Abstract
The incidence of invasive diseases, including meningitis caused by Haemophilus influenzae type b (Hib) was markedly decreased after routine immunization of Hib vaccine through diverse schedules in many countries. The purpose of this study was to evaluate the immunogenicity and safety of Hib conjugate vaccines in Korean children before the implementation of a national immunization program against Hib in Korea. A multicenter controlled trial was performed on two different Hib vaccines in Korean children. A total of 319 infants were enrolled: 199 infants were immunized with the Hib polysaccharide conjugated to the tetanus toxoid (PRP-T) and 120 infants with the Hib polysaccharide conjugated to the outer-membrane protein of Neisseria meningitides (PRP-OMP). Immunogenicity was evaluated by enzyme-linked immunosorbent assay (ELISA) and serum bactericidal assay. Both vaccines showed good immunologic responses after primary immunization. After 2 doses of PRP-T or PRP-OMP, 78.9% and 91.7% of infants achieved an antibody level of ≥1.0 µg/mL, respectively. Both vaccines were safe and well-tolerated. No serious adverse events were observed. Thus, Hib conjugate vaccines appear to be safe and show good immunogenicity in Korean infants. These results will be important reference data for the implementation of Hib vaccine in the national immunization program of Korea.
Keywords: Haemophilus, Vaccines, Immunogenicity, Safety
INTRODUCTION
Haemophilus influenzae type b (Hib) was the leading cause of bacterial meningitis and a major cause of other serious invasive diseases among children aged <5 yr before Hib conjugate vaccines became available in 1988 (1). After routine use of Hib conjugate vaccines, dramatic reductions in the incidence of invasive diseases have been noted (2). For example, in the U.S. all infants were recommended to receive Hib conjugate vaccines starting at age 2 months; by 1996, the incidence of Hib invasive disease among children aged <5 yr declined by >99% (3).
However, the efficacy and effectiveness of a vaccine depends on a variety of factors, including the local disease burden, genetic susceptibility, age, type of vaccine, vaccination schedule, etc. In order to introduce the Hib vaccine into a country, these factors must be considered with discretion. An important factor is the extent of the local Hib disease burden. Before the introduction of Hib conjugate vaccine, the incidence of invasive Hib disease in the Apache Indians and Alaskan natives was very high as 250 and 400 to 600 cases per 100,000 children under 5 yr of age, respectively (4, 5). In Spain and Sweden, the incidence was 12 to 54 cases per 100,000 children under age 5 yr, respectively. Hib meningitis incidence rates in Asia were reported <20 per 100,000 children younger than 5 yr of age (2). Another important factor is the type of vaccine. There are four different conjugate vaccines commercially available against Hib disease; with a continuous release in various combinations with other vaccines. Differences are determined by the conjugate protein and the method to conjugate the polyribosylribitol phosphate (PRP). The first conjugate produced was the diphtheria toxoid conjugate (PRP-D), followed by mutant diphtheria toxin conjugate (PRP-CRM), meningococcal outer membrane protein conjugate (PRP-OMP) and tetanus toxoid conjugate (PRP-T). All four Hib conjugate vaccines have been shown to be highly efficacious against invasive Hib disease and safe in clinical trials; however, there are differences in the immune response to each of the vaccines. In infants, PRP-T and PRP-CRM elicit high levels of anti-PRP antibody after 3-dose primary series, whereas PRP-OMP produces a significant immune response after one injection (6). Therefore, PRP-OMP was chosen for populations that had a higher proportion of disease in the first 6 months of life. Difference in vaccine response between populations is another important factor. Even with the same Hib conjugate vaccine (PRP-D), differences in vaccine performance were observed in Alaska and Finland. The efficacy in Finnish infants under 2 yr of age was 94% whereas there was no demonstrable efficacy in the Alaskan population (7). The influence of ethnic difference on immunologic responses has also been demonstrated in other studies (8, 9).
Hib conjugate vaccines were very safe and highly effective in a wide variety of epidemiologic settings in more than 13 yr worldwide experience (2). By the end of 2005, Hib vaccines were part of the routine infant immunization program in 101 countries (10). To introduce the Hib conjugate vaccine into the national immunization program in Korea, a nationwide study on the epidemiologic status in relation to the disease burden of invasive Hib diseases as well as cost-effectiveness study is urgent. Moreover, the appropriate schedule should be determined.
The objective of this study was to evaluate the immunogenicity and safety of two Hib conjugate vaccines (PRP-T and PRP-OMP) as a primary series in Korean infants and especially evaluate the immunogenicity after 2 doses of the Hib conjugate vaccine to examine the possibility of adopting 2 doses as a primary series.
