Abstract
Animal studies indicate that monosodium glutamate (MSG) can induce hypothalamic lesions and leptin resistance, possibly influencing energy balance, leading to overweight. This study examines the association between MSG intake and overweight in the human species. We conducted a cross-sectional study of 752 healthy Chinese (48.7% women), ages 40 to 59 years, randomly sampled from three rural villages in north and south China. The great majority of participants prepared their foods at home, without use of commercially processed foods. Diet was assessed with four in-depth multi-pass 24-hour recalls. Participants were asked to demonstrate MSG amounts added in food preparation. Amounts shaken out were weighed by trained interviewers. Overweight was defined as body mass index ≥25.0 kg/m2 or ≥23.0 (based on World Health Organization recommendations for Asian populations). Eighty-two percent of participants used MSG. Average intake was 0.33 gram/day (standard deviation=0.40). With adjustment for potential confounders including physical activity and total energy intake, MSG intake was positively related to body mass index. Prevalence of overweight was significantly higher in MSG users than non-users. For users in the highest tertile of MSG intake compared to non-users, the multivariable-adjusted odds ratios of overweight (body mass index ≥23.0 and ≥25.0) were 2.10 (95% CI, 1.13–3.90, P for trend across four MSG categories=0.03) and 2.75 (95% CI, 1.28–5.95, P=0.04). This research provides human data that MSG intake may be associated with increased risk of overweight independent of physical activity and total energy intake.
Keywords: monosodium glutamate, overweight, leptin resistance, hypothalamic obesity, population study
INTRODUCTION
Obesity is a major health disorder for people of both genders, all age groups, and all ethnic and socioeconomic backgrounds.1 There are diverse approaches for control of body weight, but the fundamental key is energy balance. Previous studies suggest that diet composition may affect energy balance and/or energy-related metabolic pathways.2, 3
Monosodium glutamate (MSG), the sodium salt of glutamic acid, is a food additive used as a flavoring agent to enhance taste. It is frequently added to processed foods and shaken onto foods during preparation, particularly in Asian cuisine. Although the evaluations conducted by the U.S. Food and Drug Administration (FDA) and some other organizations concluded that MSG was a safe food ingredient for the general population,4 none of them answered the question: is MSG consumption healthy? Data from animal studies suggest a possible link between MSG and overweight/obesity.5–8 Weight gain was significantly greater in MSG-treated mice compared to controls even with consumption of similar amounts of food.5 A potential explanation for the MSG-obesity link is altered regulatory mechanisms that affect fat metabolism.9, 10
Worldwide MSG consumption has increased dramatically in recent decades.11, 12 MSG has become a health concern with respect to epidemic overweight/obesity in addition to possible allergenic effects. However, we are unaware of published studies on MSG intake in relation to obesity or overweight in the human species, possibly due to difficulties in quantifying MSG intake, particularly in industrialized countries where it is widely added in commercial food processing.
In China, MSG is a common food additive. In the International Study of Macro-/Micro-nutrients and Blood Pressure (INTERMAP), MSG added by Chinese participants in home food preparation was quantitatively estimated in 1997. The primary purpose of collecting MSG data in INTERMAP was to study the association of sodium intake and blood pressure. Based on the suggestive findings from animal experimental studies, we used the INTERMAP data to examine the association between MSG intake and body mass index (BMI) in apparently healthy middle-aged Chinese adults.
RESEARCH METHODS AND PROCEDURES
Design and Participants
INTERMAP is an international, cross-sectional basic epidemiologic investigation aiming to clarify unanswered questions on the role of dietary factors in the etiology of unfavorable blood pressure patterns prevailing for most middle-aged and older individuals of diverse ethnic-racial and socio-demographic backgrounds. INTERMAP recruited 4,680 men and women ages 40–59 years from 17 randomly selected diverse population samples in four countries (China [n=839], Japan [n=1,145], U.K. [n=501], and U.S.A. [n=2,195]). Three rural samples (two north, one south) were surveyed in China.
