [Google Scholar]
- 19.Calfee CS, Katz PP, Yelin EH, et al. The influence of perceived control of asthma on health outcomes. Chest. 2006;130:1312–18. doi: 10.1378/chest.130.5.1312. [DOI] [PubMed] [Google Scholar]
- 20.Eisner MD, Yelin EH, Katz PP, et al. Risk factors for work disability in severe adult asthma. Am J Med. 2006;119:884–91. doi: 10.1016/j.amjmed.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 21.Cigarette smoking among adults—United States, 1997. MMWR Morb Mortal Wkly Rep. 1999;48:993–6. [PubMed] [Google Scholar]
- 22.Eisner MD, Yelin EH, Trupin L, et al. The influence of chronic respiratory conditions on health status and work disability. Am J Public Health. 2002;92:1506–13. doi: 10.2105/ajph.92.9.1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.American Thoracic Society Standardization of spirometry— 1987 update. Statement of the American Thoracic Society. Am Rev Respir Dis. 1987;136:1285–98. doi: 10.1164/ajrccm/136.5.1285. [DOI] [PubMed] [Google Scholar]
- 24.Standardization of spirometry, 1994 update. American Thoracic Society. Am J Respir Crit Care Med 19951521107–36. [DOI] [PubMed] [Google Scholar]
- 25.Walters JA, Wood-Baker R, Walls J, et al. Stability of the EasyOne ultrasonic spirometer for use in general practice. Respirology. 2006;11:306–10. doi: 10.1111/j.1440-1843.2006.00842.x. [DOI] [PubMed] [Google Scholar]
- 26.Perez-Padilla R, Vazquez-Garcia JC, Marquez MN, et al. The long-term stability of portable spirometers used in a multinational study of the prevalence of chronic obstructive pulmonary disease. Respir Care. 2006;51:1167–71. [PubMed] [Google Scholar]
- 27.Buist AS, McBurnie MA, Vollmer WM, et al. International variation in the prevalence of COPD (the BOLD Study): a population-based prevalence study. Lancet. 2007;370:741–50. doi: 10.1016/S0140-6736(07)61377-4. [DOI] [PubMed] [Google Scholar]
- 28.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159:179–87. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 29.Fabbri LM, Hurd SS. Global strategy for the diagnosis, management and prevention of COPD: 2003 update. Eur Respir J. 2003;22:1–2. doi: 10.1183/09031936.03.00063703. [DOI] [PubMed] [Google Scholar]
- 30.Guyatt GH, Sullivan MJ, Thompson PJ, et al. The 6-minute walk: a new measure of exercise capacity in patients with chronic heart failure. Can Med Assoc J. 1985;132:919–23. [PMC free article] [PubMed] [Google Scholar]
- 31.Sciurba F, Criner GJ, Lee SM, et al. Six-minute walk distance in chronic obstructive pulmonary disease: reproducibility and effect of walking course layout and length. Am J Respir Crit Care Med. 2003;167:1522–7. doi: 10.1164/rccm.200203-166OC. [DOI] [PubMed] [Google Scholar]
- 32.ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166:111–17. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 33.Guralnik JM, Ferrucci L, Simonsick EM, et al. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332:556–61. doi: 10.1056/NEJM199503023320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guralnik JM, Ferrucci L, Pieper CF, et al. Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol A Biol Sci Med Sci. 2000;55:M221–31. doi: 10.1093/gerona/55.4.m221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 36.Duncan PW, Weiner DK, Chandler J, et al. Functional reach: a new clinical measure of balance. J Gerontol. 1990;45:M192–7. doi: 10.1093/geronj/45.6.m192. [DOI] [PubMed] [Google Scholar]
- 37.Duncan PW, Studenski S, Chandler J, et al. Functional reach: predictive validity in a sample of elderly male veterans. J Gerontol. 1992;47:M93–8. doi: 10.1093/geronj/47.3.m93. [DOI] [PubMed] [Google Scholar]
- 38.Weiner DK, Duncan PW, Chandler J, et al. Functional reach: a marker of physical frailty. J Am Geriatr Soc. 1992;40:203–7. doi: 10.1111/j.1532-5415.1992.tb02068.x. [DOI] [PubMed] [Google Scholar]
- 39.Weiner DK, Bongiorni DR, Studenski SA, et al. Does functional reach improve with rehabilitation? Arch Phys Med Rehabil. 1993;74:796–800. doi: 10.1016/0003-9993(93)90003-s. [DOI] [PubMed] [Google Scholar]
- 40.Kendall FP, McReary EK, Provance PG. Muscles, testing and function. Williams & Wilkins; Baltimore, MD: 1993. [Google Scholar]