MATERIALS AND METHODS
Study design and subjects
A total of nine centers throughout the Republic of Korea were involved in the multicenter clinical study designed to evaluate the immunogenicity and safety of two Hib vaccines for primary immunization of infants. The study protocol was approved by the Institutional Review Board at each center and was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. After written informed consent forms were obtained from parents or guardians, healthy infants aged 6-12 weeks (43-91 days) and born after the gestational period of 37 weeks were enrolled in the study. Subjects were excluded if they had a history of Hib disease, immunosuppressive disorder, immunoglobulin therapy, blood transfusion, neurologic disease or acute or chronic disease at the time of enrollment. Enrollment began in March 2005 and ended in November 2005.
Vaccines
Two conjugate vaccines were tested; PRP-T (ActHIB®, Aventis Pasteur S.A., Lyon, France) and PRP-OMP (Liquid-PedHIB®, Merck & Co., Inc, Whitehouse Station, NJ, U.S.A.). Infants were immunized with the PRP-T vaccine at 2, 4, and 6 months of age or with the PRP-OMP vaccine at 2 and 4 months of age. Infants received a 0.5 mL injection intramuscularly on the anterolateral side of the thigh. Some children were administered other vaccines (i.e., diphtheriatetanus-acellular pertussis, inactivated poliovirus, hepatitis B, influenza or others) simultaneously on the contralateral leg. All vaccines were stored at 2 to 8℃ until vaccination.
Sera
Because one of the objectives of this study was to compare the differences of 2 doses and 3 doses for a primary vaccine schedule, infants vaccinated with the PRP-T vaccine were divided into two groups. The 1st group had serum samples collected before vaccination (Pre), at the time of the second dose (Post I) and 1 month after the third dose (Post III). The 2nd group had serum samples collected before vaccination (Pre), at the time of the third dose (Post II) and 1 month after the third dose (Post III). Infants vaccinated with the PRP-OMP vaccine had samples collected before vaccination (Pre), at the time of the second dose (Post I) and 1 month after the second dose (Post II). Sera were stored at -20℃ until analysis.
Immunogenicity
Anti-PRP antibodies were measured by an enzyme-linked immunosorbent assay (ELISA) modification previously described (11, 12). The ELISA was done at the Center for Vaccine Evaluation and Study, Ewha Medical Research Institute at Ewha Womans University. HbO-HA (Hib oligosaccharide conjugated to human serum albumin, provided by Moon Nahm, University of Alabama at Birmingham, Birmingham, AL, U.S.A.) was used as the antigen and the standard curve was generated by using reference serum lot 1983 (provided by Carl Frasch, Center for Biological Evaluation and Review, Food and Drug Administration, Bethesda, MD, U.S.A.) with a calculated IgG antibody concentration of 60.9 µg/mL. The cut-off of the ELISA for PRP was 0.15 µg/mL. Seroprotection was defined as the antibody concentration ≥1.0 µg/mL after vaccination. Seroprotection response rate was analyzed. Proportions of subjects achieving levels ≥0.15 µg/mL were also calculated.
A serum bactericidal assay (SBA) was done to evaluate functional antibodies that can efficiently bind to the PRP capsule of Hib and fix complement onto the bacterial surface to initiate phagocytosis. A standardized SBA assay, which measures the direct bactericidal activity of serum antibodies was done according to the Haemophilus bactericidal assay protocol. This protocol was previously described by Romero-Steiner et al. (13) and modified by Schlesinger et al. (14). The SBA was done with sera obtained after the 2nd dose of PRP-T and 1 month after the last vaccination in both groups. Two-fold serial dilutions of sera were made with dilution buffer made from Hanks buffer (Gibco Laboratories, Grand Island, NY, U.S.A.) with Ca++ and Mg++ and 2% Fildes enrichment (BBL Becton Dickinson and Company, Sparks, MD, U.S.A.). Ten µL of diluted serum was added in duplicate to wells of a microtiter plate. A frozen aliquot of Hib Eagan strain was diluted to yield 1,000 bacteria in a 20 µL volume of dilution buffer and added to each well. After incubation for 15 min at 37℃ in a 5% CO2 incubator, 25 µL of baby rabbit complement (Pel-Freez, Brown Deer, WI, U.S.A.) and 25 µL of dilution buffer were added to each well. After the plates were incubated for 60 min at 37℃ in a 5% CO2 incubator, 5 µL of the reaction mixture was plated on a chocolate II agar plate and was incubated for 15 hr at 37℃ in a 5% CO2 incubator.
