Abstract
The neurophysiological basis of variability in the latency of evoked neural responses has been of interest for decades. We describe a method to identify white matter pathways that may contribute to inter-individual variability in the timing of neural activity. We investigated the relation of the latency of peak visual responses in occipital cortex as measured by magnetoencephalography (MEG) to fractional anisotropy (FA) in the entire brain as measured with diffusion tensor imaging (DTI) in eight healthy young adults. This method makes no assumptions about the anatomy of white matter connections. Visual responses were evoked during a saccadic paradigm and were time-locked to arrival at a saccadic goal. The latency of the peak visual response was inversely related to FA in bilateral parietal and right lateral frontal white matter adjacent to cortical regions that modulate early visual responses. These relations suggest that biophysical properties of white matter affect the timing of early visual responses. This preliminary report demonstrates a non-invasive, unbiased method to relate the timing information from evoked-response experiments to the biophysical properties of white matter measured with DTI.
Keywords: magnetoencephalography, diffusion tensor imaging, visual evoked potentials, saccades, white matter, frontal eye field
Introduction
The neurophysiological basis of variability in the latency of evoked neural responses detected with electroencephalography (EEG) and magnetoencephalography (MEG) has been of interest for decades. A recently developed imaging technique, diffusion tensor imaging (DTI), can be used to investigate whether individual differences in the microstructural integrity of white matter tracts contributes to inter-individual variability in the timing of neural activity. White matter physiology, particularly myelination, has been proposed to contribute to Individual differences in cognitive processing speed (e.g., Luciano et al 2004) based on the well-established role of myelin thickness and axon diameter in determining conduction velocity (Waxman 1980). Recent reports of relations between DTI measures of fractional anistropy (FA) – an indirect measure of myelination (Harsan et al 2006) and other WM microstructural properties (Beaulieu 2002) – and cognitive reaction time support the proposal of a white matter contribution to variability in processing speed (Bucur et al 2007; Gold et al 2007; Madden et al 2004; Manoach et al 2007; Nestor et al 2007; Tuch et al 2005; Westerhausen et al 2006). On this basis, we reasoned that WM microstructure might also contribute to the timing of evoked neural responses, which are a more direct measure of the timing of neural responses than behavior.
We investigated the relations of FA with individual differences in the latency of peak visual responses in occipital cortex as measured by MEG in the context of a saccadic paradigm. Using this paradigm, we previously reported relations between FA and saccadic latency in patients with schizophrenia and healthy controls (Manoach et al 2007). In the present study, instead of examining relations of FA with behavior, we examined FA in relation to the latency visual responses as measured by MEG. By testing for relations in the entire brain, without making any assumptions about the anatomical connections between regions, this method relates cortical function to the microstructural integrity of white matter tracts in an unbiased manner and can reveal white matter pathways that may contribute to inter-individual variability in the timing of neural activity. The visual responses were evoked by fixating a saccadic goal and were time-locked to the end point of the saccade. Post-saccadic visual responses were chosen to maximize timing variability since previous work demonstrates significantly greater variability in visual responses in V1 neurons following fixational saccadic eye movements compared to relatively steady fixation (Gur et al 1997; Martinez-Conde et al 2000). Since early visual responses are influenced by modulatory top-down signals (Moore and Armstrong 2003; Ruff et al 2006), we hypothesized that inter-individual differences in the conduction velocity of fibers from modulatory areas would contribute to variation in response latency. Thus, our aim was to identify regions that may modulate early visual responses by correlating FA with the timing of the peak early visual response. More generally, this method can be applied to investigate the contribution of white matter microstructural integrity to variability in the latencies of EEG or MEG evoked responses non-invasively in human subjects.
Experimental Procedures
Participants
Eight healthy young adults (4 male; mean age: 22 ± 2) participated. We restricted our sample to young subjects within a restricted range of ages given the documented relation of increasing age with decreasing FA (Pfefferbaum et al 2000; Salat et al 2005). All subjects gave written informed consent. The study was approved by the Human Research Committee at Massachusetts General Hospital and adhered to the principles of the Declaration of Helsinki.
Magnetoencephalography Acquisition and Eye Movement Measurements
Whole head MEG signals (102 magnetometers, 204 planar gradiometers) were recorded in a magnetically shielded room (IMEDCO, Hagendorf, Switzerland) using a dc-SQUID Neuromag™ VectorView system (Elekta-Neuromag, Helsinki, Finland). The data were filtered to a 0.1 – 200 Hz bandpass and sampled at 600 Hz. To allow registration of MEG and MRI data, the sites of four head-position indicator (HPI) coils that were attached to the scalp were digitized using a 3Space Fastrak digitizer (Polhemus, Colchester, VT, USA) integrated with the Vectorview system. The horizontal and vertical components of eye-movements were recorded concurrently with MEG, using two pairs of bipolar electro-oculogram (EOG) electrodes.
