Abstract
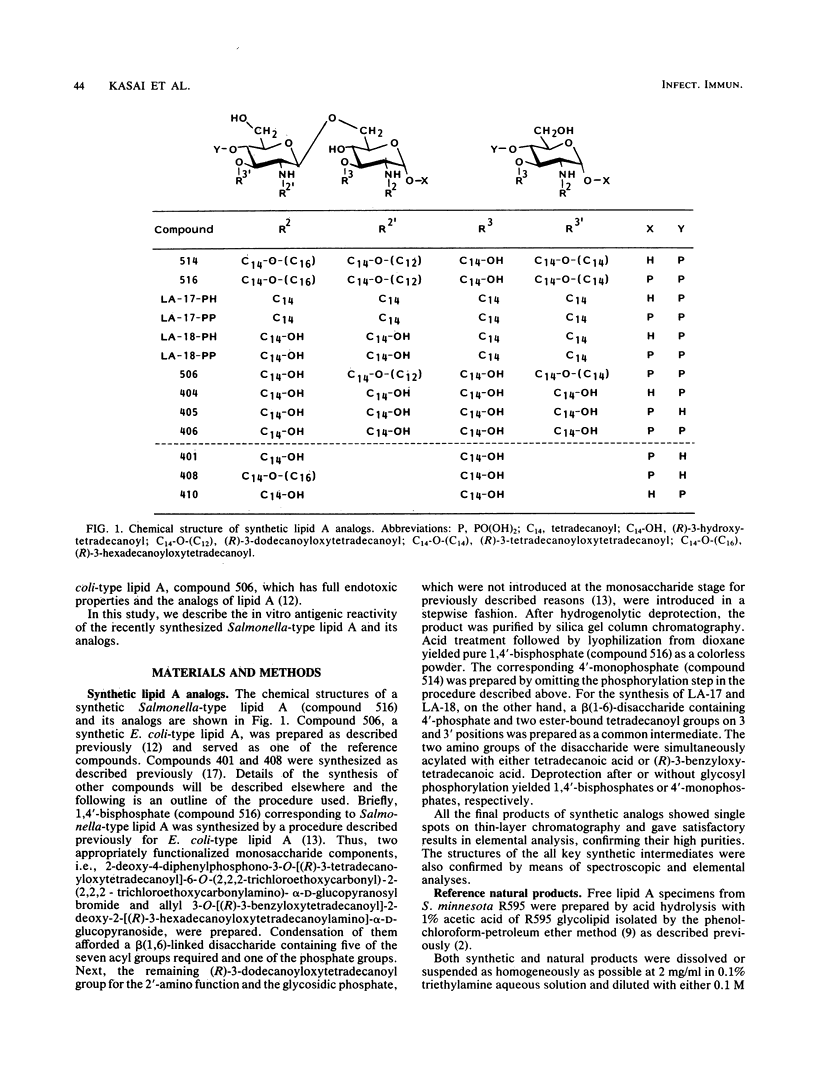
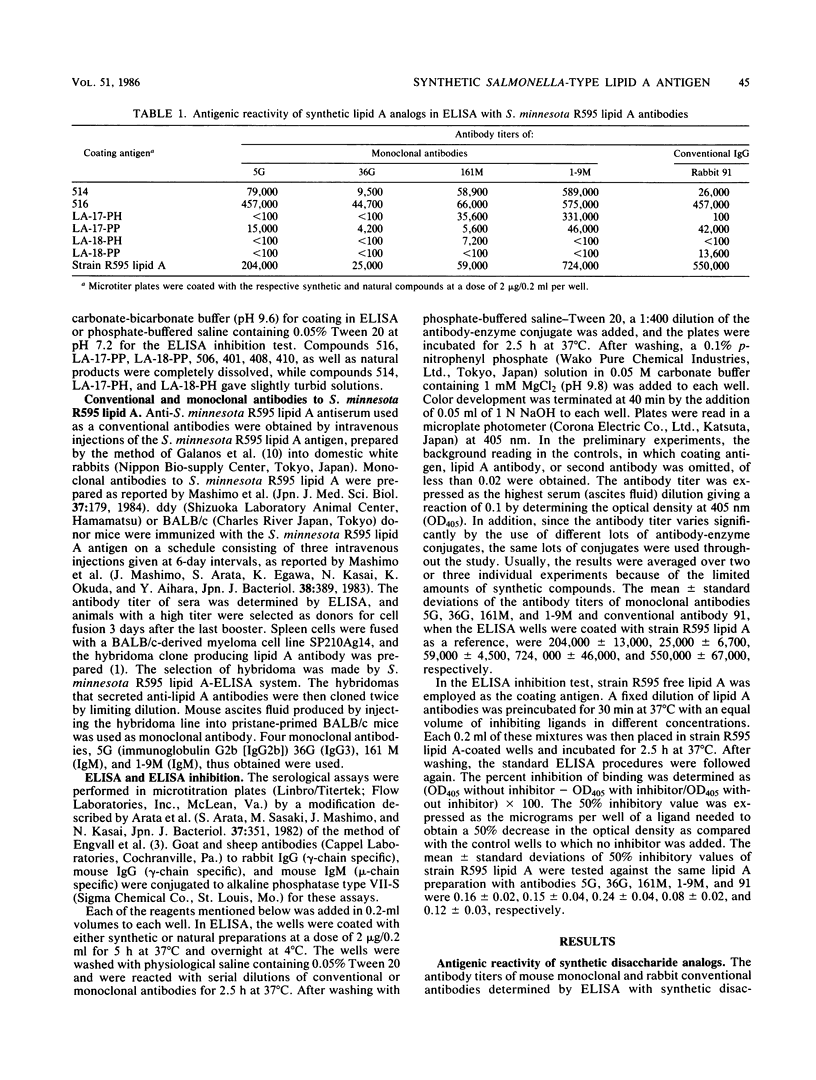
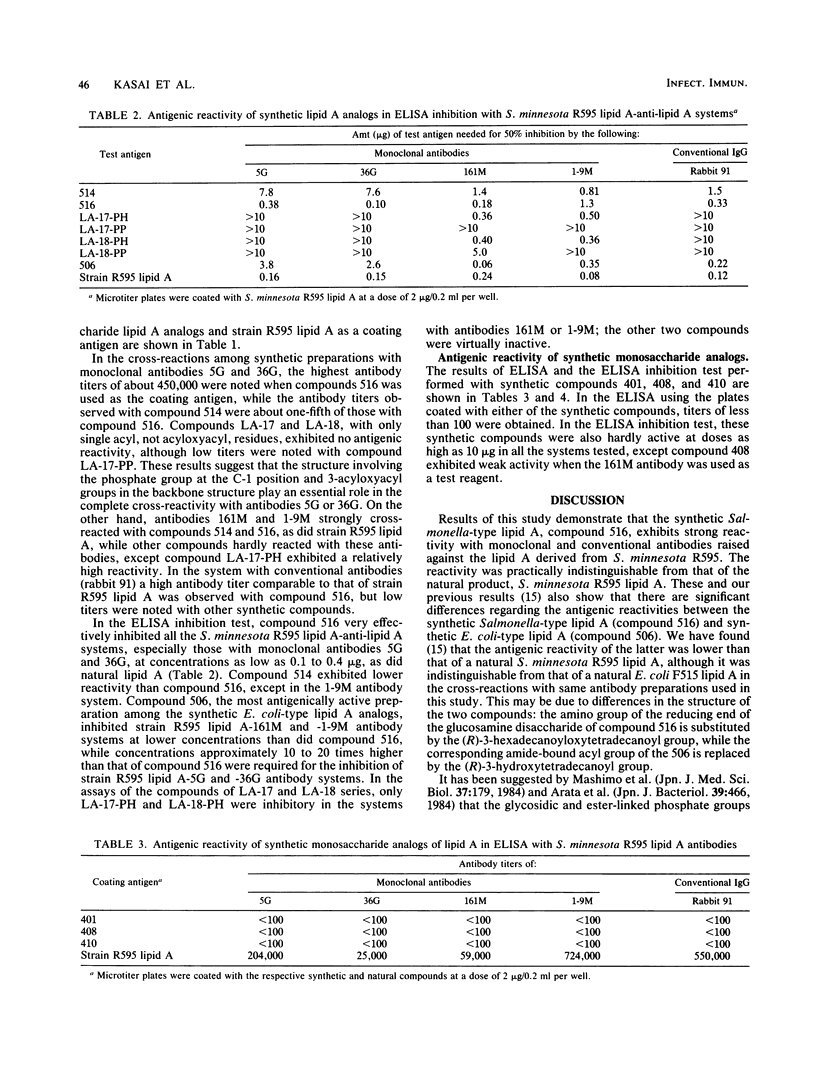
A synthetic compound (compound 516), beta(1-6)-linked D-glucosamine disaccharide 1,4'-bisphosphate, which is acylated by (R)-3-hexadecanoyloxytetradecanoyl, (R)-3-hydroxytetradecanoyl, (R)-3-dodecanoyloxytetradecanoyl, and (R)-3-tetradecanoyloxytetradecanoyl groups at positions 2,3,2', and 3', respectively, exhibited in vitro antigenic reactivity of high specificity comparable to that of free lipid A from Salmonella minnesota R595. This was confirmed by an enzyme-linked immunosorbent assay and an enzyme-linked immunosorbent assay inhibition test with monoclonal and conventional antibodies. The results of comparative analysis performed with several synthetic lipid A analogs as well as three monosaccharide derivatives suggested that the complete structure involving both phosphate groups at the C-1 and C-4' positions and the 3-acyloxyacyl groups at the C-2, C-2', and C-3' positions of the glucosamine disaccharide are required for the expression of the serological specificity of Salmonella-type lipid A. This was deduced from the observations that compound 506, a synthetic Escherichia coli-type lipid A which has the same structure as that of compound 516, except that 3-hydroxytetradecanoyl group is substituted for an acyloxyacyl residue at the C-2 position, exhibited significantly reduced antigenic reactivity as compared with compound 516 and