INTERMAP design, data collection and measurement procedures, and participant characteristics have been published.13–15 Briefly, participants were asked to attend the local research center four times: two visits on consecutive days, two visits again on average three weeks later. Dietary data were collected at each visit with the in-depth multi-pass 24-hour recall method.14 All foods and drinks consumed in the previous 24 hours, including dietary supplements, were recorded during a detailed interview by a trained and certified dietary interviewer. In countries other than the U.S., dietary data were first entered onto standard forms, then coded and computerized.14 At the country level, coded recalls were logged into batches of 30, with a 10% random sample selected for recoding by the Country Nutritionist. Percent line error was calculated [(sum of lines with error/total lines of coding) x 100]. If this error rate exceeded 6%, the batch was recoded locally and subjected to this quality control procedure again until it passed. Nutrient data were derived from country-specific food tables standardized and enhanced by the Nutrition Coordinating Center (NCC), University of Minnesota.14, 16 Dietary quality control procedures indicated generally high data quality.14
Two 24-hour urine specimens, timed at the research center, were collected on average three weeks apart.17 Measurements included 24-hour urinary excretion of sodium, potassium, calcium, magnesium, and creatinine. Questionnaire data were obtained by interview on demographic and other possible confounding factors including physical activity, smoking, medical history, medication use, et al.13 Participants were asked if they were on a special diet for weight loss, weight gain, vegetarianism, salt reduction, diabetes mellitus, fat modification, or other reasons. BMI was calculated as weight (kg)/height (m)2 measured at visits one and three.
Of 839 Chinese participants, 28 were on a special diet, 42 had diabetes or cardiovascular disease, and 17 had diabetes or cardiovascular disease and were on a special diet. These participants were excluded from the analyses. Cancer patients were excluded by design in INTERMAP. After these exclusions, a total of 752 apparently healthy Chinese men and women remained.
The study received institutional review board approval. All participants gave written informed consent.
MSG Assessment
MSG is a common additive to Chinese food, shaken into foods during cooking. For most Chinese INTERMAP participants, who were from rural areas, all foods were prepared in their households; they seldom ate processed or restaurant prepared food. Thus the MSG measurements were reasonable estimates of total exposure to this additive. For INTERMAP Japanese, British, and American participants, MSG intake was more likely to be from commercially processed and restaurant foods, hence more difficult to quantify in these samples.
To assess MSG intake, Chinese INTERMAP participants were asked whether they used MSG in food preparation. MSG users were asked to demonstrate the amount added during food preparation, using actual MSG. For male participants, the wife was asked, if the food was prepared by her, to demonstrate amount of MSG added. Amounts shaken out were weighed by trained interviewers using a precise scale. For those who used soy sauce, the amount of MSG contained was derived from the labeling or recipes provided by the manufacturers. If participants reported that they had commercially processed foods during the survey, recipes were obtained by the interviewers from the grocery store or food booth. If participants ate restaurant foods, interviewers visited restaurants; chefs were interviewed and asked to demonstrate amounts of MSG added to the dishes. Average MSG intake from four 24-hour recalls per person was used in the analyses.
Overweight Definition
In accordance with recommendations of the World Health Organization (WHO)18 and the International Association for the Study of Obesity for Asian populations,19 overweight was defined as BMI ≥23.0 kg/m2 and BMI ≥25.0.
Statistical Analysis
To examine the association of MSG intake with overweight, we used logistic regression to estimate the odds ratios for overweight defined by BMI ≥23.0 and BMI ≥25.0. MSG non-users served as reference group; MSG users were divided into tertiles according to MSG intake. Odds ratios of overweight were calculated by comparing each MSG intake group to non-users. Median MSG intake in each group was used as a continuous variable to test for trend across groups. In addition, multiple linear regression analysis was used to assess dose-response relation between MSG intake and BMI. Gender specific analyses were also done.
Because of limited literature, dietary and non-dietary potential confounders were identified based mainly on statistical tests. We calculated partial correlations between MSG and BMI respectively with individual nutrients and 24-hour urinary excretions of sodium and potassium with adjustment for age, gender, and sample. Potential confounders were added sequentially to regression models to explore if odds ratios were substantially different with and without adding a particular variable. Adjustments were made with three successive models: model 1 – adjustment for age, gender, sample; model 2 – model 1 variables plus smoking status (never, former, current <20 cigarettes per day, and current ≥20 cigarettes per day), physical activity (hours of moderate activity and hours of heavy activity per day, two variables), 24-hour excretion of sodium, total energy intake; model 3 – model 2 variables plus five nutrients, expressed as caloric density: animal protein, saturated fat, monounsaturated fat, total available carbohydrate, and fiber.
P values were two-sided with p≤0.05 considered statistically significant. SAS software (version 9; SAS Institute, Inc., Cary, North Carolina) was used for analyses.
RESULTS
Of the 752 Chinese participants, 82.4% (84.2% men and 80.6% women) used MSG. Average intake of MSG was 0.33 gram/day (standard deviation=0.40). Men had a slightly higher MSG intake than women (0.36 and 0.30 gram/day). Average BMI was 22.3 (standard deviation=2.7) for men, and 23.6 (3.6) for women. Age-, gender-, and sample-adjusted characteristics of study participants are presented in Table 1. MSG users had higher BMI and were more likely to be overweight compared to non-users. Also, MSG users generally had higher intakes of animal protein, fats, cholesterol, and calories; lower intakes of vegetable protein, total carbohydrate, starch, fiber, and magnesium than non-users.
