Abstract
Numerous studies have established the association of MUC4 with the progression of cancer and metastasis. An aberrant expression of MUC4 is reported in precancerous lesions indicating its early involvement in the disease process; however, its precise role in cellular transformation has not been explored. MUC4 contains many unique domains and is proposed to impact on cell signaling pathways and behavior of the tumor cells. In the present study, to decipher its oncogenic potential of MUC4, we stably expressed the MUC4 mucin in NIH3T3 mouse fibroblast cells. Stable ectopic expression of MUC4 resulted in increased growth, colony formation and motility of NIH3T3 cells in vitro and tumor formation in nude mice, when cells were injected subcutaneously. Microarray analysis demonstrated increased expression of several growth- and mitochondrial energy production-associated genes in MUC4-expressing NIH3T3 cells. In addition, expression of MUC4 in NIH3T3 cells resulted in enhanced levels of oncoprotein ErbB2 and its phosphorylated form (pY1248-ErbB2). In conclusion, our studies provide the first evidence that MUC4 alone induces cellular transformation and indicates a novel role of MUC4 in cancer biology.
Keywords: Mucin, MUC4, Cellular transformation, Cancer, Fibroblast cells
Introduction
MUC4 is a member of the membrane-bound mucin family (1;2). It was cloned from a human tracheobronchial cDNA library and pancreatic tumor cell line and its complete genomic organization has been established (25 exons/introns over 65 kb) (3). MUC4 is synthesized as a single polypeptide chain of approximately 930 kDa and hypothesized to be cleaved at a GDPH proteolytic cleavage site generating two subunits, the mucin type subunit MUC4α, and a transmembrane subunit MUC4β. MUC4α possesses three putative functional domains, tandem-repeat (TR), nidogen-like (NIDO), and adhesion-associated domain in MUC4 and other proteins (AMOP), while MUC4β has three epidermal growth factor (EGF)- like domains and a short cytoplasmic tail (CT) (3).
MUC4 is normally expressed by the luminal epithelial cells of the stomach, colon, lung, trachea, cervix, and prostate (4;5), while it is not or minimally expressed by gall bladder, biliary epithelial cells, intrahepatic bile ducts, liver and pancreas (4;6;7). An overexpression of MUC4 is, however, observed in pancreatic, lung, breast, colon and ovarian malignancies, suggesting its pathological significance (6;8-10). Furthermore, the association of MUC4 with the poor prognosis of the pancreatic, lung and bile duct cancer patients has also been reported (11-13). Using a MUC4-knockdown and overexpression cell models, we have demonstrated that MUC4 potentiates pancreatic tumor cell growth and metastasis by altering the behavioral properties of the tumor cells (14-16). Importantly, our recent studies have revealed that MUC4 interacts with the receptor tyrosine kinase, HER2 and regulates its expression by post-translational mechanisms (17). HER2 is an established oncoprotein and is involved in growth and malignant properties of the cancer cells (18). An aberrant expression of MUC4 in pancreatic cancer is detected early i.e. in precancerous lesions, and correlates with the disease advancement (19;20). All these findings indicate that MUC4 may play important role in the early and late events of cancer progression.
In the present study, we have carried out a set of experiments to define the role of MUC4 in oncogenic transformation. MUC4 was ectopically over-expressed in NIH3T3 mouse fibroblast cells by stable transfection and its effect on the cellular phenotype was determined by performing in vitro and in vivo functional assays. Ectopic expression of MUC4 in NIH3T3 cells resulted in increased growth, colony formation, and motility of the cells. Furthermore, MUC4-expressing NIH3T3 cells spontaneously formed tumors in nude mice in majority of cases (73%) when injected subcutaneously. An enhanced expression of ErbB2, its phosphorylated form and phosphorylated form of ERK were observed in MUC4-expressing NIH3T3 cells. Additionally, ectopic expression of MUC4 was found to alter the expression of several growth and mitochondrial energy production-associated genes. Together, all these observations support a role of MUC4 in cancer pathogenesis and provide first evidence for its oncogenic action.
Material and Methods
Cell Culture and Transfection
NIH3T3 cells were cultured in DMEM supplemented with 10% fetal bovine serum and antibiotics (penicillin and streptomycin-100 μg/ml). Cells were grown at 37°C with 5% CO2 in a humidified atmosphere. pSecTagC-MUC4 plasmid, previously designed in our lab (16), were used for attaining the ectopic expression of MUC4 in NIH3T3 mouse fibroblast cells by stable transfection using FuGENE6™ Transfection Reagent (Roche Diagnostics, Indianapolis, IN). Cells were also transfected with empty pSecTagC vector to obtain a control population. The zeocin-resistant colonies were isolated by the ring cloning method, expanded and maintained in medium supplemented with 400μg/ml zeocin (Invitrogen, Carlsbad, CA).
Immunoblot Assay
The NIH3T3 derived clones were processed for protein extraction and western blotting using standard procedures. Cell lysates were prepared as described previously (15). Protein concentrations were determined using a BIO-RADD/C protein estimation kit. For MUC4, the proteins (20 μg) were resolved by electrophoresis on a 2% SDS-agarose gel under reducing conditions. For ß-actin, ErbB2 and p1248ErbB2 and Erk1/2, SDS-PAGE (10%) was performed under similar conditions. Resolved proteins were transferred onto the polyvinylidene difluoride (PVDF) membrane and blocked in 5% non-fat milk in phosphate buffered saline (PBS) for 2 h. and subjected to the standard immunodetection procedure using specific antibodies. For ß-actin immunodetection, anti-ß-actin mouse monoclonal antibody (Sigma, St. Louis, MO) in dilution of 1:2000 (used as internal control); and for MUC4 immunodetection, anti-MUC4 mouse monoclonal antibody (8G7, generated in our laboratory) in dilution of 1:1000 was used. For ErbB2, p1248ErbB2, ERK,and phosphorylated ERK immunodetection, anti-ErbB2 (Santa Crutz Biotecnology, Santa Cruz, CA), anti-p1248ErbB2 (Upstate, San Francisco, CA), anti-ERK1/2 (Santa Crutz Biotecnology, Santa Cruz, CA), anti-p ERK1/2 (Cell Signaling Tech, Danvers, MA) rabbit polyclonal antibodies in dilution of 1:1000 were used. The membranes were incubated for 4 h at room temperature, followed by 6 × 10 min washes in TBST (50mM Tris-HCl pH 7.4, 150 mM NaCl, and 0.05% Tween-20). Further, the membranes were incubated in Horseradish peroxidase-conjugated secondary antibodies (Amersham Biosciences, Buckinghamshire, United Kingdom) (diluted at 1:2000 in PBS) for 1 h at room temperature, followed by six washes in TBST. The blots were processed with ECL Chemiluminescence kit (Amersham Biosciences, Buckinghamshire, United Kingdom), and the signal was detected by exposing the processed blots to X-ray films (Biomax Films, Kodak, NY).