that the replacement by the hydrogen atom of the phosphoryl group at the C-1 position or by 3-hydroxytetradecanoyl or tetradecanoyl groups of acyl residues at the 2, 3, 2', and 3' positions of compound 516 results in a marked reduction of reactivity with monoclonal antibodies 5G and 36G. Similar results were obtained by assays with conventional rabbit antibodies, but the structural difference between compounds 516 and 506 could not be distinguished by these polyclonal antibodies. The results of cross-reactions among synthetic analogs with monoclonal antibodies 161M and 1-9M, which have been confirmed to exhibit different serological specificities from the 5G or 36G antibody, also suggested that the nature and linkage of fatty acyl residues as well as the backbone structure of lipid A play an important role in determining serological specificity of the lipid A molecule.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aihara Y., Tadokoro I., Katoh K., Minami M., Okuda K. T cell allotypic determinants encoded by genes linked to the immunoglobulin heavy chain locus. I. Establishment of monoclonal antibodies against allotypic determinants. J Immunol. 1983 Jun;130(6):2920–2925. [PubMed] [Google Scholar]
- Egawa K., Kasai N. Endotoxic glycolipid as a potent depressor of the hepatic drug-metabolizing enzyme systems in mice. Microbiol Immunol. 1979;23(2):87–94. doi: 10.1111/j.1348-0421.1979.tb00444.x. [DOI] [PubMed] [Google Scholar]