The bactericidal titer of a serum sample was defined as the dilution of the serum sample that resulted in half as many colonies as were seen with controls. If an undiluted serum sample killed 50% of colonies, then the bactericidal titer was 4 in our system. A control serum sample (in house QC sera which has an assigned value of anti-PRP antibody concentration) was included in each assay to monitor assay reproducibility.
Safety
After each vaccination, infants were observed for approximately 30 min. Parents or guardians received a diary card to record the occurrence and intensity of specific solicited local reactions (tenderness, redness, and swelling) and systemic reactions (fever, irritability, drowsiness, and loss of appetite) on the day of vaccination and for three subsequent days. Injection site redness or swelling was recorded in the exact diameter. Redness and swelling measured ≥5 mm in diameter were considered clinically relevant. Fever was defined as axillary body temperature ≥37.5℃. The intensity of symptoms was graded on a scale of 0-3. Grade 3 symptoms were defined as follows: for local tenderness, spontaneously painful limb; for local redness and swelling, diameter ≥30 mm; for fever, axillary temperature ≥39.0℃; for irritability, crying that could not be comforted or preventing normal activity; for drowsiness, drowsiness that prevents normal activity; for loss of appetite, not eating at all; and for any other adverse event, preventing normal activity.
Other serious adverse events were also monitored. Adverse events were defined as a visit to a medical doctor or emergency room or any medication required for any reason. Parents or guardians were asked to record severe adverse events occurring within 30 days following each vaccine administration and to contact the investigator immediately if the infant manifested any serious signs or symptoms that need immediate medical attention.
Statistical analysis
Primary immunogenicity analyses were based on the according-to-protocol cohort, which included all subjects who met the eligibility criteria and those who complied with the procedures of the protocol. All safety analyses were descriptive and based on the modified intent-to-treat cohort, which included all vaccinated infants for whom safety data were available.
Serologic calculations were performed on logarithmically transformed data. Antibody responses were assessed by calculating the geometric mean titers (GMT) with their 95% confidence intervals (CI) and seroprotection response rates. Antibody concentrations below the cut-off value of 0.15 µg/mL were arbitrarily assigned a value corresponding to half the cut-off value of the test as 0.08 µg/mL. The results were compared with the Students t-test and chi-square test (p<0.05). A correlation between anti-PRP antibody titers by the ELISA and bacteridical titer by SBA were evaluated. If bacteria were not killed with an undiluted serum sample, then the bactericidal titer were arbitrarily assigned a value corresponding to half the cut-off value of the test as 2.
The analysis of safety was performed based on the percentage of the incidence of each local and systemic reaction over all doses. The results were compared with the chi-square test (p<0.05).
RESULTS
Subjects
A total of 319 Korean infants were enrolled in the primary vaccination study: 199 in the PRP-T group and 120 in the PRP-OMP group. Approximately half of the subjects (53.9%) were male. One hundred forty nine (74.9%) infants in the PRP-T group and 72 (60.0%) infants in the PRP-OMP group completed the study. Sixteen injections with the PRP-T and 34 injections with the PRP-OMP were eliminated from the safety analysis due to missing of essential data or safety documentation. None of these eliminated subjects reported a serious adverse event. Fifty cases (25.1%) in the PRP-T group and 48 cases (40.0%) in the PRP-OMP group were withdrawn during the study for the following reasons: move out of the district (n=2), lost to follow-up (n=40), parent request (n=27), violation of protocol (n=7), medication during the study that may have an effect on the immunogenicity analysis (n=3), and others (n=19).
Immunogenicity
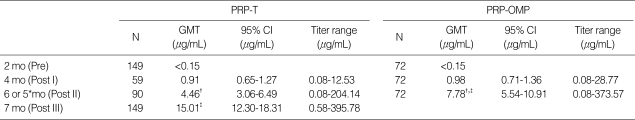
Results of the immunogenicity analysis are shown in Table 1 and 2. In both groups the GMT increased significantly after each dose. In the comparison between the two groups, the prevaccination GMT was similar in each group. Few subjects actually had measurable antibody levels. Two months after the first dose, at the age of 4 months, the GMT was higher in the PRP-OMP group but not with a significant difference. After the 2nd dose, the GMT differed significantly in the 2 groups. The GMT in the PRP-OMP group was significantly higher than the PRP-T group (7.78 vs. 4.46 µg/mL, p<0.05). However, the GMT of anti-PRP antibodies 1 month after completion of the primary vaccination course was significantly higher in the PRP-T vaccines than in the recipients of PRP-OMP (15.01 vs. 7.78 µg/mL, p<0.05).
Table 1.
Geometric mean antibody concentrations after primary vaccination
*, Those who were immunized with a PRP-OMP; †, compared PRP-T with PRP-OMP after 2 doses, p<0.05; ‡, compared PRP-T with PRP-OMP 1 month after primary series, p<0.05.