- 41.Hamilton AL, Killian KJ, Summers E, et al. Muscle strength, symptom intensity, and exercise capacity in patients with cardiorespiratory disorders. Am J Respir Crit Care Med. 1995;152:2021–31. doi: 10.1164/ajrccm.152.6.8520771. [DOI] [PubMed] [Google Scholar]
- 42.Bernard S, LeBlanc P, Whittom F, et al. Peripheral muscle weakness in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;158:629–34. doi: 10.1164/ajrccm.158.2.9711023. [DOI] [PubMed] [Google Scholar]
- 43.Gosker HR, Lencer NH, Franssen FM, et al. Striking similarities in systemic factors contributing to decreased exercise capacity in patients with severe chronic heart failure or COPD. Chest. 2003;123:1416–24. doi: 10.1378/chest.123.5.1416. [DOI] [PubMed] [Google Scholar]
- 44.Gosselink R, Troosters T, Decramer M. Peripheral muscle weakness contributes to exercise limitation in COPD. Am J Respir Crit Care Med. 1996;153:976–80. doi: 10.1164/ajrccm.153.3.8630582. [DOI] [PubMed] [Google Scholar]
- 45.Andrews AW, Thomas MW, Bohannon RW. Normative values for isometric muscle force measurements obtained with hand-held dynamometers. Phys Ther. 1996;76:248–59. doi: 10.1093/ptj/76.3.248. [DOI] [PubMed] [Google Scholar]
- 46.Adams LS, Greene LW, Topoozian E. American Association of Hand Therapists clinical assessment recommendations. Churchill Livingstone; New York, NY: 1999. [Google Scholar]
- 47.Mathiowetz V, Weber K, Volland G, et al. Reliability and validity of grip and pinch strength evaluations. J Hand Surg [Am] 1984;9:222–6. doi: 10.1016/s0363-5023(84)80146-x. [DOI] [PubMed] [Google Scholar]
- 48.Sternfeld B, Ngo L, Satariano WA, et al. Associations of body composition with physical performance and self-reported functional limitation in elderly men and women. Am J Epidemiol. 2002;156:110–21. doi: 10.1093/aje/kwf023. [DOI] [PubMed] [Google Scholar]
- 49.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–83. [PubMed] [Google Scholar]
- 50.Cleveland WS. Robust locally weighted regression and smoothing scatterplots. J Am Stat Assoc. 1979;74:829–36. [Google Scholar]
- 51.Ottenheijm CA, Heunks LM, Dekhuijzen PN. Diaphragm muscle fiber dysfunction in chronic obstructive pulmonary disease: toward a pathophysiological concept. Am J Respir Crit Care Med. 2007;175:1233–40. doi: 10.1164/rccm.200701-020PP. [DOI] [PubMed] [Google Scholar]
- 52.Skeletal muscle dysfunction in chronic obstructive pulmonary disease. A statement of the American Thoracic Society and European Respiratory Society. Am J Respir Crit Care Med. 1999;159(4 Pt 2):S1–40. doi: 10.1164/ajrccm.159.supplement_1.99titlepage. [DOI] [PubMed] [Google Scholar]
- 53.Yende S, Waterer GW, Tolley EA, et al. Inflammatory markers are associated with ventilatory limitation and muscle dys-function in obstructive lung disease in well functioning elderly subjects. Thorax. 2006;61:10–16. doi: 10.1136/thx.2004.034181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Man WD, Hopkinson NS, Harraf F, et al. Abdominal muscle and quadriceps strength in chronic obstructive pulmonary disease. Thorax. 2005;60:718–22. doi: 10.1136/thx.2005.040709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Decramer M, Lacquet LM, Fagard R, et al. Corticosteroids contribute to muscle weakness in chronic airflow obstruction. Am J Respir Crit Care Med. 1994;150:11–16. doi: 10.1164/ajrccm.150.1.8025735. [DOI] [PubMed] [Google Scholar]
- 56.Gosselink R, Decramer M. Peripheral skeletal muscles and exercise performance in patients with chronic obstructive pulmonary disease. Monaldi Arch Chest Dis. 1998;53:419–23. [PubMed] [Google Scholar]
- 57.Engelen MP, Schols AM, Does JD, et al. Skeletal muscle weakness is associated with wasting of extremity fat-free mass but not with airflow obstruction in patients with chronic obstructive pulmonary disease. Am J Clin Nutr. 2000;71:733–8. doi: 10.1093/ajcn/71.3.733. [DOI] [PubMed] [Google Scholar]
- 58.Rantanen T, Masaki K, Foley D, et al. Grip strength changes over 27 years in Japanese-American men. J Appl Physiol. 1998;85:2047–53. doi: 10.1152/jappl.1998.85.6.2047. [DOI] [PubMed] [Google Scholar]
- 59.Maltais F, LeBlanc P, Whittom F, et al. Oxidative enzyme activities of the vastus lateralis muscle and the functional status in patients with COPD. Thorax. 2000;55:848–53. doi: 10.1136/thorax.55.10.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sue DY. Peripheral muscle dysfunction in patients with COPD: comparing apples to apples? Chest. 2003;124:1–4. doi: 10.1378/chest.124.1.1. [DOI] [PubMed] [Google Scholar]