Saccadic task
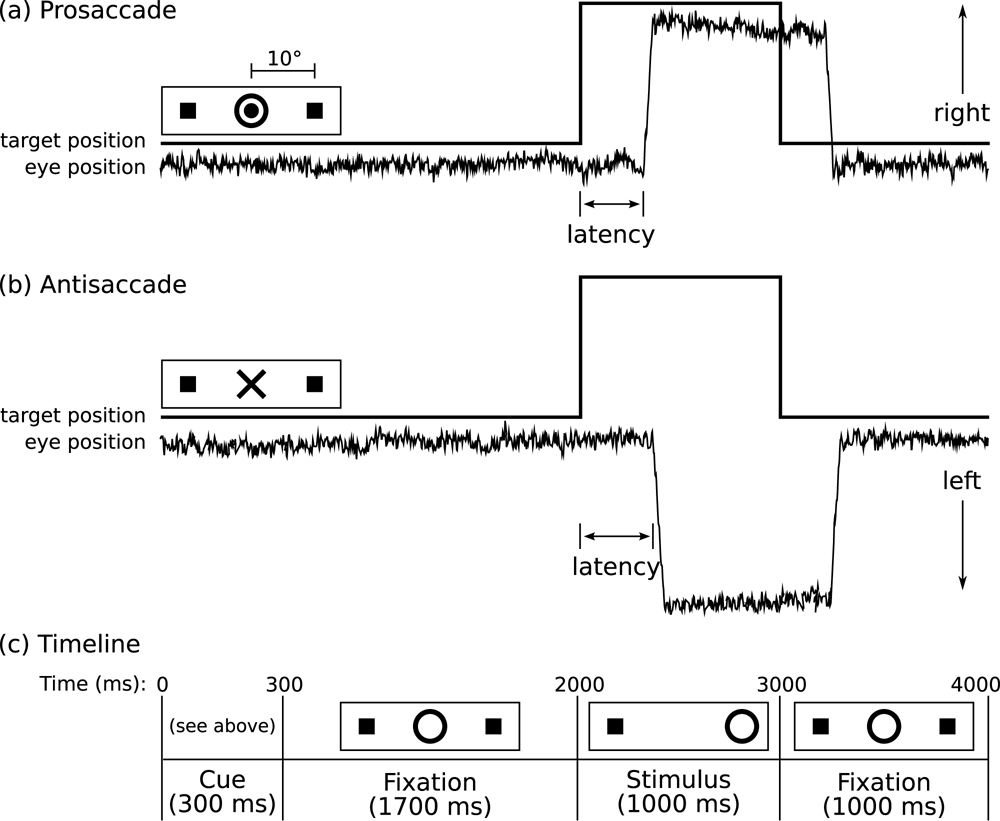
Figure 1 provides a graphic depiction of the task and a description of task parameters. During MEG acquisition, participants performed a saccadic task. Visual stimuli were generated using the Vision Shell programming platform (www.visionshell.com) and presented with a Digital Light Processing (DLP) InFocus 350 projector, through an opening in the wall, onto a back-projection screen placed 102 cm in front of the participant inside the magnetically shielded room. Each participant performed eight runs of the saccadic task with short breaks in between. Each run was 5 minutes 22 seconds and consisted of a pseudorandom sequence of prosaccade, antisaccade, and fixation trials. Prosaccade trials required participants to make a saccade to a suddenly appearing visual stimulus, and antisaccade trials required participants to make a saccade in the opposite direction, to a stimulus that remained on the screen for the duration of the experiment. The saccadic trials were balanced for right and leftward movements and lasted 4000 ms. The total experiment lasted approximately one hour and generated a total of 278 prosaccade, 285 antisaccade, and 107 fixation trials.
Figure 1.
Saccadic Paradigm. Saccadic trials lasted 4000 ms and began with an instructional cue at screen center. For half of the participants, an orange ring was the cue for a PS trial and a blue × the cue for an AS trial. These cues were reversed for the rest of the participants. The cue was flanked horizontally by two small green squares of 0.2° side that marked the potential locations of stimulus appearance, 10° left and right of center. These squares remained on the screen for the duration of each run. At 300 ms the instructional cue was replaced by a green fixation ring at screen center with a diameter of 0.4° and luminance of 20 cd/m2. After 1700 ms the ring shifted to one of the two stimulus locations, right or left, with equal probability. This ring was the stimulus to which participants responded. The green ring remained in the peripheral location for 1000 ms and then returned to the center where participants were instructed to return their gaze for 1000 ms. Fixation intervals were simply a continuation of the fixation display that constituted the final second of the previous saccadic trial.
MRI and DTI Acquisition
Images were collected using a 3.0 Tesla Siemens Trio MRI scanner (Siemens Medical System, Iselin, NJ). Two T1-weighted high-resolution structural images were acquired in the sagittal plane for slice prescription, spatial normalization, and cortical surface reconstruction using a high resolution 3D magnetization prepared rapid gradient echo (MP-RAGE) sequence (repetition time (TR), 2530 ms; echo spacing, 7.25 ms; echo time (TE), 3 ms; flip angle 7°) with an in-plane resolution of 1.3 mm and 1 mm slice thickness.
Single-shot EPI DTI data were acquired using a twice-refocused spin echo sequence (Reese et al 2003). The sequence parameters were repetition time (TR) = 8400 ms, echo time (TE) = 82 ms, b = 700 s/mm2, NEX=1, 72 diffusion directions; 128×128 matrix; 2×2 mm in-plane resolution; 64 axial oblique (AC-PC) slices; 2mm (0mm gap) slice thickness, scan duration 12’44”. The n=72 diffusion directions were obtained using the electrostatic shell algorithm (Jones 2004). The slices were oriented in the intercommissural (AC-PC) plane. The eddy current distortions between diffusion weightings were typically less than two voxels.
Analysis of Eye Movement Data
The EOG data were low-pass filtered at 30 Hz and scored in MATLAB (Mathworks, Natick, MA) using a partially automated program that determined the directional accuracy of each saccade with respect to the required response, the latency of saccadic initiation, and the endpoint of the saccade. For each trial, saccadic onset was defined as the point preceding peak velocity at which the horizontal eye-position trace deviated from fixation (Figure 2). To determine this point an automated algorithm started at the point of peak velocity and searched the eye-position trace backwards to fixation. The endpoint of the saccade was defined as the point following peak velocity at which the eye reached fixation. Fixation was defined as the time point at which the slope of the eye-position trace was zero as determined by evaluating the slope in relation to the surrounding time points, a moving window of 5 ms. Algorithm results were visually inspected to ensure accuracy. Only trials with saccades in the desired direction and latencies between 130 and 800 ms were considered correct, and only correct saccades were included in the regression analysis. The cutoff of 130 ms excluded anticipatory saccades, which are executed too quickly to be a valid response to the appearance of the target (Doricchi et al 1997; Fischer and Breitmeyer 1987; Straube et al 1999).