- Engvall E., Perlmann P. Enzyme-linked immunosorbent assay, Elisa. 3. Quantitation of specific antibodies by enzyme-labeled anti-immunoglobulin in antigen-coated tubes. J Immunol. 1972 Jul;109(1):129–135. [PubMed] [Google Scholar]
- Galanos C., Freudenberg M. A., Jay F., Nerkar D., Veleva K., Brade H., Strittmatter W. Immunogenic properties of lipid A. Rev Infect Dis. 1984 Jul-Aug;6(4):546–552. doi: 10.1093/clinids/6.4.546. [DOI] [PubMed] [Google Scholar]
- Galanos C., Lüderitz O., Rietschel E. T., Westphal O., Brade H., Brade L., Freudenberg M., Schade U., Imoto M., Yoshimura H. Synthetic and natural Escherichia coli free lipid A express identical endotoxic activities. Eur J Biochem. 1985 Apr 1;148(1):1–5. doi: 10.1111/j.1432-1033.1985.tb08798.x. [DOI] [PubMed] [Google Scholar]
- Galanos C., Lüderitz O., Westphal O. A new method for the extraction of R lipopolysaccharides. Eur J Biochem. 1969 Jun;9(2):245–249. doi: 10.1111/j.1432-1033.1969.tb00601.x. [DOI] [PubMed] [Google Scholar]
- Galanos C., Lüderitz O., Westphal O. Preparation and properties of antisera against the lipid-A component of bacterial lipopolysaccharides. Eur J Biochem. 1971 Dec 22;24(1):116–122. doi: 10.1111/j.1432-1033.1971.tb19661.x. [DOI] [PubMed] [Google Scholar]
- Galanos C., Roppel J., Weckesser J., Rietschel E. T., Mayer H. Biological activities of lipopolysaccharides and lipid A from Rhodospirillaceae. Infect Immun. 1977 May;16(2):407–412. doi: 10.1128/iai.16.2.407-412.