Table 1.
Age-, sex- sample - adjusted characteristics of study participants (752 healthy Chinese adults) by MSG intake
| Characteristics | Non-users of MSG | MSG users | P value | ||
|---|---|---|---|---|---|
| Tertile 1 | Tertile 2 | Tertile 3 | |||
| Number of participants (%) | 132 (17.6) | 201 (26.7) | 215 (28.6) | 204 (27.1) | -- |
| Education, years | 5.2 (0.23) | 5.5 (0.20) | 5.3 (0.17) | 5.7 (0.20) | 0.21 |
| BMI, kg/m2 | 22.3 (0.28) | 22.7 (0.24) | 23.1 (0.21) | 23.5 (0.25) | 0.01 |
| % BMI ≥ 23.0 | 34.5 | 40.0 | 46.1 | 54.4 | 0.01 |
| % BMI ≥ 25.0 | 12.3 | 20.2 | 25.8 | 30.3 | < 0.01 |
| Smoking status, % | |||||
| Never | 54.4 | 54.7 | 53.4 | 56.0 | 0.72 |
| Former | 5.7 | 5.3 | 10.8 | 8.2 | |
| <20 cigarettes/day | 12.1 | 11.2 | 10.6 | 9.2 | |
| ≥20 cigarettes/day | 27.8 | 28.8 | 25.2 | 26.6 | |
| Heavy activity, hours/day | 1.9 (0.28) | 2.0 (0.24) | 2.3 (0.22) | 2.0 (0.25) | 0.73 |
| Moderate activity, hours/day | 4.2 (0.29) | 4.2 (0.25) | 4.2 (0.22) | 4.0 (0.25) | 0.98 |
| Urinary sodium, mmol/24 hours | 239 (6.79) | 219 (5.82) | 220 (5.21) | 224 (6.02) | 0.09 |
| Urinary potassium, mmol/24 hours | 39.6 (1.16) | 38.2 (0.99) | 36.7 (0.89) | 39.0 (1.03) | 0.14 |
| Daily nutrient intake | |||||
| Glutamic acid, % kcal | 2.9 (0.04) | 2.9 (0.03) | 2.9 (0.03) | 3.0 (0.03) | 0.23 |
| Alcohol, % kcal | 2.0 (0.53) | 2.4 (0.46) | 3.3 (0.41) | 3.1 (0.47) | 0.23 |
| Total protein, % kcal | 12.1 (0.15) | 12.3 (0.13) | 12.5 (0.11) | 12.7 (0.13) | 0.08 |
| Animal protein, % kcal | 1.9 (0.17) | 2.3 (0.15) | 2.7 (0.13) | 3.1 (0.15) | < 0.01 |
| Vegetable protein, % kcal | 10.2 (0.11) | 10.0 (0.09) | 9.8 (0.08) | 9.6 (0.10) | < 0.01 |
| Total fat, % kcal | 17.2 (0.51) | 18.8 (0.44) | 20.4 (0.39) | 22.4 (0.45) | < 0.01 |
| SFA, % kcal | 4.0 (0.17) | 4.7 (0.14) | 5.2 (0.13) | 5.9 (0.15) | < 0.01 |
| MFA, % kcal | 6.7 (0.24) | 7.5 (0.20) | 8.2 (0.18) | 9.3 (0.21) | < 0.01 |
| PFA, % kcal | 5.4 (0.18) | 5.5 (0.15) | 5.9 (0.14) | 6.1 (0.16) | 0.03 |
| n-3 fatty acids, % kcal | 0.47 (0.02) | 0.53 (0.02) | 0.53 (0.02) | 0.54 (0.02) | 0.10 |
| n-6 fatty acids, % kcal | 5.0 (0.17) | 5.0 (0.14) | 5.4 (0.13) | 5.6 (0.15) | 0.04 |
| Cholesterol, mg/1000 kcal | 61.2 (7.46) | 79.8 (6.40) | 94.7 (5.72) | 101.2 (6.62) | < 0.01 |
| Keys dietary lipid score | 13.2 (0.88) | 16.8 (0.75) | 19.2 (0.67) | 21.1 (0.78) | < 0.01 |
| Total available carbohydrate, % kcal | 68.7 (0.79) | 66.4 (0.67) | 63.7 (0.60) | 61.8 (0.70) | < 0.01 |
| Starch, % kcal | 60.4 (0.84) | 58.3 (0.72) | 55.6 (0.64) | 52.7 (0.74) | < 0.01 |
| Estimated total sugars, % kcal | 8.3 (0.47) | 8.1 (0.40) | 8.2 (0.36) | 9.2 (0.41) | 0.25 |
| Calcium, mg/1000 kcal | 144 (4.63) | 145 (3.98) | 151 (3.55) | 152 (4.11) | 0.49 |
| Magnesium, mg/1000 kcal | 166 (2.67) | 154 (2.29) | 155 (2.05) | 152 (2.37) | < 0.01 |
| Phosphorus, mg/1000 kcal | 457 (6.09) | 437 (5.22) | 441 (4.67) | 441 (5.40) | 0.06 |
| Fiber, g/1000 kcal | 16.0 (0.32) | 14.1 (0.27) | 13.6 (0.25) | 13.5 (0.28) | < 0.01 |
| Total energy, kcal | 1965 (42) | 1951 (36) | 2031 (33) | 2216 (38) | < 0.01 |
Data are mean (standard error), unless otherwise specified. SFA: Saturated fatty acids; MFA: monounsaturated fatty acids; PFA: polyunsaturated fatty acids. Keys dietary lipid score = 1.35 (2SFA-PFA) + 1.5 Cholesterol1/2.