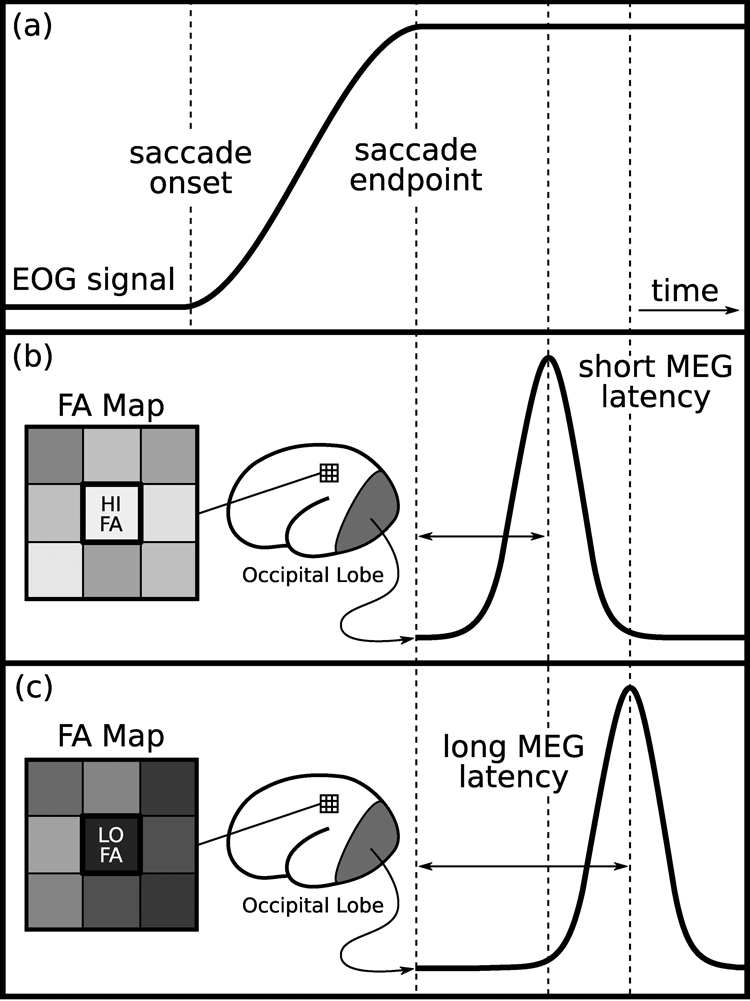
Figure 2.
Schematic illustration of method and findings: (a) Idealized trace of the horizontal eye-position over time with saccade onset and endpoint indicated. The latency measure was the timing of the peak occipital MEG evoked response occurring between 70 and 130 ms following the saccade endpoint. (b, c) Schematic illustrations of FA values in a grid of voxels in subcortical white matter regions that were correlated with the latency of the peak MEG evoked visual response in occipital cortex. (b) Low FA values correspond to long latencies and (c) high FA values correspond to short latencies.
MEG Analysis
MEG data were bandpass filtered at 0.1 – 30Hz. Consistently noisy channels were identified by visual inspection and omitted from analysis. Each participant’s waveforms were averaged to the endpoint of the saccade. Only trials meeting amplitude criteria (maximum peak-to-peak amplitude; magnetometers: 10,000fT, gradiometers: 3000fT/cm) were included in the averaged waveforms. A 200 ms interval prior to the beginning of each trial was used as a baseline and subtracted from each epoch before the trial was added to the average. For source estimation, a 3D structural image was created for each participant by averaging the two MP-RAGE scans after correcting for motion. The structural image was segmented and inflated using FreeSurfer software (Dale et al 1999; Fischl et al 1999). The source space for MEG analysis was seeded onto the cortical surface using approximately 3000 dipolar current sources per hemisphere. The forward solution was calculated using a single-compartment boundary element model (Hamalainen and Sarvas 1989) with the inner skull surface segmented from MRI data. An L2 minimum norm estimate with noise-normalization was applied to the averaged MEG signals, yielding dynamic statistical parametric maps (dSPM) (Dale et al 2000; Hamalainen and Ilmoniemi 1984). In calculating the average dipole waveforms, the dipole orientation was approximately constrained to the cortical normal direction by setting source variances for the transverse current components to be 0.4 times the variance of the currents normal to the cortical surface (Lin et al 2006a; Lin et al 2006b).
We considered data from prosaccade and antisaccade trials separately and also averaged them to derive latency measures. The maximum dSPM activation within 70–130 ms following the end of the saccade (re-fixation on the saccadic goal) was identified within the occipital cortex of each hemisphere. The latency of the neuromagnetic visual response for each participant was derived from the occipital vertex with the maximum response in either hemisphere.
DTI Preprocessing
Raw diffusion data were corrected for head motion and residual eddy current distortion by registering all images to the first acquired T2 image. Using the FLIRT tool (Jenkinson and Smith 2001) with a 12-df global affine transformation, the DTI images were registered using a mutual information cost function, available through the FSL software library (www.fmrib.ox.ac.uk/fsl). Trilinear interpolation was used for the resampling. Inter-subject registration of individual FA maps to the Montreal Neurological Institute (MNI305) atlas (Collins et al 1994) was performed using the average of the two 3D structural images. The resulting transformation was applied to individual FA volumes. The MNI-normalized FA volumes were smoothed with a 3D Gaussian kernel with 6-mm full-width at half-maximum (FWHM) and 6-mm spatial extent. Voxels with trace diffusion >6 µm2/ms were not included in the smoothing operation in an effort to minimize the partial volume contribution from cerebrospinal fluid. FA was calculated for each voxel using in-house software.