Confocal Immunofluorescence Microscopy
For immunofluorescence staining, cells were grown at low density on sterilized cover slips for 20 h. Cells were first washed with 0.1 M HEPES containing Hanks buffer and then fixed in ice-cold methanol at −20° C for two min. Methanol-fixed cells were blocked in 10% goat serum containing 0.05% Tween-20 for 30 min at room temperature for nonspecific blocking, followed by incubation with the anti-MUC4 monoclonal antibody (8G7), diluted (1:100) in PBS, for 90 min at room temperature. Cells were washed 4 to 5 times for 5 min with PBS containing 0.05% Tween 20 (PBS-T) and then incubated with FITC-conjugated goat anti-mouse secondary antibodies (Jackson Immuno Research laboratories Inc., West Grove, PA) for 60 min. Cells were again washed 4 to 5 times for 5 min with PBS-T and mounted on glass slides in anti-fade Vectashield mounting medium (Vector Laboratories, Burlingame, CA). Immunostaining was observed under a ZEISS confocal laser-scanning microscope, and representative photographs were captured digitally using 510 LSM software.
Growth Kinetics Assay
2.5 × 104 cells in 2 ml of DMEM medium containing 1.0 % FBS, were seeded in six-well plates and allowed to grow for different time intervals. The growth of the cells was monitored by counting the number of cells using a hemocytometer everyday for 10 days. The cell population doubling time (Td) was calculated during the exponential growth phase (192 h to 240 h) using the following formula: Td = 0.693 t / ln (Nt / N0) (15), where t is time difference (in h), Nt is the cell number at time t (240h), and N0 is the cell number at initial time (192h).
Colony-Forming Assay
Cells were seeded in triplicate at a density of 2.5 × 103 cells/10-cm dishes in DMEM medium containing 1.0 % FBS. After 2 weeks of growth, the cells were fixed and stained with crystal violet stain (0.1%, w/v) in 20 nM 4-morpholinepropanesulfonic acid (Sigma Chemicals, St. Louis, MO). Cells in 10 random fields of view at X100 magnification were counted and expressed as the average number of cells/field of view.
In-vivo Tumorigenicity Assay
To test the tumorigenicity, the MUC4-transfected NIH3T3 cells along with the control cells were harvested from subconfluent cultures by a brief exposure to 0.25% trypsin and 0.02% EDTA. Trypsinization was stopped with medium containing 10% fetal bovine serum, and the cells were washed once in PBS. Cell viability and count were determined by trypan blue staining using a hemocytometer. Cells were resuspended in a normal saline solution at a concentration of 10×106 cells/ml. Single cell suspensions of >90% viability was used for the injections. Immunodeficient mice were purchased from the Animal Production Area of the National Cancer Institute-Frederick Cancer Research and Development Center (Frederick, MD). The mice were housed in pathogen-free conditions, fed sterile water and food ad libitum and treated in accordance with the Institutional Animal Care and Use Committee (IACUC) guidelines. Viable MUC4-transfected or empty vector-transfected NIH3T3 cells (1×106), suspended in a normal saline solution, were injected subcutaneously in immunodeficient mice (n=15). To quantify tumor growth, two perpendicular tumor dimensions (a and b) were measured twice a week with a vernier caliper, and tumor surface area (mm3) were calculated by the following formula (V= (a) × (b) 2/ 2) (21) for each time period. Tumor volumes were compared between mice injected with NIH3T3/MUC4 and NIH3T3/pSecTag cells. Differences in tumor-free survival were displayed using Kaplan-Meier plots and survival curves were compared using the log-rank test. All mice were sacrificed on day 36 after transplantation, and the presence of metastatic lesions in different organs were also determined.
Motility Assay
For motility assays, 1×106 cells were plated in the top chamber of non-coated polyethylene teraphthalate membranes (6-well insert, pore size 8 mm; Becton Dickinson). The cells were incubated for 24 h, and the cells that did not migrate through the pores in the membrane were removed by scraping the membrane with a cotton swab. Cells that traversed through the membrane pores were stained with a Diff-Quick cell staining kit (Dade Behring, Inc., Newark, DE). Cells in 10 random fields of view at X100 magnification were counted and expressed as the average number of cells/field of view. Two independent experiments were done in each case. The data were represented as the average of the two independent experiments with the standard deviation (SD) of the average indicated.
