Abstract
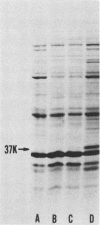
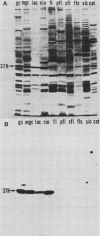
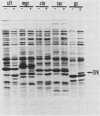
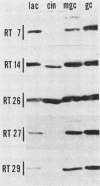
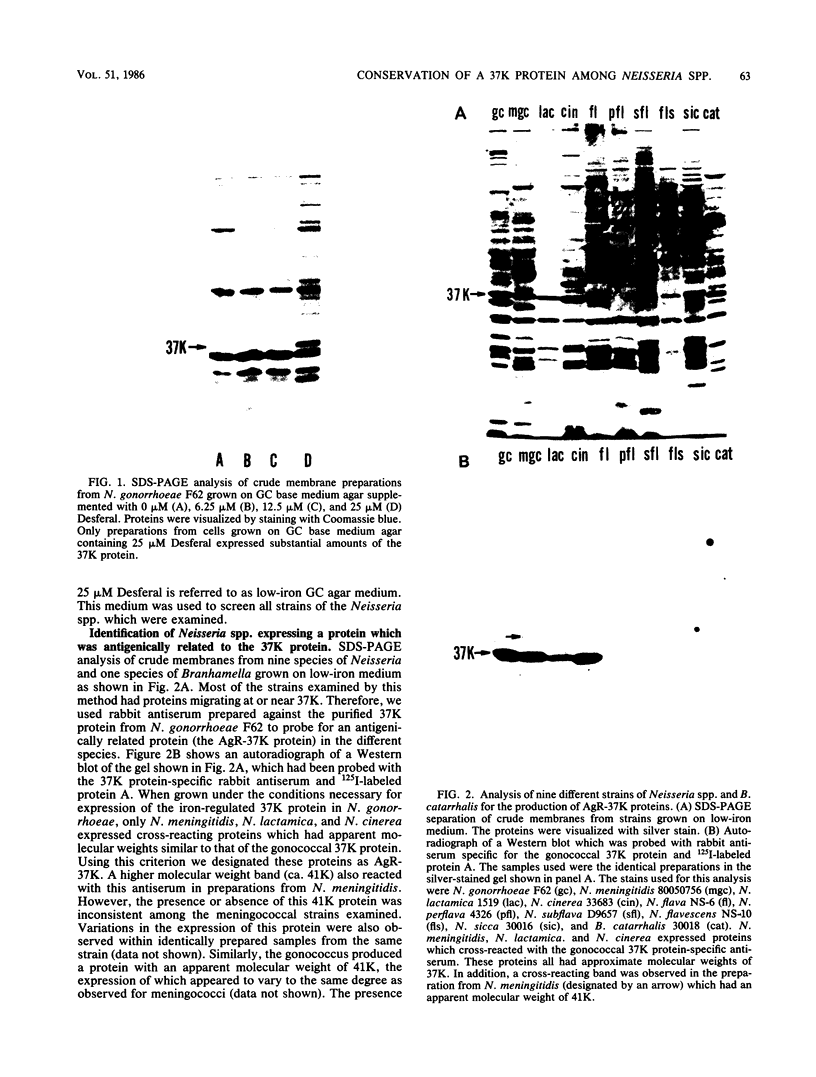
Several iron-regulated proteins of Neisseria gonorrhoeae have been reported. One of these, a 37,000-molecular-weight protein (37K protein), appears to be common to all gonococcal isolates. Recently, the occurrence of a similar protein has also been noted in N. meningitidis. The gonococcal 37K protein has been purified and used to produce both rabbit monospecific antiserum and murine monoclonal antibodies. Using these antibody reagents, we analyzed 57 strains from nine species of Neisseria and the closely related organism Branhamella catarrhalis for the presence of proteins antigenically related to the gonococcal 37K protein. Strains grown on medium with low iron content were probed for antigenic reactivity by Western blot techniques and an enzyme-linked immunosorbent assay. Proteins which cross-reacted with the rabbit monospecific antiserum were designated as AgR-37K proteins. The data indicated that the AgR-37K proteins were conserved among the 40 strains of N. gonorrhoeae, N. meningitidis, N. lactamica, and N. cinerea tested. Seventeen strains from other species of Neisseria and Branhamella did not express AgR-37K proteins with the exception of one N. subflava isolate. All AgR-37K proteins appeared to be regulated by the amount of available iron in the growth medium. Murine monoclonal antibodies were used to probe the antigenic heterogeneity of the AgR-37K proteins from different Neisseria spp. Two of seven monoclonal antibodies were broadly cross-reactive, recognizing the AgR-37K proteins from all species examined. The remaining five monoclonal antibodies were more discriminating, recognizing the AgR-37K proteins from certain species. The antigenic conservation of these AgR-37K proteins, particularly among the pathogenic members of the genus Neisseria, may imply that these proteins serve a common function in pathogenicity.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blake M. S., Gotschlich E. C. Purification and partial characterization of the opacity-associated proteins of Neisseria gonorrhoeae. J Exp Med. 1984 Feb 1;159(2):452–462. doi: 10.1084/jem.159.2.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake M. S., Johnston K. H., Russell-Jones G. J., Gotschlich E. C. A rapid, sensitive method for detection of alkaline phosphatase-conjugated anti-antibody on Western blots. Anal Biochem. 1984 Jan;136(1):175–179. doi: 10.1016/0003-2697(84)90320-8. [DOI] [PubMed] [Google Scholar]
- Buchanan T. M. Antigenic heterogeneity of gonococcal pili. J Exp Med. 1975 Jun 1;141(6):1470–1475. doi: 10.1084/jem.141.6.1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CATLIN B. W., CUNNINGHAM L. S. Transforming activities and base contents of deoxyribonucleate preparations from various Neisseriae. J Gen Microbiol. 1961 Oct;26:303–312. doi: 10.1099/00221287-26-2-303. [DOI] [PubMed] [Google Scholar]