FA-Latency Regression Analysis
FA values were regressed on the peak MEG latency for each voxel in whole-brain FA volume. To correct the regression map for multiple comparisons, we ran 10,000 Monte Carlo simulations of the smoothing, averaging, and resampling parameters of the regression data using synthesized white Gaussian noise data. This determines the likelihood that a cluster of a certain size would be found by chance for our starting threshold of p ≤ 0.001 and yields a corrected p-value ≤ 0.05. Significant clusters are visualized as an overlay on horizontal slices of a representative MNI-normalized individual T1 volume (Figure 4). MNI coordinates for the voxel with the maximum correlation within each cluster were transformed to standard Talairach space using an algorithm developed by Matthew Brett (http://imaging.mrc-cbu.cam.ac.uk/imaging/MniTalairach).
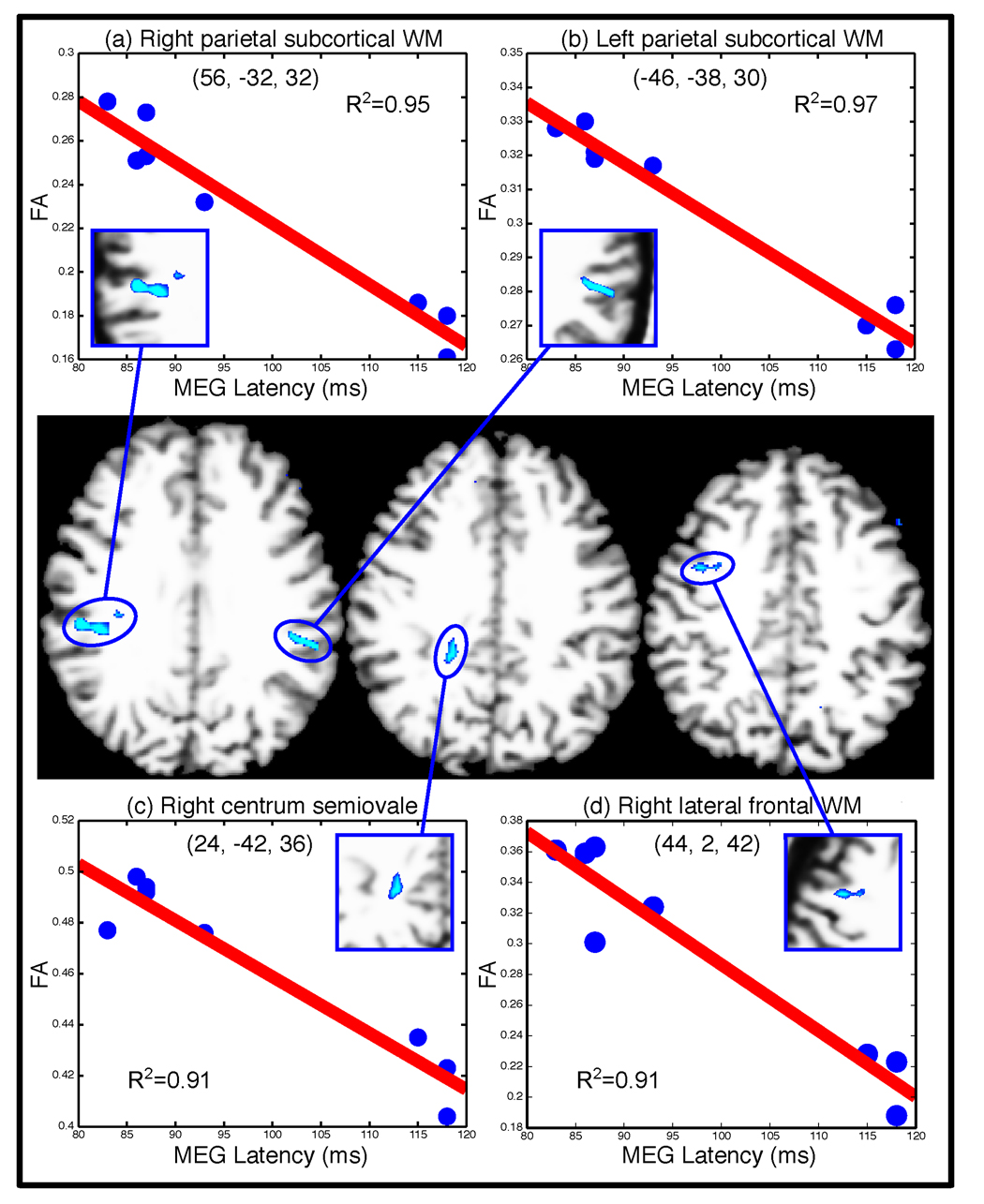
Figure 4.
Regions showing significant FA-latency correlations with scatter plots. Statistical maps are displayed at p ≤ 0.001 on horizontal slices of an MNI-normalized representative T1 volume. (a–d) Scatter plots of FA and MEG latency with regression lines and R-squared values for bilateral parietal, right lateral frontal, and right centrum semiovale maxima. Talairach coordinates for the voxel of maximum correlation are provided (x, y, z). Approximate dimensions of each region of correlation in the group map in mm are lateral parietal: right:18 × 6 × 4 mm; left: 16 × 6 × 8 mm; right centrum semiovale: 6 × 12 × 6 mm; right lateral frontal: 12 × 8 × 6 mm. WM = white matter.
Results
The latencies of the peak of the earliest MEG evoked responses to the saccadic target in occipital lobe were almost identical for prosaccade and antisaccade trials and yielded similar correlations with FA, so we present only the analyses that used the combined data. Figure 3 presents the current dipole estimates from which latency measurements were derived for each participant. These show a range of latencies for the peak response within the 70 to 130 ms time window. As displayed in Figure 4, FA was significantly related to the latency of the occipital MEG evoked response (p ≤ 0.001 uncorrected; p ≤ 0 .05 corrected for multiple comparisons) in the right centrum semi-ovale (24, −42, 36) and in white matter regions at or near the gray-white border of the lateral parietal cortex bilaterally (right: 56, −32, 32; left: −46, −38, 30) and the right lateral frontal cortex near the middle and inferior frontal gyri (44, 2, 42).