Oligonucleotide Array Gene Expression Analysis
Mouse oligonucleotide array containing probes for around 10,800 genes were constructed at the Microarray Core Facility of University of Nebraska Medical Center. Total RNA was isolated from MUC4 and empty vector transfected NIH3T3 cells by RNeasy mini kit (Qiagen, Valencia, CA). Spotted microarrays were used to determine differential gene expression between NIH3T3/MUC4 and NIH3T3/pSecTag samples. The design had NIH3T3/MUC4 and NIH3T3/pSecTag samples competitively hybridised to three arrays. To generate the fluorescently-labeled, single-stranded cDNA target, total RNA (750 ng) was reverse transcribed to generate cDNA, followed by in vitro-transcription to generate amino-allyl (aRNA) utilizing the Amino-Allyl Message Amp kit (Ambion, Austin TX). aRNA (5 ug) was coupled with either CY5 or CY3 dye as per manufacturers suggestion, mixed and co-hybridized (in 20 ul of Ambion hybridization buffer) to microarray slides for 16 hours at 42 °C The slides were washed per manufacturers suggestions and scanned using an Axon 4000b scanner to generate .tiff images. The images were extracted using GenePix software and resultant .GPR files used for downstream analysis. Differentially expressed genes were identified using BRB Array Tools developed by Dr. Richard Simon and Amy Peng (Biometric Research Branch of the National Cancer Institute, National Institutes of Health, Bethesda, MD).
Several filters and normalization were applied prior to analysis. Spots were excluded if both the red and green channels had values less than 100, if only one of the red or green channels was less than 100, it was increased to the threshold of 100. Median background was subtracted and Log2 transformation was applied to all ratios. Normalization was then done to “center” each array using lowess smoother and genes were excluded if any of the spots were missing or filtered out for any of the samples. Random-variance paired t-tests were used to determine which genes are differentially expressed between NIH3T3/MUC4 and NIH3T3/pSecTag samples, comparing the log red (NIH3T3/MUC4) and green (NIH3T3/pSecTag) channel intensities. The random-variance paired t-test allows sharing information among genes about variation without assuming that all genes have the same variance, which gives a more accurate estimate of the variability when sample sizes are small. A significance level of 0.001 was selected to help limit the false discovery rate due to multiple comparisons.
Quantitative reverse transcription-polymerase chain reaction (Q-RT-PCR)
Total RNA (2 μg) from each of the derived NIH3T3 cell lines was reverse-transcribed using the first-strand cDNA synthesis kit (Perkin-Elmer, Branchburg, NJ) and oligo-d(T) primers according to the manufacturer's instructions. Real-time PCR amplifications were carried out with 100 ng of first-strand cDNA in 10-μl reaction volumes. The reaction mixture was subjected to a two step cyclic program (95 °C for 10 min followed by 40 cycles of 95 °C for 15 s and 60 °C for 1 min) as per manufacturer's protocol on ABI 7500 sequence detection system (Applied Biosystems, Foster City, CA) with SYBR chemistry. Pre-designed PCR primers for Tln, Sna, Nek6, and GAPDH were purchased from a commercial source (Superarray Biosciences Corporation, Frederick, MD). The relative fold difference in gene expression was calculated by ΔΔCT method (22) using GAPDH as a normalization control.
Results
Constitutive over-expression of MUC4 in mouse fibroblast NIH3T3 cells
To investigate the transforming ability of MUC4, mouse fibroblast NIH3T3 cells that do not express endogenous Muc4, were transfected with human MUC4 expression plasmid (pSecTag-MUC4) (16) or empty pSecTag vector (as a control) and stable cell clones were selected. The clones were expanded and screened for MUC4 expression by immunoblot analysis. Two clones that exhibited high level of MUC4 (Figure 1a) were pooled together and further characterized by confocal analysis (Figure 1b). The pooled populations of MUC4-transfected (NIH3T3/MUC4) and empty vector-transfected (NIH3T3/pSecTag) cells were monitored for 1−2 months for stable expression and utilized to study the oncogenic potential of MUC4 and its effect on cellular phenotype by performing in vitro and in vivo functional assays.
Figure 1.
Western blot and confocal immunofluorescence analyses of MUC4 expression in empty vector- and MUC4-transfected NIH3T3 cells. (a) A total of 20 μg protein from cell extracts was resolved by electrophoresis on a 2% SDS-agarose gel, transferred to polyvinylidene difluoride membrane, and incubated with anti-MUC4 MAb (8G7). The membrane was then probed with horseradish peroxidase-labeled goat anti-mouse immunoglobulin. Immunoblot of ß-actin, obtained from 10% SDS-PAGE, was used as an internal control. The signal was detected using an electrochemiluminescence reagent kit. (b) Cells were grown at low density on sterilized cover slips and processed for the immunofluorescence procedure after fixation in methanol for 10 minutes. Slides were incubated with MUC4 MAb (8G7), followed by FITC conjugated secondary antibody and were observed under a ZEISS confocal laser-scanning microscope.
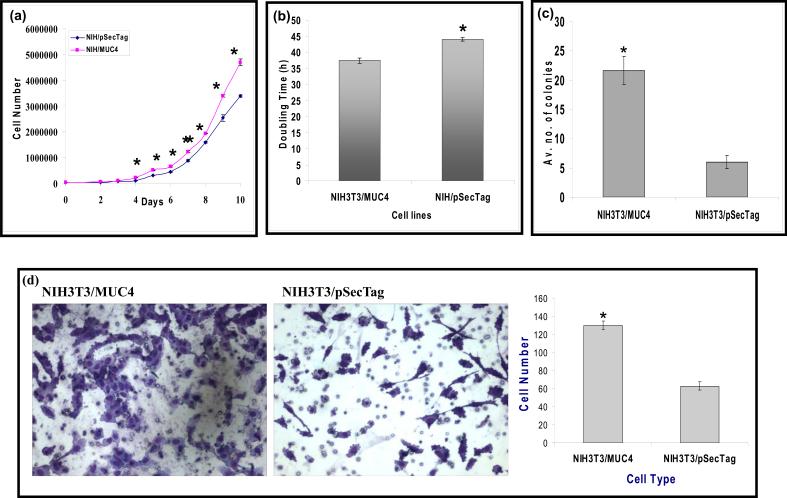
Ectopic expression of human MUC4 enhances the growth kinetics and clonogenicity of NIH3T3 cells
To determine whether MUC4 influences the growth of NIH3T3 cells, NIH3T3/MUC4 and NIH3T3/pSecTag cells were seeded at low density (25 × 103 cells/well of six well plate) and growth rates were determined by cell counting at different time intervals (Figure 2a). NIH3T3/MUC4 cells showed an enhanced growth rate as compared to the empty vector-transfected (NIH3T3/pSecTag) cells. Population doubling times were calculated from the growth curve during the exponential phase (192−240 h). NIH3T3/MUC4 cells displayed a significant decrease (P<0.05) in doubling time compared with the control cells. NIH3T3/MUC4 cells had a doubling time of ≈ 37 h, while it was recorded ≈ 44 h for the control cells (Figure 2b). To assess the effects of MUC4 expression on the plating efficiency of NIH3T3 cells, NIH3T3/MUC4 and NIH3T3/pSecTag cells were analyzed by colony formation assay. Cells were seeded at very low density (2.5 × 103 cells/10-cm dish) and after 2 week, cells were fixed and stained with 0.1% crystal violet and representative photographs were taken. NIH3T3/MUC4 cells showed significantly enhanced colony formation when compared with the vector-transfected cells (Figure 2c).