- 61.Schols AM, Buurman WA, van den Brekel AJ Staal, et al. Evidence for a relation between metabolic derangements and increased levels of inflammatory mediators in a subgroup of patients with chronic obstructive pulmonary disease. Thorax. 1996;51:819–24. doi: 10.1136/thx.51.8.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Couillard A, Maltais F, Saey D, et al. Exercise-induced quadriceps oxidative stress and peripheral muscle dysfunction in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2003;167:1664–9. doi: 10.1164/rccm.200209-1028OC. [DOI] [PubMed] [Google Scholar]
- 63.Fink G, Moshe S, Goshen J, et al. Functional evaluation in patients with chronic obstructive pulmonary disease: pulmonary function test versus cardiopulmonary exercise test. J Occup Environ Med. 2002;44:54–8. doi: 10.1097/00043764-200201000-00009. [DOI] [PubMed] [Google Scholar]
- 64.McGavin CR, Gupta SP, McHardy GJ. Twelve-minute walking test for assessing disability in chronic bronchitis. Br Med J. 1976;1:822–3. doi: 10.1136/bmj.1.6013.822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.McGavin CR, Artvinli M, Naoe H, et al. Dyspnoea, disability, and distance walked: comparison of estimates of exercise performance in respiratory disease. Br Med J. 1978;2:241–3. doi: 10.1136/bmj.2.6132.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Poulain M, Durand F, Palomba B, et al. 6-minute walk testing is more sensitive than maximal incremental cycle testing for detecting oxygen desaturation in patients with COPD. Chest. 2003;123:1401–7. doi: 10.1378/chest.123.5.1401. [DOI] [PubMed] [Google Scholar]
- 67.Lacasse Y, Goldstein R, Lasserson TJ, et al. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2006;(4):CD003793. doi: 10.1002/14651858.CD003793.pub2. (DOI: 10.1002/14651858.CD003793.pub2).
- 68.Eisner MD, Balmes J, Katz PP, et al. Lifetime environmental tobacco smoke exposure and the risk of chronic obstructive pulmonary disease. Environ Health. 2005;4:7. doi: 10.1186/1476-069X-4-7. (DOI: 10.1186/ 1476-069X-4-7).
- 69.Corrigan D, Bohannon RW. Relationship between knee extension force and stand-up performance in community-dwelling elderly women. Arch Phys Med Rehabil. 2001;82:1666–72. doi: 10.1053/apmr.2001.26811. [DOI] [PubMed] [Google Scholar]
- 70.Bohannon RW. Hand-held compared with isokinetic dynamometry for measurement of static knee extension torque (parallel reliability of dynamometers) Clin Phys Physiol Meas. 1990;11:217–22. doi: 10.1088/0143-0815/11/3/004. [DOI] [PubMed] [Google Scholar]
- 71.Reed RL, Den Hartog R, Yochum K, et al. A comparison of hand-held isometric strength measurement with isokinetic muscle strength measurement in the elderly. J Am Geriatr Soc. 1993;41:53–6. doi: 10.1111/j.1532-5415.1993.tb05949.x. [DOI] [PubMed] [Google Scholar]
- 72.Agre JC, Magness JL, Hull SZ, et al. Strength testing with a portable dynamometer: reliability for upper and lower extremities. Arch Phys Med Rehabil. 1987;68:454–8. [PubMed] [Google Scholar]
- 73.Hurley BF.Age, gender, and muscular strength J Gerontol A Biol Sci Med Sci 199550 Spec No:41–4. [DOI] [PubMed] [Google Scholar]
- 74.Fried LP, Bandeen-Roche K, Chaves PH, et al. Preclinical mobility disability predicts incident mobility disability in older women. J Gerontol A Biol Sci Med Sci. 2000;55:M43–52. doi: 10.1093/gerona/55.1.m43. [DOI] [PubMed] [Google Scholar]
- 75.Fried LP, Young Y, Rubin G, et al. Self-reported preclinical disability identifies older women with early declines in performance and early disease. J Clin Epidemiol. 2001;54:889–901. doi: 10.1016/s0895-4356(01)00357-2. [DOI] [PubMed] [Google Scholar]
- 76.Rantanen T, Guralnik JM, Sakari-Rantala R, et al. Disability, physical activity, and muscle strength in older women: the Women’s Health and Aging Study. Arch Phys Med Rehabil. 1999;80:130–5. doi: 10.1016/s0003-9993(99)90109-0. [DOI] [PubMed] [Google Scholar]
- 77.Takazawa K, Arisawa K, Honda S, et al. Lower-extremity muscle forces measured by a hand-held dynamometer and the risk of falls among day-care users in Japan: using multinomial logistic regression analysis. Disabil Rehabil. 2003;25:399–404. doi: 10.1080/0963828031000090416. [DOI] [PubMed] [Google Scholar]