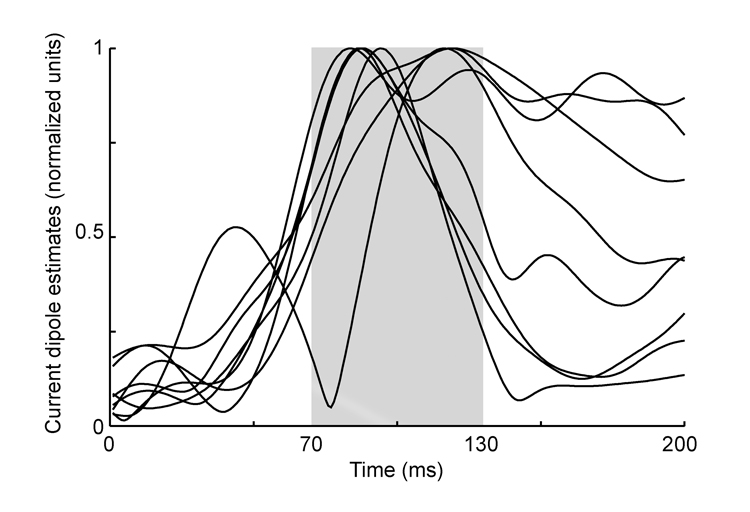
Figure 3.
Current dipole estimates as a function of time for each participant in normalized units. The latencies of peaks between 70 and 130 ms (shaded area) from the saccade end point were measured.
To determine whether the correlated regions lay in white matter for each participant, we overlaid the significant cluster on the averaged high-resolution anatomical scan for each participant. With the exception of the centrum semiovale, which lay entirely in white matter for all participants, the more superficial subcortical regions contained voxels in both gray and white matter. To rule out partial average volume effects (due to voxels that either spanned gray and white matter or fell in entirely in gray matter for only some participants) as accounting for our results, we conducted the correlations with latency using only voxels that lay entirely in white matter. To this end, we segmented each participant’s high resolution anatomical scan into gray and white matter using FreeSurfer software (Fischl et al 2002) and overlaid the regions of significant correlation from the averaged group data. Within each region, an average FA value was calculated using only the voxels that lay entirely in white matter for each participant. Using these FA values, the correlations of FA with latency of the MEG evoked response remained significant in all of the regions identified: lateral parietal cortex bilaterally (right: R2=0.91, p<0.01; left: R2=0.86, p<0.01) and right lateral frontal cortex (R2=.89, p<0.01).
Discussion
Across individuals, faster latencies of peak evoked neuromagnetic fields in occipital cortex were related to greater FA in areas thought to contain fibers from cortical regions that modulate early visual responses: posterior parietal cortex and frontal eye field (FEF). We interpret these relations to reflect that white matter fibers linking these ocular motor regions to early visual regions transmit information that modulates the visual response, and that the speed of transmission depends on white matter properties, such as myelination and axon diameter, that contribute to FA (Waxman 1980). Increased myelination would correspond to increased conduction velocity in fibers from top-down regions and thus a reduced latency of the visual evoked response. While there are prior reports of relations between FA and physiological measures such as the amplitude of steady-state visual evoked potentials (Butler et al 2005), measures of functional connectivity (Boorman et al 2007), and task-related BOLD signal (Baird et al 2005; Olesen et al 2003), this is the first report of relations between FA and the timing of evoked responses. Although it will be important to validate these findings with larger samples and well-established visual paradigms, these relations suggest that biophysical properties of white matter affect the timing of early visual responses. More importantly, this preliminary report demonstrates a non-invasive method to relate the timing information from evoked-response experiments to the biophysical properties of white matter measured with DTI using a whole brain approach.
Factors that may contribute to individual differences in white matter physiology among healthy individuals are largely unknown, but likely include gene expression (Michailov et al 2004). Individual differences in white matter physiology, particularly myelination have been proposed to contribute to variation in information processing speed (Luciano et al 2004) consistent with recent reports of correlations between FA and cognitive reaction time (Bucur et al 2007; Gold et al 2007; Madden et al 2004; Manoach et al 2007; Nestor et al 2007; Tuch et al 2005; Westerhausen et al 2006). Here we report relations between FA and the latency of evoked neural responses, which are presumably more proximal measures of neuronal function than are behavioral measures of latency. These relations may be based on the speed of axonal transmission from frontal and parietal regions that modulate early visual responses.
Classic peaks in event-related potentials such as the P1, P170 and N200 are thought to arise from sources within the occipital, parietal, and temporal lobes due to sensory and intermediate levels of processing. The neuromagnetic responses in this study originated in the occipital lobe and may represent an equivalent of the P100 or P1m component of the visual evoked response, in our case representing a response to foveating a stimulus following a saccade. Early visual responses are usually measured in response to a stimulus appearing in foveal vision during fixation. The standard deviation of the peak latency for early visual responses is approximately 15 ms, with some dependence on the experimental design (Fahle and Bach 2006). Variability in the latency of early visual responses has been attributed to small saccadic eye movements that occur during fixation (Gur et al 1997; Martinez-Conde et al 2000). Since fixational microsaccades and longer-range saccades rely on overlapping circuitry, we reasoned that longer range saccades might also give rise to variability of visual responses both within and across individuals. In the present study the latency of the evoked magnetic responses ranged from 83 to 118 ms and there was a division between participants showing short vs. long latencies (see plots in Figure 2). While a larger sample might have produced a more continuous distribution of latencies, the division in latency may also reflect that the visual responses from short and long latency participants arose from different occipital regions. The limited spatial resolution of our MEG source modeling approach does not allow us to exclude this explanation of our findings. However, if this were the case, we would not expect to see strong and specific inverse correlations between the latency of these visual responses and FA in the white matter presumed to underlie top-down ocular motor regions.