Figure 2.
Functional characterization of MUC4-expressing and empty vector-transfected NIH3T3 cells in vitro (a) Growth curve was plotted for cell number versus time of incubation of transfected cells in vitro. A total of 25000 cells were seeded at 0 h, and cell number in different cell lines was counted at an interval of 24 h up to 10 days. Mean ± SE; n = 3. (b) Cell population doubling time was calculated from the cell growth during the exponential growth phase (192−240 h) according to the equation Td = 0.693t/ln(Nt/N0), where t is time (in h), Nt is the cell number at time t, and N0 is the cell number at initial time. Mean ± SE; n = 3; *, P < 0.05. (c) Colony formation assay was performed by seeding 2500 cells in 10-cm dishes and grown for 2 weeks. Cells were fixed and stained with 0.1% crystal violet and representative photographs were taken for NIH3T3/MUC4 and NIH3T3/pSecTag cells. The NIH3T3/MUC4 cells showed significant increase in colony formation as compared to control NIH3T3/pSecTag cells. Mean ± SE; n = 20; *P < 0.003. (d) Motility assay was performed by plating the cells onto non-coated membranes and subsequent incubation for 24 h. Medium containing 10% fetal bovine serum in the lower chamber was used as a chemoattractant. Cells that migrated through the pores were fixed, stained and representative fields were photographed under bright field microscopy with 100X magnification. The number of cells transversing the membrane was determined by averaging 10 random fields of view at x100 and expressed as the average number of cells/field of view and is the average of two independent experiments. The NIH3T3/MUC4 cells showed significant increase (two folds approximately) in cell motility as compared to control NIH3T3/pSecTag cells. Mean ± SE; n = 20; *P < 0.04×10−7.
MUC4 expression enhances motility of NIH3T3 cells
To examine the effect of MUC4 expression on NIH3T3 cells on behavioral properties, a motility assay was performed by utilizing uncoated porous membranes of 8.0 mm pore diameter. The number of cells migrated to the lower surface of the porous membrane under chemoattractive stimulus of fetal bovine serum (FBS) in the lower chamber was approximately two fold greater in MUC4-expressing NIH3T3/MUC4 cells in comparison with control NIH3T3/pSecTag cells (Figure 2d). This data indicated that expression of MUC4 enhanced motility in NIH3T3 cells.
Expression of MUC4 induces oncogenic transformation of NIH3T3 cells
To test the hypothesis that MUC4 plays a role in cellular transformation and potentiation of tumor development, we examined the tumorigenic potential of NIH3T3/MUC4 and NIH3T3/pSecTag cells in vivo. A total of 30 immunodeficient mice were injected subcutaneously with NIH3T3/MUC4 and NIH3T3/pSecTag cells (1×106) and tumor growth was measured twice a week in two independent experiments. Tumor volumes were calculated from bidimensional measurements at each time point and mice were sacrificed on day 36 post-injection. Results were expressed as differences in tumor-free survival and tumor volumes were also compared between these two groups. The tumor-free survival experience was significantly decreased in NIH3T3/MUC4 cells injected mice (p<0.001). The mean tumor free survival time for NIH3T3/MUC4 mice (n=15) was only 17.6 days as compared to 34.7 days for control animals (n=15) (Figure 3a). Only seven percent of mice injected with MUC4-expressing NIH3T3 cells showed tumor free survival on 36th day of implantation of cells as compared to 73 percent of mice injected with control cells. Furthermore, tumor volumes were significantly increased in mice injected with NIH3T3/MUC4 cells compared with the mice injected with empty vector-transfected NIH3T3/pSecTag cells (p<0.05) (Figure 3b). No observable metastasis, however, was observed in any of the control- or NIH3T3/MUC4 cells-injected mice.
Figure 3.
MUC4 induces tumorigenicity in NIH3T3 cells. NIH3T3/MUC4 and NIH3T3/pSecTag cells (1×106) were injected subcutaneously into the nude mice. Tumor volumes were calculated from bidimensional measurements at each time point. Results were expressed as differences in tumor-free survival and tumor volumes between mice injected with NIH3T3/MUC4 and NIH3T3/pSecTag cells; each group includes 15 mice per group. (a) Differences in tumor free survival were measured using Kaplan-Meier plots and survival curves were compared using the log-rank test. (b) Tumor volumes were compared as (mean ± SE) between these groups.