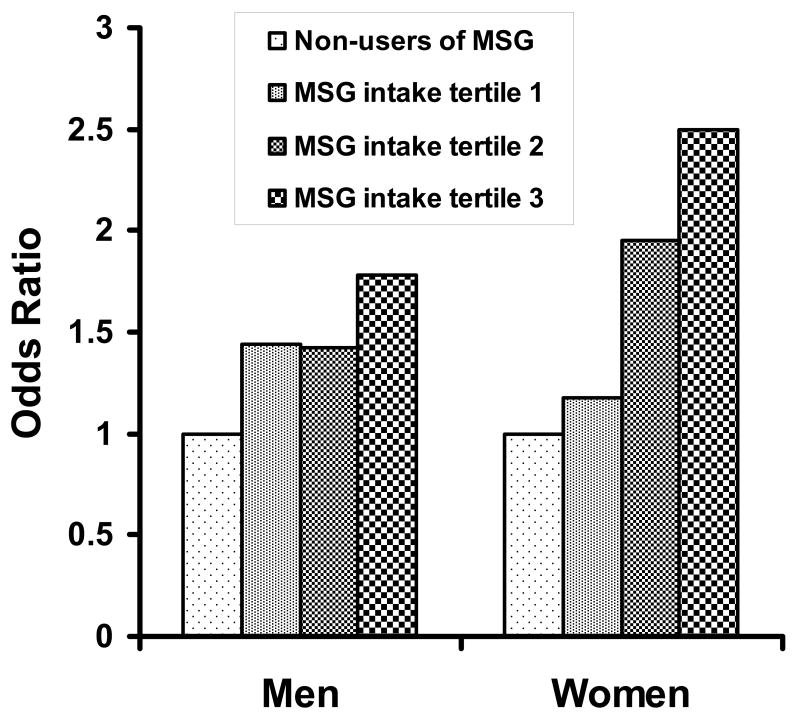
With adjustment for potential confounders, MSG intake was positively related to BMI. With 1 gram higher MSG intake, the estimate was BMI higher by 0.61 kg/m2 (P=0.08). Prevalence of overweight was significantly higher for MSG users than non-users: with BMI ≥23.0 as criterion for overweight, the multivariable odds ratio (model 3) for MSG users in the highest tertile of MSG intake compared to non-users was 2.10 (95% CI, 1.13–3.90, P for trend across the four MSG categories=0.03); with BMI ≥ 25.0, it was 2.75 (95% CI, 1.28–5.95, P for trend=0.04) (Table 2). Positive associations between MSG intake and overweight prevailed for men and women, slightly more pronounced in women (Figure 1), but interaction test between MSG intake and gender statistically non-significant.
Table 2.