Although plausible, the explanation of top-down modulation of the timing of early visual responses rests on the presumption that the correlated areas contain fibers that originate in posterior parietal cortex and FEF and synapse in early visual areas, which is not something that we can demonstrate using these techniques. For example, we hypothesize that the right frontal region showing a significant correlation contains fibers from FEF. The putative human homologue of FEF is located in the vicinity of the precentral sulcus and gyrus (Koyama et al 2004; Paus 1996) with distinct regions in the superior and inferior portions (Luna et al 1998; Simo et al 2005). While it is possible that the region showing a correlation contains fibers from FEF, we cannot definitively pinpoint fiber origins or endpoints. The correlated region in the deep white matter of right centrum semiovale is even less regionally specific. While the centrum semiovale contains major white matter fascicles from frontal and parietal cortex, whether the region identified by the correlation analysis carries fibers from ocular motor regions is impossible to determine in the present study. While DTI-based tractography may provide suggestive evidence of fiber origins and endpoints, it requires numerous assumptions regarding crossing fibers and the location and size of seed regions. An advantage of the present technique is that it makes no such assumptions, rather it examines relations of latency to FA in the entire brain. In the present study the findings were regionally specific in that they lay in regions that could plausibly contain fibers from ocular motor regions.
Although the anatomy of white matter in humans is not well established (c.f., Schmahmann et al 2007), extensive anatomical connections between FEF, lateral intraparietal area (LIP), and occipital areas have been documented in monkeys (Andersen et al 1990; Blatt et al 1990; Cavada and Goldman-Rakic 1989). Moreover, electrical stimulation of macaque FEF neurons modulates activity in V4 neurons (Moore and Armstrong 2003). There is also evidence of top-down modulation of occipital responses in humans. Transcranial magnetic stimulation of FEF modulates both fMRI visual responses in early human retinotopic cortex (areas V1–V4) (Ruff et al 2006) and event-related potentials (ERPs) recorded from occipital electrodes (Taylor et al 2007). When applied to right posterior parietal cortex during visual search, transcranial magnetic stimulation eliminates the early phase of N2pc, an ERP generated in occipital lobe (Fuggetta et al 2006). Finally, patients with lesions of right parietal cortex show abnormal fMRI visual responses in areas V1–V4, but only under conditions of increased attentional load, suggesting a failure of top-down attentional modulation of visual responses (Vuilleumier and Driver 2007). These findings suggest that anatomical connections exist between FEF and posterior parietal cortex and early visual regions that may modulate both early and later visual responses.
It should be emphasized that some of the voxels in the correlated clusters contained gray matter. We chose not to restrict our analyses to deep subcortical white matter as it excludes white matter close to the gray-white border that emanates from specific regions, such as subcortical U-fibers. An important advantage of conducting the analysis in the whole volume is that meaningful, regionally specific correlations in these border areas can be detected. A disadvantage of sampling close to the gray-white border in a group analysis is that it results in partial average volume effects due to voxels that either span gray and white matter or that fall entirely in gray matter for only some participants given variations in cortical anatomy. The presence of gray matter in the correlated regions does not necessarily invalidate the results. Gray matter also contains myelinated fiber tracts that could contribute to the speed of neural conduction. However, since our FA values likely varied across individuals depending on how much gray matter was included, partial volume averaging represents a potential confound in our analyses. In control analyses, we were able to exclude partial volume effects as an account of our findings. Specifically, when we measured averaged FA values in individual participants based on voxels that lay entirely in white matter, the correlations with latency remained significant.
In conclusion, we observed relations between FA in parietal and frontal white matter and the latency of the MEG visual response in occipital cortex time-locked to arrival at a saccadic goal. These preliminary results suggest that the microstructural integrity of white matter connecting top-down cortical regions to early visual areas contributes to inter-individual variability of the timing of visual evoked responses. More generally, we introduce a noninvasive method that can illuminate the contribution of white matter physiology to inter-individual variability in the latency of evoked potentials in health and in neuropathologic conditions.
Acknowledgements
David S. Tuch’s current address is Novartis Pharma AG, Basel, Switzerland. Support was provided by the National Institute for Mental Health (K08 MH067966, R01 MH67720), National Center for Research Resources (P41-RR14075), Mental Illness and Neuroscience Discovery (MIND) Institute, (DOE-DE-FG02-99ER62764); National Alliance for Research in Schizophrenia and Affective Disorder; the Canada Research Chair program, and a Michael Smith Foundation for Health Research Senior Scholarship.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andersen RA, Asanuma C, Essick G, Siegel RM. Corticocortical connections of anatomically and physiologically defined subdivisions within the inferior parietal lobule. J Comp Neurol. 1990;296:65–113. doi: 10.1002/cne.902960106. [DOI] [PubMed] [Google Scholar]