MUC4 expression enhances the expression of ErbB2 and its downstream signaling in NIH3T3 cells
In our previous studies, a reduced level of total and phosphorylated (at Tyr1248) HER2 protein was found in MUC4 down-regulated CD18/HPAF cells (14;15). Furthermore, we have recently provided evidence that MUC4 interacts with HER2 and regulates its expression (17). In other studies, the rat homologue of MUC4 (rMuc4) has also been shown to interact with ErbB2/HER2/neu and induce its limited phosphorylation (23). On the basis of these observations, we wanted to determine whether MUC4 regulates the expression of ErbB2 protein and thus of its phosphprylated form (p1248ErbB2) in NIH3T3 cells. Our results clearly show that NIH3T3/MUC4 cells have increased levels of total and phosphorylated ErbB2 protein as compared to the control NIH3T3/pSecTag cells (Figure 4). MUC4-associated increased expression and activity of HER2 has been shown to be associated with enhanced phosphorylation of ERK (17). Consistent with our previous observations, NIH3T3/MUC4 cells also showed an increased phosphorylation of ERKs (p42/44 MAPK) as compared to the control NIH3T3/pSecTag cells.
Figure. 4.

Effect of MUC4 on ErbB2 and its downstream signaling. Expression of ErbB2, ERK, and phosphorylated form of ErbB2 and ERK in NIH3T3 cell sublines was examined by immunoblot analysis. The protein was isolated from subconfluent cultures and 30μg of protein from each cell extract was resolved by SDS-PAGE (10%), transferred to the polyvinylidene difluoride membrane and probed with antibodies against ErbB2, phospho-tyr1248 ErbB2, ERK, pERK, and β-actin (internal control). MUC4-transfected NIH3T3 cells showed up-regulated ErbB2, phosphorylated-ErbB2, and pERK expression compared with empty vector-transfected NIH3T3 cells.
MUC4-associated alterations of gene expression in NIH3T3 cells
DNA oligonucleotide microarrays representing around 10,800 genes were used to identify genes regulated by MUC4 and potentially responsible for tumorigenic potential of MUC4-expressing NIH3T3/MUC4 cells and its effect on the cellular phenotype. The mRNA expression profile of NIH3T3/MUC4 cells was compared with that of NIH3T3/pSecTag cells. Among all the genes, 59 showed at least 1.8 fold change in expression after normalization. Out of these genes, a few selected genes that were either over-expressed or down-regulated in the MUC4-expressing cells are listed in Table 1. Analysis of the data revealed that several growth and energy production associated genes were up-regulated in the MUC4-expressing cells. Additionally, several genes associated for cell motility were also altered. Genes of particular importance, that were differentially expressed in MUC4-expressing NIH3T3 cells, were those encoding for nek6, sna, gas5, S100A11, cox3, ND1, pkp3, HM13, trim16, atf7ip, and talin. To validate our microarray data, expression of few randomly selected differentially-expressed genes was examined by quantitative RT-PCR (Figure S1). The results of the RT-PCR were in complete agreement with the microarray data, indicating that these alterations in gene expression could be functionally implicated in MUC4-mediated cellular transformation and phenotypic changes of NIH3T3 cells.
Table 1.
List of selected differentially expressed genes in NIH3T3/MUC4 cells as compared to control (NIH3T3/pSecTag) cells
| Genes upregulated | |||
|---|---|---|---|
| Gene | Product | Fold change | Function |
| Cox3 | Cytochrome c oxidase subunit 3 | 8.969 | Oxidative phosphorylation |
| ND1 | Nicotinamide adenine dinucleotide dehydrogenase subunit 1 | 2.485 | Oxidative phosphorylation |
| nek6 | Nima (never in mitosis gene a)-related kinase 6 | 2.432 | Mitotic progression |
| trim16 | Tripartite motif-containing 16 | 2.058 | Regulation of cell growth |
| atf7ip | Activating transcription factor 7 interacting protein | 1.922 | Regulation of transcription |
| IL18R1 | Interleukin 18 receptor 1 | 1.912 | Regulation of cell growth |
| Sna | Snail homolog 1 | 1.909 | Repression of E-cadherin transcription |
| gas5 | Growth arrest-specific 5 | 1.841 | apoptosis inhibitor |
| S100A11 | S100 calcium binding protein a11 | 1.8 | Cell growth |
| Genes downregulated | |||
| Tln | Talin | 0.499 | Cell adhesion |
| BCAP | b cell phosphoinositide 3-kinase adaptor | 0.489 | Regulation of PI3K Signaling |
| Pkp3 | Plakophilin 3 | 0.471 | Cell adhesion |
| atp8a1 | Atpase, aminophospholipid transporter 8a1 | 0.467 | Transport of aminophospholipids |
| HM13 | Histocompatibility minor 13 | 0.461 | Antigen recognition |
| HTR1B | 5-hydroxytryptamine receptor1B | 0.427 | Neurotransmittor |
Discussion
Mucins are high molecular weight glycoproteins secreted by epithelial cells for the lubrication and protection of vulnerable surfaces. Mucins are also believed to play an important role in the pathogenesis of benign and malignant diseases of secretory epithelial cells (24). Our previous studies have demonstrated the specific and differential expression of MUC4 in pancreatic adenocarcinomas as compared to the normal pancreas or chronic pancreatitis (6). Furthermore, we have observed 100% incidence of MUC4 over-expression in early stage of ovarian cancer, whereas only a faint staining was observed in some cases of non-neoplastic ovary (25). An upregulation of MUC4 has also been observed in variety of other human adenocarcinomas such as squamous cell carcinoma, lung carcinoma, mammary and colon cancer (9;10;26). De novo expression of MUC4 in precancerous pancreatic intraepithelial neoplasias and a progressive increase in its expression with disease advancement, implicates MUC4 as an important player in the early and late phases of pancreatic cancer development (20). In fact, our earlier studies have clearly demonstrated the pathogenic functions of MUC4 in pancreatic cancer progression (14;15). Here, we have presented data demonstrating the oncogenic potential of MUC4 and thus its role in early stages of cancer development. The findings from the present study showed that the ectopic expression of MUC4 increased in vitro growth and colony formation in NIH3T3 cells and induced spontaneous tumor formation in vivo. MUC4 expression was also associated with increased motility of cells. Moreover, ectopic expression of MUC4 was found to alter the expression of several growth and energy production associated genes.