Odds ratio and 95% confidence interval for overweight by MSG intake
| Non-users of MSG | MSG Users | P value | |||
|---|---|---|---|---|---|
| Tertile 1 | Tertile 2 | Tertile 3 | |||
| Number of participants | 132 | 201 | 215 | 204 | -- |
| Median MSG intake (range), g/d | 0.00 (0.00–0.00) | 0.08 (0.01–0.15) | 0.28 (0.15–0.45) | 0.70 (0.45–3.23) | -- |
| BMI ≥ 23.0 | |||||
| No. of participants with BMI ≥ 23.0 | 56 | 98 | 97 | 85 | -- |
| Model 1 | 1.0 | 1.27 (0.80–2.00) | 1.67 (1.03–2.72) | 2.50 (1.43–4.38) | < 0.01 |
| Model 2 | 1.0 | 1.42 (0.88–2.30) | 1.84 (1.10–3.06) | 2.51 (1.40–4.52) | < 0.01 |
| Model 3 | 1.0 | 1.30 (0.80–2.12) | 1.59 (0.94–2.72) | 2.10 (1.13–3.90) | 0.03 |
| BMI ≥ 25.0 | |||||
| No. of participants with BMI ≥ 25.0 | 25 | 55 | 52 | 42 | -- |
| Model 1 | 1.0 | 1.61 (0.92–2.82) | 2.33 (1.28–4.25) | 3.37 (1.68–6.75) | < 0.01 |
| Model 2 | 1.0 | 1.83 (1.03–3.25) | 2.59 (1.39–4.83) | 3.21 (1.55–6.65) | < 0.01 |
| Model 3 | 1.0 | 1.77 (0.98–3.20) | 2.36 (1.23–4.52) | 2.75 (1.28–5.95) | 0.04 |
Model 1: adjustment for age, gender, and sample;
Model 2: model 1 with additional adjustment for smoking status, heavy activity, moderate activity, total energy intake, and 24-hour excretion of sodium;
Model 3: model 2 with additional adjustment for intakes (expressed as caloric density) of animal protein, saturated fat, monounsaturated fat, total carbohydrate, and fiber.
P value is test for trend across four categories.
Figure.
Odds ratio of overweight (BMI ≥ 23.0 kg/m2) by gender according to MSG intake.
adjustment for age, gender, and sample, smoking status, heavy activity, moderate activity, total energy intake, 24-hour excretion of sodium, and intakes (expressed as caloric density) of animal protein, saturated fat, monounsaturated fat, carbohydrates and fiber.
In sensitivity analysis including all 839 INTERMAP Chinese participants, the associations of MSG intake with BMI were somewhat attenuated (data not shown). Also, since 7% (n=54) participants reported in at least one visit that they ate commercially processed foods or restaurant foods, we excluded those participants in further sensitivity analyses; associations between MSG intake and BMI remained (data not shown).
DISCUSSION
This cross-sectional study is, to the best of our knowledge, the first to examine the association in human population samples between MSG intake and overweight. A positive relation prevailed with control for physical activity, total energy intake, and other possible confounders. Although animal studies have indicated for decades that MSG intake may cause overweight or obesity, data on the human species have been lacking, possibly because MSG is widely used in commercially processed foods, hence it is difficult to measure intake, particularly in developed countries. INTERMAP Chinese participants were from rural areas in China. In 1997 when the population-based INTERMAP field surveys were done, most foods eaten by Chinese participants were prepared in their households (by themselves or by their spouses). Thus, they lent themselves particularly to the investigation of MSG intake in relation to overweight/obesity.
These positive associations between MSG intake and overweight are generally consistent with data from animal studies. In 1969, it was first reported that weight gain in 4 months was significantly greater in both male and female mice with large-dose MSG-administration immediately post-birth compared to controls, even though the controls had slightly larger food consumption.5 Hypothalamic lesions were found in the MSG-treated animals. The investigator suggested that a regulatory mechanism affecting fat metabolism in the mouse, other than appetite disturbance, should be considered as linking MSG intake with obesity. Recently, the MSG-obesity hypothesis has been re-formulated, i.e., chronic MSG intake may intoxicate arcuate nucleus neurons and disrupt the hypothalamic signaling cascade of leptin action, causing leptin resistance related to overweight/obesity.9, 10
MSG ingestion as a cause of hypothalamic damage has been debated for decades. Some animal studies suggest that injection of MSG may induce neuronal necrosis in several brain regions including the hypothalamus.5, 20–25 Since destruction of hypothalamic neurons in animals results in a complex neuroendocrine deficiency syndrome, concern has arisen that ingestion of MSG by humans may contribute to occurrence of neuroendocrinopathies.26, 27 Most of the evidence on hypothalamic lesions and appetite regulation was provided from laboratory animals that were injected with MSG at neonatal age. While the deleterious effects of MSG injection on hypothalamic neuronal circuitries have been well documented, limited studies have been published on the effects of orally administered MSG.12, 28 Some researchers are unconvinced that oral MSG affects appetite regulation.29 Further studies are clearly needed. Nevertheless, the findings from our study support the judgment against MSG supplementation of human nutrition.