- Baird AA, Colvin MK, Vanhorn JD, Inati S, Gazzaniga MS. Functional connectivity: integrating behavioral, diffusion tensor imaging, and functional magnetic resonance imaging data sets. J Cogn Neurosci. 2005;17:687–693. doi: 10.1162/0898929053467569. [DOI] [PubMed] [Google Scholar]
- Beaulieu C. The basis of anisotropic water diffusion in the nervous system - a technical review. NMR Biomed. 2002;15:435–455. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- Blatt GJ, Andersen RA, Stoner GR. Visual receptive field organization and corticocortical connections of the lateral intraparietal area (area LIP) in the macaque. J Comp Neurol. 1990;299:421–445. doi: 10.1002/cne.902990404. [DOI] [PubMed] [Google Scholar]
- Boorman ED, O'Shea J, Sebastian C, Rushworth MF, Johansen-Berg H. Individual differences in white-matter microstructure reflect variation in functional connectivity during choice. Curr Biol. 2007;17:1426–1431. doi: 10.1016/j.cub.2007.07.040. [DOI] [PubMed] [Google Scholar]
- Bucur B, Madden DJ, Spaniol J, Provenzale JM, Cabeza R, White LE, Huettel SA. Age-related slowing of memory retrieval: Contributions of perceptual speed and cerebral white matter integrity. Neurobiol Aging. 2007 doi: 10.1016/j.neurobiolaging.2007.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler PD, Zemon V, Schechter I, Saperstein AM, Hoptman MJ, Lim KO, et al. Early-stage visual processing and cortical amplification deficits in schizophrenia. Arch Gen Psychiatry. 2005;62:495–504. doi: 10.1001/archpsyc.62.5.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavada C, Goldman-Rakic PS. Posterior parietal cortex in rhesus monkey: II. Evidence for segregated corticocortical networks linking sensory and limbic areas with the frontal lobe. J Comp Neurol. 1989;287:422–445. doi: 10.1002/cne.902870403. [DOI] [PubMed] [Google Scholar]
- Collins DL, Neelin P, Peters TM, Evans AC. Automatic 3D intersubject registration of MR volumetric data in standardized Talairach space. J Comput Assist Tomogr. 1994;18:192–205. [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Dale AM, Liu AK, Fischl BR, Buckner RL, Belliveau JW, Lewine JD, Halgren E. Dynamic statistical parametric mapping: combining fMRI and MEG for high-resolution imaging of cortical activity. Neuron. 2000;26:55–67. doi: 10.1016/s0896-6273(00)81138-1. [DOI] [PubMed] [Google Scholar]
- Doricchi F, Perani D, Inoccia C, Grassi F, Cappa SF, Bettinardi V, et al. Neural control of fast-regular saccades and antisaccades: an investigation using positron emission tomography. Exp Brain Res. 1997;116:50–62. doi: 10.1007/pl00005744. [DOI] [PubMed] [Google Scholar]
- Fahle M, Bach M. Origin of the VEP Potentials. In: Heckenlively JR, Arden GB, editors. Principles and Practice of Clinical Electrophysiology of Vision. 2nd Edition. Cambridge, MA: MIT Press; 2006. pp. 207–234. [Google Scholar]
- Fischer B, Breitmeyer B. Mechanisms of visual attention revealed by saccadic eye movements. Neuropsychologia. 1987;25:73–83. doi: 10.1016/0028-3932(87)90044-3. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999;9:195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- Fuggetta G, Pavone EF, Walsh V, Kiss M, Eimer M. Cortico-cortical interactions in spatial attention: A combined ERP/TMS study. J Neurophysiol. 2006;95:3277–3280. doi: 10.1152/jn.01273.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold BT, Powell DK, Xuan L, Jiang Y, Hardy PA. Speed of lexical decision correlates with diffusion anisotropy in left parietal and frontal white matter: Evidence from diffusion tensor imaging. Neuropsychologia. 2007;45:2439–2446. doi: 10.1016/j.neuropsychologia.2007.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur M, Beylin A, Snodderly DM. Response variability of neurons in primary visual cortex (V1) of alert monkeys. J Neurosci. 1997;17:2914–2920. doi: 10.1523/JNEUROSCI.17-08-02914.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamalainen MS, Ilmoniemi R. Interpreting measured magnetic fields of the brain: estimates of current distribution. Helsinki: University of Technology, Dept. of Technical Physics Report; 1984. p. TKK-F-A559. [Google Scholar]
- Hamalainen MS, Sarvas J. Realistic conductivity geometry model of the human head for interpretation of neuromagnetic data. IEEE Trans Biomed Eng. 1989;36:165–171. doi: 10.1109/10.16463. [DOI] [PubMed] [Google Scholar]
- Harsan LA, Poulet P, Guignard B, Steibel J, Parizel N, de Sousa PL, et al. Brain dysmyelination and recovery assessment by noninvasive in vivo diffusion tensor magnetic resonance imaging. J Neurosci Res. 2006;83:392–402. doi: 10.1002/jnr.20742. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Med Image Anal. 2001;5:143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Jones DK. The effect of gradient sampling schemes on measures derived from diffusion tensor MRI: a Monte Carlo study. Magn Reson Med. 2004;51:807–815. doi: 10.1002/mrm.20033. [DOI] [PubMed] [Google Scholar]
- Koyama M, Hasegawa I, Osada T, Adachi Y, Nakahara K, Miyashita Y. Functional magnetic resonance imaging of macaque monkeys performing visually guided saccade tasks: comparison of cortical eye fields with humans. Neuron. 2004;41:795–807. doi: 10.1016/s0896-6273(04)00047-9. [DOI] [PubMed] [Google Scholar]
- Lin FH, Belliveau JW, Dale AM, Hamalainen MS. Distributed current estimates using cortical orientation constraints. Hum Brain Mapp. 2006a;27:1–13. doi: 10.1002/hbm.20155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin FH, Witzel T, Ahlfors SP, Stufflebeam SM, Belliveau JW, Hamalainen MS. Assessing and improving the spatial accuracy in MEG source localization by depth-weighted minimum-norm estimates. Neuroimage. 2006b;31:160–171. doi: 10.1016/j.neuroimage.2005.11.054. [DOI] [PubMed] [Google Scholar]