PRP-T, polyribosylribitol phosphate-tetanus toxoid conjugate; PRP-OMP, polyribosylribitol phosphate-meningococcal outer membrane protein conjugate; N, number of subjects in the according-to-protocol cohort for immunogenicity with available results; GMT, geometric mean titer; 95% CI, 95% confidence interval; mo, month.
Table 2.
Seroprotection rates after primary vaccination
*, Those who were immunized with a PRP-OMP; †, compared PRP-T with PRP-OMP after 2 doses, p<0.05; ‡, compared PRP-T with PRP-OMP 1 month after primary series, p<0.05.
PRP-T, polyribosylribitol phosphate-tetanus toxoid conjugate; PRP-OMP, polyribosylribitol phosphate-meningococcal outer membrane protein conjugate; N, number of subjects in the according-to-protocol cohort for immunogenicity with available results; 95% CI, 95% confidence interval; mo, month.
The seroprotection response rate increased significantly with each dose in both PRP-T and PRP-OMP groups. The proportion of infants achieving anti-PRP antibody concentrations ≥1.0 µg/mL after the second dose was significantly higher in infants primed with PRP-OMP than those primed with PRP-T (p<0.05) and 1 month after completion of the primary vaccination course, it was significantly higher in the PRP-T group compared with the PRP-OMP group (97.3 vs. 91.7%, p<0.05). However, in both vaccine groups, the proportion of infants with anti-PRP antibody concentrations ≥1.0 µg/mL was higher than 70% after the second dose.
Serum bactericidal activity specific for Hib was done randomly on 62 sera. Twenty sera were collected after 2 doses of PRP-T, 21 collected after 3 doses, and 21 collected after 2 doses of PRP-OMP. Samples were obtained randomly from subjects with low to high titers of anti-PRP antibodies.
In Fig. 1, comparison of anti-PRP IgG titer with SBA titers in sera after 2 doses of PRP-T vaccine (n=20 serum samples) (A), in sera after 3 doses of PRP-T vaccine (n=21 serum samples) (B) and in sera after 2 doses of PRP-OMP vaccine (n=21 serum samples) (C) are shown. The dotted line represents the detection limit of anti-PRP IgG antibody titer, 0.15 µg/mL or serum bactericidal titer, 2. After 2 and 3 doses of PRP-T vaccine, there was one sample with an antibody titer ≥0.15 µg/mL but undetectable bactericidal activity, respectively. After 2 doses of PRP-OMP vaccine, there were three samples with an antibody titer ≥0.15 µg/mL but undetectable bactericidal activity. All the other samples showed bactericidal activity. The PRP-T and PRP-OMP vaccines in both groups showed comparable correlation between the ELISA and SBA titers.
Fig. 1.
Comparison of anti-PRP IgG titer with SBA titers in sera after 2 doses of PRP-T vaccine (n=20 serum samples) (A), sera after 3 doses of PRP-T vaccine (n=21 serum samples) (B) and sera after 2 doses of PRP-OMP vaccine (n=21 serum samples) (C). The vertical and horizontal dotted lines represent the detection limit of anti-PRP IgG antibody assay (0.15 µg/mL) and serum bactericidal assay (2), respectively.
Safety
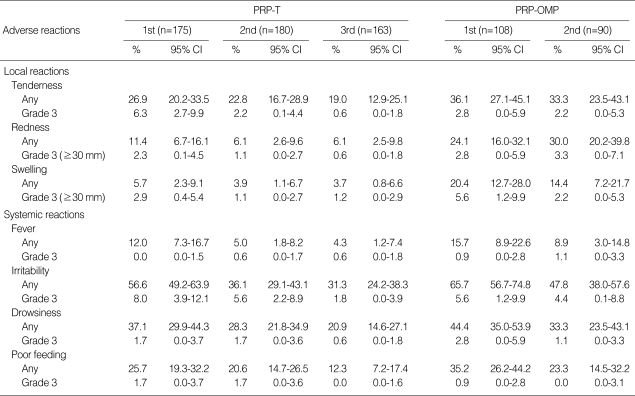
The safety evaluation was conducted on all enrolled infants. Both vaccines were safe and well tolerated (Table 3). No serious adverse reactions were reported. The incidence of grade 3 solicited symptoms was low in both groups. The incidence of solicited local reactions such as redness and swelling were higher in the infants immunized with PRP-OMP compared with the PRP-T. However, there was no significant difference in other local or systemic reactions between the two vaccines. The most frequent solicited local reaction was injection-site tenderness and redness, irritability was the most common systemic symptom. The incidence of fever of grade 3 intensity (axillary temperature ≥39.0℃) was 0.0-1.1% with both vaccines for all doses. No substantial increase in the incidence of symptoms was observed for subsequent doses in either group. In contrast, the incidence of any reports of fever, irritability, drowsiness, and poor feeding, tended to decrease with successive doses.