Cellular transformation involves the increased expression or activity-promoting mutation(s) in growth-enhancing genes and/or deletion/mutational inactivation of the growth suppressor genes (27). Mucins, MUC4 and MUC1 (another trans-membrane mucin), are now well recognized for their growth promoting activity (24;28). In fact, MUC1 was also shown to induce oncogenic transformation (29). The role of MUC4 in cellular transformation, however, was not defined thus far, and therefore, the present study provides first evidence on the role of MUC4 in early phase of oncogenesis. Another important observation in the present study was an enhanced expression of ErbB2 and its phosphorylated form (pY1248-ErbB2) in MUC4-expressing NIH3T3 cells as compared to the control cells. This was consistent with our previous reports in pancreatic cancer cells (15;17). ErbB2 is a receptor tyrosine kinase and its over-expression has been shown to correlate with aggressiveness, prognosis and invasive behavior of various tumors (30). In other studies, SMC/Muc4 (the rat homologue of MUC4) was also shown to act as an intra-membrane ligand for ErbB2/HER2/neu, inducing its limited phosphorylation (31). Quantitation of ErbB2 transcripts did not show an overexpression (data not shown), which substantiates our recent finding that MUC4 affects ErbB2 expression post-translationally and increases the stability of ErbB2 protein (17). Interestingly, NIH3T3/MUC4 cells showed an increased phosphorylation of ERKs (p42/44MAPK) as compared to control NIH3T3/pSecTag cells. The HER2-mediated activation of the ERK pathway plays a crucial role in mediating transformation and cell proliferation. It will be of interest to examine the correlation between the expression of MUC4, HER2 and other downstream mediators and their activation status in clinical samples to support the pathogenic relevance.
An altered expression of genes is observed in response to ectopic expression of MUC4 in NIH3T3 cells (Table 1). MUC4 expression in NIH3T3 cells up-regulated the expression of cox3 and ND1, proteins associated with mitochondrial ATP production. Various genes coding for mitochondrial proteins have also been previously identified as being up-regulated in MUC4 expressing pancreatic cancer cells (16). It has been postulated that MUC4-mediated increase in mitochondrial proteins is associated with an increase in metabolic activity and thereby decrease the cancer cell death (16). Although NIH3T3/MUC4 cells showed increased in vitro growth compared to control cells, we did not find any significant difference in cell death. An up-regulation of sna and down-regulation of talin and pkp3, which are implicated in cell motility, was also observed. Sna is a transcription factor which induces epithelial-mesenchymal transitions responsible for acquisition of motile and invasive properties during tumor progression (32;33). Pkp3 is a protein of desmosomal plaque and all histological grades of squamous cell carcinoma tissues were shown to be negative for pkp3 expression (34). Talin is an actin-binding cytoskeletal protein that plays an essential role in the formation of integrin cell adhesion proteins. A recent study showed complete de-expression of talin in endometroid carcinoma tissues (35). An up-regulation of the nek6, S100A11, gas5, IL18R1 and trim16 was also reported in MUC4-overexpressing NIH3T3 cells compared with control cells. All these proteins are key regulators of cell growth. Nek6 is a protein kinase, critical for cell cycle progression (36) and calcium-binding protein S100A11 has been shown to be correlated with the growth of human uterine leiomyoma (37). Gas5 is regarded as a useful marker for growth arrest, proliferation, and differentiation in the developing embryo (38). It has been identified as significantly up-regulated in benzo(a)pyrene treated smooth muscle cells as an apoptosis inhibitor for survival of cells by cDNA microarray analysis (39). Trim and IL18R proteins are involved in a variety of biological processes including cell growth and cancer pathogenesis (40;41). Hence, these altered genes might be responsible for MUC4-mediated induction of cell transformation.
In conclusion, our data provide first evidence on the role of MUC4 mucin in cellular transformation. We demonstrate that MUC4 induces tumor formation in vivo and results in an enhanced cell growth, colony formation, motility and a change in expression of various growth and mitochondrial energy production-associated genes. Furthermore, we also show that expression of MUC4 is associated with enhanced levels of oncoprotein ErbB2 and its phosphorylated form. (pY1248-ErbB2). An increased phosphorylation of ERK1/2, downstream targets of ErbB2, was also observed. The events downstream of MUC4 that promote tumorigenesis are still unclear but could conclude enhancing conditions for cell proliferation. A model (Figure 5) has been proposed to depict the possible mechanism of MUC4 in oncogenic transformation. Hence, MUC4 mucin presents an attractive target for cancer prevention and therapeutics with the potential of inhibiting the initiation and growth of human cancer cells.
Figure 5.
Schematic representation of the proposed mechanism of MUC4 in oncogenic transformation. We propose that MUC4 causes cellular transformation by impacting on cellular proliferation (MUC4-ErbB2-Grb2/sos-Ras-Raf1-MEK-Erk1/2) and cell death (increased expression of mitochondrial energy production genes COX3, ND1). In addition, increased expression of growth promoting genes Nek6, S100A11 might also be responsible for oncogenic transformation of NIH3T3 cells.
Acknowledgements
We thank Erik Moore for technical support, Dr. Subhankar Chakraborty for help with real-time quantitative PCR, Microarray Core Facility for gene expression analysis and the Confocal Facility for imaging. This work was supported by grants from the US Department of Defense (OC04110) and National Institutes of Health (RO1 CA78590). The UNMC Microarray Core Facility receives partial support from the NIH grant number P20 RR016469 from the INBRE Program of the National Center for Research Resources.