In addition, studies have indicated an important role for leptin in regulating food intake and energy balance.30, 31 Leptin receptor mRNA is present in the hypothalamus, postulated as a potential site of action for leptin.32 Leptin production is increased in animal models of obesity associated with experimentally induced hypothalamic damage, including the MSG-treated model.33 One study found that leptin suppressed body weight gain in controls but did not suppress weight gain in MSG-treated rats.6 This finding suggests that destruction of neurons in the hypothalamus by MSG can attenuate the actions of leptin. Another study reported that leptin significantly inhibited food intake and caused weight loss in control rats whereas MSG-treated rats were unresponsive to leptin treatment.7 The authors suggested that the hypothalamic arcuate nucleus is essential for mediating the anorectic effects of leptin influencing energy balance. Human hypothalamic obesity has been reported in humans due to hypothalamic damage from tumour, 34, 35 as well as in animals after neonatal administration of MSG.5, 36 A recent study reported that MSG maintains its toxicity in animals when administered orally, and that MSG at concentrations only slightly surpassing those found in everyday human food has potential for damaging hypothalamic regulation of appetite.12
An alternative mechanism for the possible linkage of MSG to obesity is that MSG may regulate adipsin and thereby change body composition. Adipsin is synthesized and secreted by adipocytes. Depressed adipsin expression has been associated with obesity in animal models.37 Mice treated with MSG had 50% lower serum adipsin and over 2-fold higher percentage of body fat than lean controls.38
A positive association between MSG intake and overweight may be a result of greater voracity since MSG or leptin resistance can stimulate appetite. In the INTERMAP Chinese samples, participants with higher MSG intake tended to have higher total energy intake. However, our findings are independent of total energy intake. Presumably, the weight gain independent of total energy intake and physical activity was due to decreased non-exercise energy expenditure, e.g., thermogenesis.39, 40
Our findings are unlikely to be due to chance. The multivariable adjusted positive associations are statistically significant, and prevailed for men and women. Also, the associations were present using either the international standardized cut-off (BMI ≥25.0) or the WHO recommendation (BMI ≥23.0) for overweight for Asian populations.
One concern is the accuracy of the MSG measurement. Similar to other food additives such as table salt (NaCl), MSG intake is difficult to quantify accurately with existing dietary assessment instruments. However, our estimation of MSG intake in INTERMAP is unlikely to be substantially biased. First, all foods eaten by the great majority of INTERMAP Chinese participants were exclusively prepared in their households. Second, the amounts of MSG added to foods during preparation appear to be habitual and were demonstrable by participants in the diet assessment. Third, although estimates of absolute intake of MSG may have been limited in precision, we apparently were able to identify MSG users and non-users. In fact, the prevalence of overweight for MSG users was significantly higher compared to MSG non-users, in addition to the positive trend for relation of amount of MSG intake to BMI. Finally, measurement error is likely to be non-differential, tending to dilute any observed association.
Another relevant question is: why are Asian populations relatively lean since MSG use is popular in Asian cuisine? A possible explanation is that an adverse effect of MSG intake on body weight may be attenuated by lower caloric density of foods and other lifestyle factors such as greater physical activity in Asian populations compared to Western. For example, the average amount of heavy activity per day for INTERMAP rural Chinese participants was 2.4 hours (standard deviation=3.7) for men and 1.7 hours (3.2) for women, indicating relatively higher energy expenditure in this group.
In this study, our ability to examine the relation of MSG intake and obesity was limited by small number of persons (3%) with BMI ≥30.0. However, the partial correlation between MSG intake and BMI approached significance. Hence it is reasonable to infer that MSG intake is also associated with higher prevalence of obesity. This study is also limited by lack of data on leptin and adipsin concentrations. In addition, the INTERMAP findings are cross-sectional, but nevertheless unique; this topic has not been previously pursued in human population samples.
In conclusion, we found a positive relation of MSG intake to BMI that persisted with controlling for physical activity and total energy intake among apparently healthy Chinese adults. MSG intake was significantly related to prevalence of overweight. This study is of public health interest because MSG is increasingly used worldwide. This study also provides the first human data on this issue and raises a concern about MSG use and body weight in addition to allergenic effects. Further studies are needed to determine reproducibility of these findings, elucidate their etiopathogenetic pathway, and amass the evidence needed to assess whether the relation between MSG intake and body weight is causal.
Acknowledgments
This work was supported by the NIH grants 2-R01-HL50490 and R21DK073812; by the Chicago Health Research Foundation; and by national and local agencies in China, Japan, and the United Kingdom.
The authors thank their colleagues from Beijing, Shanxi Province, and Guangxi Zhuang Autonomous Region, and colleagues at other INTERMAP research facilities. A partial listing of these colleagues is given in reference 12.