Table 3.
Incidence of adverse reactions reported during the 4-day follow-up period following vaccination
Grade 3 tenderness, spontaneously painful; grade 3 fever, axillary temperature ≥39℃; grade 3 irritability, crying that could not be comforted/preventing normal activity; grade 3 drowsiness, drowsiness that prevents normal activity; grade 3 poor feeding, not eating at all.
PRP-T, polyribosylribitol phosphate-tetanus toxoid conjugate; PRP-OMP, polyribosylribitol phosphate-meningococcal outer membrane protein conjugate; 95% CI, 95% confidence interval.
DISCUSSION
This study aimed to evaluate the immunogenicity and safety of two Hib conjugate vaccines, PRP-T and PRP-OMP, in Korean infants. We found that both PRP-OMP and PRP-T vaccines elicited significant antibody responses after a primary series of 2 or 3 doses, respectively, and were safe and well-tolerated without serious adverse reactions.
Studies on Hib conjugate vaccines for infants in Korea showed a good response to the vaccine (15-19). However, these previous studies were done on limited numbers of infants in limited districts. Also, most studies were done on PRP-T and there are no studies on PRP-OMP. In this study, we compared the immunogenicity and safety of two Hib conjugate vaccines, PRP-T and PRP-OMP, in a multicenter controlled clinical trial of 9 centers throughout Korea. The results of PRP-T vaccine are comparable to the studies done earlier in Korea (16-19). But there are no comparable data with this study on the immunogenicity of PRP-OMP in Korea.
The results of this study show that Korean infants had higher GMTs compared to published data from the U.S. or European infants (6, 20, 21). Correlates of protection from invasive Hib diseases are derived from studies of natural immunity, plain-polysaccharide vaccine responses and passive immunization (22). The concentration of anti-PRP antibody ≥0.15 µg/mL is commonly considered predictive of immunity and ≥1 µg/mL is considered predictive of longer-term protection from invasive disease (23). After a primary series of PRP-T at 2, 4, and 6 months of age and PRP-OMP vaccine at 2 and 4 months of age, infants with an anti-PRP antibody titer ≥0.15 µg/mL were 100% and 98.6%, respectively, and infants with titer ≥1.0 µg/mL were 97.3% and 91.7%, respectively.
Another important finding of this study is that PRP-T vaccines have resulted in comparable immunogenicity after the first 2 doses of primary series. According to the World Health Organization (WHO) recommendations, effective Hib vaccines have been found to induce ≥1.0 µg/mL anti-PRP antibody in 70% or more of the infants 1 month after the completion of the primary immunization series (24). On applying this criterion to our study, the results were acceptable after 2 doses of PRP-T as well as PRP-OMP vaccines.
Population-based surveillance studies for invasive bacterial disease in Korea show that Hib meningitis incidence rates may be lower than those in other regions. Considering the cost-benefit effectiveness with highly priced conjugate vaccines, Hib conjugate vaccines have not yet been included in the routine immunization schedule in Korea. However, regarding the worldwide data of the effectiveness and safety of Hib vaccines (2) and the serious clinical consequences of the invasive Hib diseases (1), it would be advisable to consider adopting Hib vaccine in the routine immunization schedule.
There are ways to overcome the high cost of conjugate vaccines. The number of doses could be lowered. Instead of four total doses, several European countries use only two doses for the primary series with a booster in the second year of life (25), and they have reported good results (26). Another alternative is to use smaller antigen doses. In a study of fractional dose regimens, 91% to 100% of Chilean infants immunized with one-half or one-third of a full dose of Hib conjugate developed an immune response which was comparable to those immunized with the full dose (27). Given that the PRP-T vaccine shows an acceptable rate of immunogenicity even after 2 doses and PRP-OMP shows good immunogenicity with recommended 2 doses of primary series, a modification of the existing regimen of PRP-T or use of PRP-OMP could be a possibility in the routine immunization schedule in Korea, where Hib vaccine is often limited and not included in the national immunization program because of the high cost of the vaccine.
The present study evaluates the serum bactericidal activities, a measure of functional antibody activity. There was a good correlation between anti-PRP antibody titers with the serum bactericidal titers in both vaccines. Thus, while the antibodies elicited reached levels considered protective for invasive Hib disease, they also elicited functional antibody activities. Functional antibodies were elicited after 2 doses of the PRP-OMP and PRP-T conjugate vaccines, also.