Reference List
- 1.Moniaux N, Nollet S, Porchet N, Degand P, Laine A, Aubert JP. Complete sequence of the human mucin MUC4: a putative cell membrane-associated mucin. Biochem J. 1999;338:325–33. [PMC free article] [PubMed] [Google Scholar]
- 2.Nollet S, Moniaux N, Maury J, et al. Human mucin gene MUC4: organization of its 5'-region and polymorphism of its central tandem repeat array. Biochem J. 1998;332:739–48. doi: 10.1042/bj3320739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chaturvedi P, Singh AP, Batra SK. Structure, evolution, and biology of the MUC4 mucin. FASEB J. 2008;22:966–81. doi: 10.1096/fj.07-9673rev. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Price-Schiavi SA, Perez A, Barco R, Carraway KL. Cloning and characterization of the 5' flanking region of the sialomucin complex/rat Muc4 gene: promoter activity in cultured cells. Biochem J. 2000;349:641–9. doi: 10.1042/0264-6021:3490641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Singh AP, Chauhan SC, Bafna S, et al. Aberrant expression of transmembrane mucins, MUC1 and MUC4, in human prostate carcinomas. Prostate. 2006;66:421–9. doi: 10.1002/pros.20372. [DOI] [PubMed] [Google Scholar]
- 6.Andrianifahanana M, Moniaux N, Schmied BM, et al. Mucin (MUC) gene expression in human pancreatic adenocarcinoma and chronic pancreatitis: a potential role of MUC4 as a tumor marker of diagnostic significance. Clin Cancer Res. 2001;7:4033–40. [PubMed] [Google Scholar]
- 7.Shibahara H, Tamada S, Higashi M, et al. MUC4 is a novel prognostic factor of intrahepatic cholangiocarcinoma-mass forming type. Hepatology. 2004;39:220–9. doi: 10.1002/hep.20031. [DOI] [PubMed] [Google Scholar]
- 8.Davidson B, Baekelandt M, Shih I. MUC4 is upregulated in ovarian carcinoma effusions and differentiates carcinoma cells from mesothelial cells. Diagn Cytopathol. 2007;35:756–60. doi: 10.1002/dc.20771. [DOI] [PubMed] [Google Scholar]
- 9.Hanaoka J, Kontani K, Sawai S, et al. Analysis of MUC4 mucin expression in lung carcinoma cells and its immunogenicity. Cancer. 2001;92:2148–57. doi: 10.1002/1097-0142(20011015)92:8<2148::aid-cncr1557>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 10.Rossi EA, McNeer RR, Price-Schiavi SA, et al. Sialomucin complex, a heterodimeric glycoprotein complex. Expression as a soluble, secretable form in lactating mammary gland and colon. J Biol Chem. 1996;271:33476–85. doi: 10.1074/jbc.271.52.33476. [DOI] [PubMed] [Google Scholar]
- 11.Saitou M, Goto M, Horinouchi M, et al. MUC4 expression is a novel prognostic factor in patients with invasive ductal carcinoma of the pancreas. J Clin Pathol. 2005;58:845–52. doi: 10.1136/jcp.2004.023572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tamada S, Shibahara H, Higashi M, et al. MUC4 is a novel prognostic factor of extrahepatic bile duct carcinoma. Clin Cancer Res. 2006;12:4257–64. doi: 10.1158/1078-0432.CCR-05-2814. [DOI] [PubMed] [Google Scholar]
- 13.Tsutsumida H, Goto M, Kitajima S, et al. MUC4 expression correlates with poor prognosis in small-sized lung adenocarcinoma. Lung Cancer. 2007;55:195–203. doi: 10.1016/j.lungcan.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 14.Chaturvedi P, Singh AP, Moniaux N, et al. MUC4 mucin potentiates pancreatic tumor cell proliferation, survival, and invasive properties and interferes with its interaction to extracellular matrix proteins. Mol Cancer Res. 2007;5:309–20. doi: 10.1158/1541-7786.MCR-06-0353. [DOI] [PubMed] [Google Scholar]
- 15.Singh AP, Moniaux N, Chauhan SC, Meza JL, Batra SK. Inhibition of MUC4 expression suppresses pancreatic tumor cell growth and metastasis. Cancer Res. 2004;64:622–30. doi: 10.1158/0008-5472.can-03-2636. [DOI] [PubMed] [Google Scholar]
- 16.Moniaux N, Chaturvedi P, Varshney GC, et al. Human MUC4 mucin induces ultra-structural changes and tumorigenicity in pancreatic cancer cells. Br J Cancer. 2007;97:345–57. doi: 10.1038/sj.bjc.6603868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chaturvedi P, Singh AP, Chakraborty S, et al. MUC4 mucin interacts with and stabilizes the HER2 oncoprotein in human pancreatic cancer cells. Cancer Res. 2008;68:2065–70. doi: 10.1158/0008-5472.CAN-07-6041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hsieh AC, Moasser MM. Targeting HER proteins in cancer therapy and the role of the non-target HER3. Br J Cancer. 2007;97:453–7. doi: 10.1038/sj.bjc.6603910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Park HU, Kim JW, Kim GE, et al. Aberrant expression of MUC3 and MUC4 membrane-associated mucins and sialyl Le(x) antigen in pancreatic intraepithelial neoplasia. Pancreas. 2003;26:e48–e54. doi: 10.1097/00006676-200304000-00022. [DOI] [PubMed] [Google Scholar]
- 20.Swartz MJ, Batra SK, Varshney GC, et al. MUC4 expression increases progressively in pancreatic intraepithelial neoplasia. Am J Clin Pathol. 2002;117:791–6. doi: 10.1309/7Y7N-M1WM-R0YK-M2VA. [DOI] [PubMed] [Google Scholar]
- 21.Nishiyama N, Okazaki S, Cabral H, et al. Novel cisplatin-incorporated polymeric micelles can eradicate solid tumors in mice. Cancer Res. 2003;63:8977–83. [PubMed] [Google Scholar]