References
- 1.Flegal KM, et al. Prevalence and trends in obesity among US adults, 1999–2000. Jama. 2002;288(14):1723–7. doi: 10.1001/jama.288.14.1723. [DOI] [PubMed] [Google Scholar]
- 2.Zemel MB. Role of calcium and dairy products in energy partitioning and weight management. Am J Clin Nutr. 2004;79(5):907S–912S. doi: 10.1093/ajcn/79.5.907S. [DOI] [PubMed] [Google Scholar]
- 3.Raben A, et al. Meals with similar energy densities but rich in protein, fat, carbohydrate, or alcohol have different effects on energy expenditure and substrate metabolism but not on appetite and energy intake. Am J Clin Nutr. 2003;77(1):91–100. doi: 10.1093/ajcn/77.1.91. [DOI] [PubMed] [Google Scholar]
- 4.S. Food and Drug Administration. FDA and Monosodium Glutamate (MSG) [Accessed January 22, 2008]; Available at: http://www.cfsan.fda.gov/~lrd/msg.html.
- 5.Olney JW. Brain lesions, obesity, and other disturbances in mice treated with monosodium glutamate. Science. 1969;164(880):719–21. doi: 10.1126/science.164.3880.719. [DOI] [PubMed] [Google Scholar]
- 6.Dawson R, et al. Attenuation of leptin-mediated effects by monosodium glutamate-induced arcuate nucleus damage. Am J Physiol. 1997;273(1 Pt 1):E202–6. doi: 10.1152/ajpendo.1997.273.1.E202. [DOI] [PubMed] [Google Scholar]
- 7.Tang-Christensen M, et al. The arcuate nucleus is pivotal in mediating the anorectic effects of centrally administered leptin. Neuroreport. 1999;10(6):1183–7. doi: 10.1097/00001756-199904260-00005. [DOI] [PubMed] [Google Scholar]
- 8.Bergen HT, et al. Hyperphagia and weight gain after gold-thioglucose: relation to hypothalamic neuropeptide Y and proopiomelanocortin. Endocrinology. 1998;139(11):4483–8. doi: 10.1210/endo.139.11.6324. [DOI] [PubMed] [Google Scholar]
- 9.Hermanussen M, Tresguerres JA. Does high glutamate intake cause obesity? J Pediatr Endocrinol Metab. 2003;16(7):965–8. doi: 10.1515/jpem.2003.16.7.965. [DOI] [PubMed] [Google Scholar]
- 10.Hermanussen M, Tresguerres JA. Does the thrifty phenotype result from chronic glutamate intoxication? A hypothesis. J Perinat Med. 2003;31(6):489–95. doi: 10.1515/JPM.2003.075. [DOI] [PubMed] [Google Scholar]
- 11.Kawakita T. Kirk-Othmer Encyclopedia of Chemical Technology. 4. Vol. 2. Inter-Science-Wiley; 1992. L-monosodium glutamate (MSG) p. 571. [Google Scholar]
- 12.Hermanussen M, et al. Obesity, voracity, and short stature: the impact of glutamate on the regulation of appetite. Eur J Clin Nutr. 2005 doi: 10.1038/sj.ejcn.1602263. [DOI] [PubMed] [Google Scholar]
- 13.Stamler J, et al. INTERMAP: background, aims, design, methods, and descriptive statistics (nondietary) J Hum Hypertens. 2003;17(9):591–608. doi: 10.1038/sj.jhh.1001603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dennis B, et al. INTERMAP: the dietary data--process and quality control. J Hum Hypertens. 2003;17(9):609–22. doi: 10.1038/sj.jhh.1001604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou BF, et al. Nutrient intakes of middle-aged men and women in China, Japan, United Kingdom, and United States in the late 1990s: the INTERMAP study. J Hum Hypertens. 2003;17(9):623–30. doi: 10.1038/sj.jhh.1001605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schakel SF, Sievert YA, Buzzard IM. Sources of data for developing and maintaining a nutrient database. J Am Diet Assoc. 1988;88(10):1268–71. [PubMed] [Google Scholar]
- 17.Elliott P, Stamler R. Manual of operations for “INTERSALT”, an international cooperative study on the relation of sodium and potassium to blood pressure. Control Clin Trials. 1988;9(2 Suppl):1S–117S. [PubMed] [Google Scholar]
- 18.Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363(9403):157–63. doi: 10.1016/S0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
- 19.International Obesity Task Force (on behalf of the Steering Committee) Western Pacific Region. Sydney, Australia: Health Communications Australia Pty Limited; 2002. The Asia-Pacific Perspective: Redefining Obesity and Its Treatment. [Google Scholar]