The incidence of local and systemic symptoms occurring after the primary series of immunization was similar in both vaccines. Local reactions such as tenderness and swelling were mild and transient. No increase in local reactions with subsequent injections was observed. The most common systemic reaction noted was irritability. No serious adverse events relevant to the vaccine were reported throughout the entire clinical trial.
There have not been many studies on the epidemiology of invasive Hib diseases in Korea. A population-based study was done prospectively on bacterial meningitis in Jeonbuk province from 1999 to 2001 (28). However, the Hib conjugate vaccine was already introduced into Korea on a private sector basis before the start of surveillance and the immunization rate was 15.7% for children <5 yr of age. According to the study, the Hib meningitis incidence was 6.0 (2.9-12.4) cases per 100,000 children younger than 5 yr old. This is lower than the incidence reported in the U.S. and other countries (4, 5); however, this might be due to the limitation in laboratory diagnostic aids, hospital-based epidemiologic studies, and more importantly, the fact that the consideration for Hib pneumonia may constitute a larger overall disease burden. A retrospective study based on medical records from 13 hospitals of the causative organisms of bacterial meningitis in Korean children over a 10 yr period (1986-1995) showed that among children under 16 yr of age (with the exclusion of neonates, defined as 0-28 days), H. influenzae was as common a cause as Streptococcus pneumoniae; responsible for 34.3% of the cases. Moreover, among children under 5 yr of age, H. influenzae was the most common cause of meningitis. The case fatality rate was 16.7% for H. influenzae, which was lower than that in developing countries (16-35%) (29) and higher than those in many developed countries (30). Thus, the disease burden of H. influenzae meningitis might be higher than the limited reports in Korea.
The history of Hib conjugate vaccine goes back to the late 1980s, and few vaccines in history have induced such a dramatic decline in incidence over such a short period as have the Hib conjugate vaccines (26). There are immense world-wide data on the evidence of the safety and effectiveness of the Hib conjugate vaccine (7, 31). Hib conjugate vaccines not only prevent Hib disease in individuals but also facilitate herd immunity through reduction in carriage and transmission of the organism in the community (32, 33).
Many factors are to be considered before introducing a vaccine into a country. The local epidemiology of the disease, the social cognition related to the disease and the economic status of the country. Whereas in the past, infectious diseases with high rates of mortality and complications were candidates for vaccinations, nowadays, diseases with a low rate, but with severe complications are considered. Even though the local burden of the Hib disease may be lower in Korea than in other countries, considering the effectiveness and safety of the Hib conjugate vaccine, introduction of the vaccine into the routine immunization schedule should be taken into account.
We conclude that both PRP-T and PRP-OMP conjugate vaccines are highly immunogenic and safe in Korean infants. Infants after a 2 dose of PRP-T and PRP-OMP have responses considered acceptable for effectiveness of prevention of disease. Further studies on the local Hib disease burden and cost-benefit effectiveness to determine the appropriate immunization schedule are needed.
ACKNOWLEDGMENTS
We thank Moon H. Nahm, University of Alabama at Birmingham and Carl Frasch, Center for Biological Evaluation and Review, Food and Drug Administration for providing the antigens (HboHA) and standard serum for this study.
Footnotes
This work was supported by a grant from the Korean Food and Drug Administration.