- 22.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 23.Carraway KL, Perez A, Idris N, et al. Muc4/sialomucin complex, the intramembrane ErbB2 ligand, in cancer and epithelia: to protect and to survive. Prog Nucleic Acid Res Mol Biol. 2002;71:149–85. 149–85. doi: 10.1016/s0079-6603(02)71043-x. [DOI] [PubMed] [Google Scholar]
- 24.Hollingsworth MA, Swanson BJ. Mucins in cancer: protection and control of the cell surface. Nat Rev Cancer. 2004;4:45–60. doi: 10.1038/nrc1251. [DOI] [PubMed] [Google Scholar]
- 25.Chauhan SC, Singh AP, Ruiz F, et al. Aberrant expression of MUC4 in ovarian carcinoma: diagnostic significance alone and in combination with MUC1 and MUC16 (CA125). Mod Pathol. 2006;19:1386–94. doi: 10.1038/modpathol.3800646. [DOI] [PubMed] [Google Scholar]
- 26.Weed DT, Gomez-Fernandez C, Yasin M, et al. MUC4 and ErbB2 expression in squamous cell carcinoma of the upper aerodigestive tract: correlation with clinical outcomes. Laryngoscope. 2004;114:1–32. doi: 10.1097/00005537-200408001-00001. [DOI] [PubMed] [Google Scholar]
- 27.Hoque MO, Soria JC, Woo J, et al. Aquaporin 1 is overexpressed in lung cancer and stimulates NIH-3T3 cell proliferation and anchorage-independent growth. Am J Pathol. 2006;168:1345–53. doi: 10.2353/ajpath.2006.050596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Singh AP, Chaturvedi P, Batra SK. Emerging roles of MUC4 in cancer: a novel target for diagnosis and therapy. Cancer Res. 2007;67:433–6. doi: 10.1158/0008-5472.CAN-06-3114. [DOI] [PubMed] [Google Scholar]
- 29.Li Y, Liu D, Chen D, Kharbanda S, Kufe D. Human DF3/MUC1 carcinoma-associated protein functions as an oncogene. Oncogene. 2003;22:6107–10. doi: 10.1038/sj.onc.1206732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ross JS, Fletcher JA, Linette GP, et al. The Her-2/neu gene and protein in breast cancer 2003: biomarker and target of therapy. Oncologist. 2003;8:307–25. doi: 10.1634/theoncologist.8-4-307. [DOI] [PubMed] [Google Scholar]
- 31.Komatsu M, Jepson S, Arango ME, Carothers Carraway CA, Carraway KL. Muc4/sialomucin complex, an intramembrane modulator of ErbB2/HER2/Neu, potentiates primary tumor growth and suppresses apoptosis in a xenotransplanted tumor. Oncogene. 2001;20:461–70. doi: 10.1038/sj.onc.1204106. [DOI] [PubMed] [Google Scholar]
- 32.Peinado H, Del Carmen Iglesias-de la Cruz, Olmeda D, et al. A molecular role for lysyl oxidase-like 2 enzyme in snail regulation and tumor progression. EMBO J. 2005;24:3446–58. doi: 10.1038/sj.emboj.7600781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zha YH, Mei YW, Mao L, et al. [The advantages for snail expression to promote cell migration and induce actin reorganization and to protect against the serum-deprivation-triggered apoptosis of bone marrow stem cells]. Sheng Wu Gong Cheng Xue Bao. 2007;23:645–51. [PubMed] [Google Scholar]
- 34.Papagerakis S, Shabana AH, Depondt J, Gehanno P, Forest N. Immunohistochemical localization of plakophilins (PKP1, PKP2, PKP3, and p0071) in primary oropharyngeal tumors: correlation with clinical parameters. Hum Pathol. 2003;34:565–72. doi: 10.1016/s0046-8177(03)00174-6. [DOI] [PubMed] [Google Scholar]
- 35.Slater M, Cooper M, Murphy CR. The cytoskeletal proteins alpha-actinin, Ezrin, and talin are De-expressed in endometriosis and endometrioid carcinoma compared with normal uterine epithelium. Appl Immunohistochem Mol Morphol. 2007;15:170–4. doi: 10.1097/01.pai.0000194762.78889.26. [DOI] [PubMed] [Google Scholar]
- 36.Yin MJ, Shao L, Voehringer D, Smeal T, Jallal B. The serine/threonine kinase Nek6 is required for cell cycle progression through mitosis. J Biol Chem. 2003;278:52454–60. doi: 10.1074/jbc.M308080200. [DOI] [PubMed] [Google Scholar]
- 37.Kanamori T, Takakura K, Mandai M, et al. Increased expression of calcium-binding protein S100 in human uterine smooth muscle tumours. Mol Hum Reprod. 2004;10:735–42. doi: 10.1093/molehr/gah100. [DOI] [PubMed] [Google Scholar]
- 38.Coccia EM, Cicala C, Charlesworth A, et al. Regulation and expression of a growth arrest-specific gene (gas5) during growth, differentiation, and development. Mol Cell Biol. 1992;12:3514–21. doi: 10.1128/mcb.12.8.3514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lu KP, Alejandro NF, Taylor KM, Joyce MM, Spencer TE, Ramos KS. Differential expression of ribosomal L31, Zis, gas-5 and mitochondrial mRNAs following oxidant induction of proliferative vascular smooth muscle cell phenotypes. Atherosclerosis. 2002;160:273–80. doi: 10.1016/s0021-9150(01)00581-0. [DOI] [PubMed] [Google Scholar]
- 40.Kosaka Y, Inoue H, Ohmachi T, et al. Tripartite motif-containing 29 (TRIM29) is a novel marker for lymph node metastasis in gastric cancer. Ann Surg Oncol. 2007;14:2543–9. doi: 10.1245/s10434-007-9461-1. [DOI] [PubMed] [Google Scholar]
- 41.Lorey SL, Huang YC, Sharma V. Constitutive expression of interleukin-18 and interleukin-18 receptor mRNA in tumour derived human B-cell lines. Clin Exp Immunol. 2004;136:456–62. doi: 10.1111/j.1365-2249.2004.02465.x. [DOI] [PMC free article] [PubMed] [Google Scholar]