- 20.Olney JW, Sharpe LG. Brain lesions in an infant rhesus monkey treated with monsodium glutamate. Science. 1969;166(903):386–8. doi: 10.1126/science.166.3903.386. [DOI] [PubMed] [Google Scholar]
- 21.Arees EA, Mayer J. Monosodium glutamate-induced brain lesions: electron microscopic examination. Science. 1970;170(957):549–50. doi: 10.1126/science.170.3957.549. [DOI] [PubMed] [Google Scholar]
- 22.Pizzi WJ, Barnhart JE, Fanslow DJ. Monosodium glutamate admlinistration to the newborn reduces reproductive ability in female and male mice. Science. 1977;196(4288):452–4. doi: 10.1126/science.557837. [DOI] [PubMed] [Google Scholar]
- 23.Simson EL, et al. Axon-sparing brain lesioning technique: the use of monosodium-L-glutamate and other amino acids. Science. 1977;198(4316):515–7. doi: 10.1126/science.910144. [DOI] [PubMed] [Google Scholar]
- 24.Olney JW, Ho OL. Brain damage in infant mice following oral intake of glutamate, aspartate or cysteine. Nature. 1970;227(5258):609–11. doi: 10.1038/227609b0. [DOI] [PubMed] [Google Scholar]
- 25.Olney JW. Brain damage and oral intake of certain amino acids. In: Levi G, Battistin L, Lajtha A, editors. Transport phenomena in the Nervous System: Physiological and Pathological Aspects. New York: Plenum; 1976. [Google Scholar]
- 26.Olney JW. Excitotoxic food additives--relevance of animal studies to human safety. Neurobehav Toxicol Teratol. 1984;6(6):455–62. [PubMed] [Google Scholar]
- 27.Olney JW. Food additives, excitotoxic. In: Adelman G, editor. Encyclopedia of Neuroscience. Boston: Birkhauser; 1987. pp. 436–438. [Google Scholar]
- 28.Monno A, et al. Extracellular glutamate levels in the hypothalamus and hippocampus of rats after acute or chronic oral intake of monosodium glutamate. Neurosci Lett. 1995;193(1):45–8. doi: 10.1016/0304-3940(95)11664-i. [DOI] [PubMed] [Google Scholar]
- 29.Beyreuther K, et al. Consensus meeting: monosodium glutamate - an update. Eur J Clin Nutr. 2007;61:304–313. doi: 10.1038/sj.ejcn.1602526. [DOI] [PubMed] [Google Scholar]
- 30.Campfield LA, et al. Recombinant mouse OB protein: evidence for a peripheral signal linking adiposity and central neural networks. Science. 1995;269(5223):546–9. doi: 10.1126/science.7624778. [DOI] [PubMed] [Google Scholar]
- 31.Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature. 1998;395(6704):763–70. doi: 10.1038/27376. [DOI] [PubMed] [Google Scholar]
- 32.Mercer JG, et al. Coexpression of leptin receptor and preproneuropeptide Y mRNA in arcuate nucleus of mouse hypothalamus. J Neuroendocrinol. 1996;8(10):733–5. doi: 10.1046/j.1365-2826.1996.05161.x. [DOI] [PubMed] [Google Scholar]
- 33.Frederich RC, et al. Expression of ob mRNA and its encoded protein in rodents. Impact of nutrition and obesity. J Clin Invest. 1995;96(3):1658–63. doi: 10.1172/JCI118206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bray GA. Syndromes of hypothalamic obesity in man. Pediatr Ann. 1984;13(7):525–36. [PubMed] [Google Scholar]
- 35.Pinkney J, et al. Hypothalamic obesity in humans: what do we know and what can be done? Obes Rev. 2002;3(1):27–34. doi: 10.1046/j.1467-789x.2002.00052.x. [DOI] [PubMed] [Google Scholar]
- 36.Meister B, et al. Neurotransmitters, neuropeptides and binding sites in the rat mediobasal hypothalamus: effects of monosodium glutamate (MSG) lesions. Exp Brain Res. 1989;76(2):343–68. doi: 10.1007/BF00247894. [DOI] [PubMed] [Google Scholar]
- 37.Flier JS, et al. Severely impaired adipsin expression in genetic and acquired obesity. Science. 1987;237(4813):405–8. doi: 10.1126/science.3299706. [DOI] [PubMed] [Google Scholar]
- 38.Spurlock ME, Hahn KJ, Miner JL. Regulation of adipsin and body composition in the monosodium glutamate (MSG)-treated mouse. Physiol Behav. 1996;60(5):1217–21. doi: 10.1016/s0031-9384(96)00219-3. [DOI] [PubMed] [Google Scholar]
- 39.Choi S, et al. Rats with hypothalamic obesity are insensitive to central leptin injections. Endocrinology. 1999;140(10):4426–33. doi: 10.1210/endo.140.10.7064. [DOI] [PubMed] [Google Scholar]
- 40.Considine RV, et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med. 1996;334(5):292–5. doi: 10.1056/NEJM199602013340503. [DOI] [PubMed] [Google Scholar]