References
- 1.Wenger JD, Ward JI. Haemophilus influenzae vaccine. In: Plotkin SA, Orenstein WA, editors. Vaccines. 4th ed. Philadelphia: WB Saunders Co; 2004. pp. 229–268. [Google Scholar]
- 2.Watt JP, Levine OS, Santosham M. Global reduction of Hib disease: what are the next steps? Proceedings of the meeting Scottsdale, Arizona, September 22-25, 2002. J Pediatr. 2003;143(6 Suppl):S163–S187. doi: 10.1067/s0022-3476(03)00576-6. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention (CDC) Progress toward elimination of Haemophilus influenzae type b disease among infants and children--United States, 1987-1995. MMWR Morb Mortal Wkly Rep. 1996;45:901–906. [PubMed] [Google Scholar]
- 4.Losonsky GA, Santosham M, Sehgal VM, Zwahlen A, Moxon ER. Haemophilus influenzae disease in the White Mountain Apaches: molecular epidemiology of a high risk population. Pediatr Infect Dis. 1984;3:539–547. doi: 10.1097/00006454-198411000-00012. [DOI] [PubMed] [Google Scholar]
- 5.Ward JI, Margolis HS, Lum MK, Fraser DW, Bender TR, Anderson P. Haemophilus influenzae disease in Alaskan Eskimos: characteristics of a population with an unusual incidence of invasive disease. Lancet. 1981;1:1281–1285. doi: 10.1016/s0140-6736(81)92458-2. [DOI] [PubMed] [Google Scholar]
- 6.Decker MD, Edwards KM, Bradley R, Palmer P. Comparative trial in infants of four conjugate Haemophilus influenzae type b vaccines. J Pediatr. 1992;120:184–189. doi: 10.1016/s0022-3476(05)80424-x. [DOI] [PubMed] [Google Scholar]
- 7.Heath PT. Haemophilus influenzae type b conjugate vaccines: a review of efficacy data. Pediatr Infect Dis J. 1998;17(9 Suppl):S117–S122. doi: 10.1097/00006454-199809001-00005. [DOI] [PubMed] [Google Scholar]
- 8.Siber GR, Santosham M, Reid GR, Thompson C, Almeido-Hill J, Morell A, deLange G, Ketcham JK, Callahan EH. Impaired antibody response to Haemophilus influenzae type b polysaccharide and low IgG2 and IgG4 concentrations in Apache children. N Engl J Med. 1990;323:1387–1392. doi: 10.1056/NEJM199011153232005. [DOI] [PubMed] [Google Scholar]
- 9.Santosham M, Rivin B, Wolff M, Reid R, Newcomer W, Letson GW, Almeido-Hill J, Thompson C, Siber GR. Prevention of Haemophilus influenzae type b infections in Apache and Navajo children. J Infect Dis. 1992;165(Suppl 1):S144–S151. doi: 10.1093/infdis/165-supplement_1-s144. [DOI] [PubMed] [Google Scholar]
- 10.Rossi IA, Zuber PL, Dumolard L, Walker DG, Watt J. Introduction of Hib vaccine into national immunization programmes: a descriptive analysis of global trends. Vaccine. 2007;25:7075–7080. doi: 10.1016/j.vaccine.2007.07.058. [DOI] [PubMed] [Google Scholar]
- 11.Phipps DC, West J, Eby R, Koster M, Madore DV, Quataert SA. An ELISA employing a Haemophilus influenzae type b oligosaccharide-human serum albumin conjugate correlates with the radioantigen binding assay. J Immunol Methods. 1990;135:121–128. doi: 10.1016/0022-1759(90)90264-v. [DOI] [PubMed] [Google Scholar]
- 12.Kim KH, Lim SY. Validation of enzyme immunoassay for the quantitative measurement of human IgG antibodies specific for Haemophilus influenzae type b capsular polysaccharide. Korean J Pediatr. 2007;50:143–150. [Google Scholar]
- 13.Romero-Steiner S, Spear W, Brown N, Holder P, Hennessy T, Gomez De Leon P, Carlone GM. Measurement of serum bactericidal activity specific for Haemophilus influenzae type b by using a chromogenic and fluorescent metabolic indicator. Clin Diagn Lab Immunol. 2004;11:89–93. doi: 10.1128/CDLI.11.1.89-93.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schlesinger Y, Granoff DM. Avidity and bactericidal activity of antibody elicited by different Haemophilus influenzae type b conjugate vaccines. The Vaccine Study Group. JAMA. 1992;267:1489–1494. [PubMed] [Google Scholar]
- 15.Choi SY, Kim HT, Kim YW, Kang YJ, Chung YC, Chang JK, Lee HJ. Immunogenicity of Haemophilus influenzae PRP-D conjugate vaccine in Korean infants. J Korean Pediatr Soc. 1999;42:771–777. [Google Scholar]
- 16.Chung EH, Kim YJ, Kim YK, Kim DH, Seo JW, Lee HJ. Immunogenicity and safety of a Haemophilus influenzae type b polysaccharide-tetanus toxoid conjugate vaccine (PTP-T; Hiberix™) in Korean infants. Korean J Pediatr Infect Dis. 2003;10:71–79. [Google Scholar]
- 17.Kim JS, Cho SB, Lee HR, Park SK, Hwang PH. Immunogenicity and safety of Haemophilus influenzae type b polysaccharide-tetanus toxoid conjugate vaccine (PRP-T) in Korean infants. Korean J Infect Dis. 1996;28:225–232. [Google Scholar]