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanegasaki S., Kojima Y., Matsuura M., Homma J. Y., Yamamoto A., Kumazawa Y., Tanamoto K., Yasuda T., Tsumita T., Imoto M. Biological activities of analogues of lipid A based chemically on the revised structural model. Comparison of mediator-inducing, immunomodulating and endotoxic activities. Eur J Biochem. 1984 Sep 3;143(2):237–242. doi: 10.1111/j.1432-1033.1984.tb08364.x. [DOI] [PubMed] [Google Scholar]
- Kasai N., Arata S., Mashimo J., Okuda K., Aihara Y., Kotani S., Takada H., Shiba T., Kusumoto S. In vitro antigenic reactivity of synthetic lipid A analogues as determined by monoclonal and conventional antibodies. Biochem Biophys Res Commun. 1985 Apr 30;128(2):607–612. doi: 10.1016/0006-291x(85)90089-0. [DOI] [PubMed] [Google Scholar]
- Lugowski C., Romanowska E. Biological properties of lipid A from Shigella sonnei. Eur J Biochem. 1974 Oct 1;48(1):81–87. doi: 10.1111/j.1432-1033.1974.tb03745.x. [DOI] [PubMed] [Google Scholar]
- Lüderitz O., Tanamoto K., Galanos C., McKenzie G. R., Brade H., Zähringer U., Rietschel E. T., Kusumoto S., Shiba T. Lipopolysaccharides: structural principles and biologic activities. Rev Infect Dis. 1984 Jul-Aug;6(4):428–431. doi: 10.1093/clinids/6.4.428. [DOI] [PubMed] [Google Scholar]
- Mattsby-Baltzer I., Alving C. R. Lipid A fractions analyzed by a technique involving thin-layer chromatography and enzyme-linked immunosorbent assay. Eur J Biochem. 1984 Jan 16;138(2):333–337. doi: 10.1111/j.1432-1033.1984.tb07919.x. [DOI] [PubMed] [Google Scholar]