- Luciano M, Wright MJ, Geffen GM, Geffen LB, Smith GA, Martin NG. A genetic investigation of the covariation among inspection time, choice reaction time, and IQ subtest scores. Behav Genet. 2004;34:41–50. doi: 10.1023/B:BEGE.0000009475.35287.9d. [DOI] [PubMed] [Google Scholar]
- Luna B, Thulborn KR, Strojwas MH, McCurtain BJ, Berman RA, Genovese CR, Sweeney JA. Dorsal cortical regions subserving visually guided saccades in humans: an fMRI study. Cereb Cortex. 1998;8:40–47. doi: 10.1093/cercor/8.1.40. [DOI] [PubMed] [Google Scholar]
- Madden DJ, Whiting WL, Huettel SA, White LE, MacFall JR, Provenzale JM. Diffusion tensor imaging of adult age differences in cerebral white matter: relation to response time. Neuroimage. 2004;21:1174–1181. doi: 10.1016/j.neuroimage.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Manoach DS, Ketwaroo GA, Polli FE, Thakkar KN, Barton JJ, Goff DC, et al. Reduced microstructural integrity of the white matter underlying anterior cingulate cortex is associated with increased saccadic latency in schizophrenia. Neuroimage. 2007;37:599–610. doi: 10.1016/j.neuroimage.2007.04.062. [DOI] [PubMed] [Google Scholar]
- Martinez-Conde S, Macknik SL, Hubel DH. Microsaccadic eye movements and firing of single cells in the striate cortex of macaque monkeys. Nat Neurosci. 2000;3:251–258. doi: 10.1038/72961. [DOI] [PubMed] [Google Scholar]
- Michailov GV, Sereda MW, Brinkmann BG, Fischer TM, Haug B, Birchmeier C, et al. Axonal neuregulin-1 regulates myelin sheath thickness. Science. 2004;304:700–703. doi: 10.1126/science.1095862. [DOI] [PubMed] [Google Scholar]
- Moore T, Armstrong KM. Selective gating of visual signals by microstimulation of frontal cortex. Nature. 2003;421:370–373. doi: 10.1038/nature01341. [DOI] [PubMed] [Google Scholar]
- Nestor PG, Kubicki M, Spencer KM, Niznikiewicz M, McCarley RW, Shenton ME. Attentional networks and cingulum bundle in chronic schizophrenia. Schizophr Res. 2007;90:308–315. doi: 10.1016/j.schres.2006.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olesen PJ, Nagy Z, Westerberg H, Klingberg T. Combined analysis of DTI and fMRI data reveals a joint maturation of white and grey matter in a fronto-parietal network. Brain Res Cogn Brain Res. 2003;18:48–57. doi: 10.1016/j.cogbrainres.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Paus T. Location and function of the human frontal eye-field: a selective review. Neuropsychologia. 1996;34:475–483. doi: 10.1016/0028-3932(95)00134-4. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV, Hedehus M, Lim KO, Adalsteinsson E, Moseley M. Age-related decline in brain white matter anisotropy measured with spatially corrected echo-planar diffusion tensor imaging. Magn Reson Med. 2000;44:259–268. doi: 10.1002/1522-2594(200008)44:2<259::aid-mrm13>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Reese TG, Heid O, Weisskoff RM, Wedeen VJ. Reduction of eddy-current-induced distortion in diffusion MRI using a twice-refocused spin echo. Magn Reson Med. 2003;49:177–182. doi: 10.1002/mrm.10308. [DOI] [PubMed] [Google Scholar]
- Ruff CC, Blankenburg F, Bjoertomt O, Bestmann S, Freeman E, Haynes JD, et al. Concurrent TMS-fMRI and psychophysics reveal frontal influences on human retinotopic visual cortex. Curr Biol. 2006;16:1479–1488. doi: 10.1016/j.cub.2006.06.057. [DOI] [PubMed] [Google Scholar]
- Salat DH, Tuch DS, Greve DN, van der Kouwe AJ, Hevelone ND, Zaleta AK, et al. Age-related alterations in white matter microstructure measured by diffusion tensor imaging. Neurobiol Aging. 2005;26:1215–1227. doi: 10.1016/j.neurobiolaging.2004.09.017. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Pandya DN, Wang R, Dai G, D'Arceuil HE, de Crespigny AJ, Wedeen VJ. Association fibre pathways of the brain: parallel observations from diffusion spectrum imaging and autoradiography. Brain. 2007 doi: 10.1093/brain/awl359. [DOI] [PubMed] [Google Scholar]
- Simo LS, Krisky CM, Sweeney JA. Functional neuroanatomy of anticipatory behavior: dissociation between sensory-driven and memory-driven systems. Cereb Cortex. 2005;15:1982–1991. doi: 10.1093/cercor/bhi073. [DOI] [PubMed] [Google Scholar]
- Straube A, Riedel M, Eggert T, Müller N. Internally and externally guided voluntary saccades in unmedicated and medicated schizophrenic patients. Part I. Saccadic velocity. Eur Arch Psychiatry Clin Neurosci. 1999;249:1–6. doi: 10.1007/s004060050058. [DOI] [PubMed] [Google Scholar]
- Taylor PC, Nobre AC, Rushworth MF. FEF TMS affects visual cortical activity. Cereb Cortex. 2007;17:391–399. doi: 10.1093/cercor/bhj156. [DOI] [PubMed] [Google Scholar]
- Tuch DS, Salat DH, Wisco JJ, Zaleta AK, Hevelone ND, Rosas HD. Choice reaction time performance correlates with diffusion anisotropy in white matter pathways supporting visuospatial attention. Proc Natl Acad Sci U S A. 2005;102:12212–12217. doi: 10.1073/pnas.0407259102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuilleumier P, Driver J. Modulation of visual processing by attention and emotion: windows on causal interactions between human brain regions. Philos Trans R Soc Lond B Biol Sci. 2007;362:837–855. doi: 10.1098/rstb.2007.2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waxman SG. Determinants of conduction velocity in myelinated nerve fibers. Muscle Nerve. 1980;3:141–150. doi: 10.1002/mus.880030207. [DOI] [PubMed] [Google Scholar]
- Westerhausen R, Kreuder F, Woerner W, Huster RJ, Smit CM, Schweiger E, Wittling W. Interhemispheric transfer time and structural properties of the corpus callosum. Neurosci Lett. 2006;409:140–145. doi: 10.1016/j.neulet.2006.09.028. [DOI] [PubMed] [Google Scholar]