- 18.Yang PS, Seo JI, Noh KT, Yoo JH, Hwang KS, Hwang KG. Studies of the change of antibody titers after vaccination of Haemophilus influenzae PRP-T conjugate vaccine. J Korean Pediatr Soc. 2002;45:987–993. [Google Scholar]
- 19.Yoo ES, Park EA, Kim GH. Natural anti-PRP antibody levels of Haemophilus influenzae type b (Hib) and changes of antibody levels after three doses of vaccination. J Korean Pediatr Soc. 1995;38:1201–1209. [Google Scholar]
- 20.Kayhty H, Eskola J, Peltola H, Ronnberg PR, Kela E, Karanko V, Saarinen L. Antibody responses to four Haemophilus influenzae type b conjugate vaccines. Am J Dis Child. 1991;145:223–227. doi: 10.1001/archpedi.1991.02160020117030. [DOI] [PubMed] [Google Scholar]
- 21.Fritzell B, Plotkin S. Efficacy and safety of a Haemophilus influenzae type b capsular polysaccharide-tetanus protein conjugate vaccine. J Pediatr. 1992;121:355–362. doi: 10.1016/s0022-3476(05)81786-x. [DOI] [PubMed] [Google Scholar]
- 22.Kayhty H. Difficulties in establishing a serological correlate of protection after immunization with Haemophilus influenzae conjugate vaccines. Biologicals. 1994;22:397–402. doi: 10.1006/biol.1994.1062. [DOI] [PubMed] [Google Scholar]
- 23.Kayhty H, Peltola H, Karanko V, Makela PH. The protective level of serum antibodies to the capsular polysaccharide of Haemophilus influenzae type b. J Infect Dis. 1983;147:1100. doi: 10.1093/infdis/147.6.1100. [DOI] [PubMed] [Google Scholar]
- 24.World Health Organization. WHO Expert Committee on Biological Standardization. Forty-ninth report. World Health Organ Tech Rep Ser. 2000;897(i-iv):1–106. [PubMed] [Google Scholar]
- 25.Peltola H, Aavitsland P, Hansen KG, Jonsdottir KE, Nokleby H, Romanus V. Perspective: a five-country analysis of the impact of four different Haemophilus influenzae type b conjugates and vaccination strategies in Scandinavia. J Infect Dis. 1999;179:223–229. doi: 10.1086/314535. [DOI] [PubMed] [Google Scholar]
- 26.Peltola H. Worldwide Haemophilus influenzae type b disease at the beginning of the 21st century: global analysis of the disease burden 25 years after the use of the polysaccharide vaccine and a decade after the advent of conjugates. Clin Microbiol Rev. 2000;13:302–317. doi: 10.1128/cmr.13.2.302-317.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lagos R, Valenzuela MT, Levine OS, Losonsky GA, Erazo A, Wasserman SS, Levine MM. Economisation of vaccination against Haemophilus influenzae type b: a randomised trial of immunogenicity of fractional-dose and two-dose regimens. Lancet. 1998;351:1472–1476. doi: 10.1016/S0140-6736(97)07456-4. [DOI] [PubMed] [Google Scholar]
- 28.Kim JS, Jang YT, Kim JD, Park TH, Park JM, Kilgore PE, Kennedy WA, Park E, Nyambat B, Kim DR, Hwang PH, Kim SJ, Eun SH, Lee HS, Cho JH, Kim YS, Chang SJ, Huang HF, Clemens JD, Ward JI. Incidence of Haemophilus influenzae type b and other invasive diseases in South Korean children. Vaccine. 2004;22:3952–3962. doi: 10.1016/j.vaccine.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 29.Nottidge VA. Haemophilus influenzae meningitis: a 5-year study in Ibadan, Nigeria. J Infect. 1985;11:109–117. doi: 10.1016/s0163-4453(85)91931-0. [DOI] [PubMed] [Google Scholar]
- 30.Schoendorf KC, Adams WG, Kiely JL, Wenger JD. National trends in Haemophilus influenzae meningitis mortality and hospitalization among children, 1980 through 1991. Pediatrics. 1994;93:663–668. [PubMed] [Google Scholar]
- 31.Levine OS, Schwartz B, Pierce N, Kane M. Development, evaluation and implementation of Haemophilus influenzae type b vaccines for young children in developing countries: current status and priority actions. Pediatr Infect Dis J. 1998;17(9 Suppl):S95–S113. doi: 10.1097/00006454-199809001-00003. [DOI] [PubMed] [Google Scholar]
- 32.Heath PT, Bowen-Morris J, Griffiths D, Griffiths H, Crook DW, Moxon ER. Antibody persistence and Haemophilus influenzae type b carriage after infant immunisation with PRP-T. Arch Dis Child. 1997;77:488–492. doi: 10.1136/adc.77.6.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rushdy A, Ramsay M, Heath PT, Azzopardi HJ, Slack MP. Infant Hib vaccination and herd immunity. J Pediatr. 1999;134:253–254. doi: 10.1016/s0022-3476(99)70438-5. [DOI] [PubMed] [Google Scholar]