- Cannon J. G., Black W. J., Nachamkin I., Stewart P. W. Monoclonal antibody that recognizes an outer membrane antigen common to the pathogenic Neisseria species but not to most nonpathogenic Neisseria species. Infect Immun. 1984 Mar;43(3):994–999. doi: 10.1128/iai.43.3.994-999.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosa J. H. The relationship of plasmid-mediated iron transport and bacterial virulence. Annu Rev Microbiol. 1984;38:69–89. doi: 10.1146/annurev.mi.38.100184.000441. [DOI] [PubMed] [Google Scholar]
- Feder H. M., Jr, Garibaldi R. A. The significance of nongonococcal, nonmeningococcal Neisseria isolates from blood cultures. Rev Infect Dis. 1984 Mar-Apr;6(2):181–188. doi: 10.1093/clinids/6.2.181. [DOI] [PubMed] [Google Scholar]
- Heckels J. E. The surface of Neisseria gonorrhoeae: isolation of the major components of the outer membrane. J Gen Microbiol. 1977 Apr;99(2):333–341. doi: 10.1099/00221287-99-2-333. [DOI] [PubMed] [Google Scholar]
- Herbert D. A., Ruskin J. Are the "nonpathogenic" Neisseriae pathogenic? Am J Clin Pathol. 1981 May;75(5):739–743. doi: 10.1093/ajcp/75.5.739. [DOI] [PubMed] [Google Scholar]
- Ison C., Glynn A. A., Bascomb S. Acquisition of new genes by oral Neisseria. J Clin Pathol. 1982 Oct;35(10):1153–1157. doi: 10.1136/jcp.35.10.1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judd R. C. 125I-peptide mapping of protein III isolated from four strains of Neisseria gonorrhoeae. Infect Immun. 1982 Aug;37(2):622–631. doi: 10.1128/iai.37.2.622-631.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Bieber L. L., Tolbert N. E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978 Jun 15;87(1):206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- Meyer T. F., Mlawer N., So M. Pilus expression in Neisseria gonorrhoeae involves chromosomal rearrangement. Cell. 1982 Aug;30(1):45–52. doi: 10.1016/0092-8674(82)90010-1. [DOI] [PubMed] [Google Scholar]
- Mickelsen P. A., Blackman E., Sparling P. F. Ability of Neisseria gonorrhoeae, Neisseria meningitidis, and commensal Neisseria species to obtain iron from lactoferrin. Infect Immun. 1982 Mar;35(3):915–920. doi: 10.1128/iai.35.3.915-920.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mietzner T. A., Luginbuhl G. H., Sandstrom E., Morse S. A. Identification of an iron-regulated 37,000-dalton protein in the cell envelope of Neisseria gonorrhoeae. Infect Immun. 1984 Aug;45(2):410–416. doi: 10.1128/iai.45.2.410-416.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse S. A., Mintz C. S., Sarafian S. K., Bartenstein L., Bertram M., Apicella M. A. Effect of dilution rate on lipopolysaccharide and serum resistance of Neisseria gonorrhoeae grown in continuous culture. Infect Immun. 1983 Jul;41(1):74–82. doi: 10.1128/iai.41.1.74-82.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neilands J. B. Microbial envelope proteins related to iron. Annu Rev Microbiol. 1982;36:285–309. doi: 10.1146/annurev.mi.36.100182.001441. [DOI] [PubMed] [Google Scholar]
- Newhall W. J., Wilde C. E., 3rd, Sawyer W. D., Haak R. A. High-molecular-weight antigenic protein complex in the outer membrane of Neisseria gonorrhoeae. Infect Immun. 1980 Feb;27(2):475–482. doi: 10.1128/iai.27.2.475-482.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandstrom E. G., Chen K. C., Buchanan T. M. Serology of Neisseria gonorrhoeae: coagglutination serogroups WI and WII/III correspond to different outer membrane protein I molecules. Infect Immun. 1982 Nov;38(2):462–470. doi: 10.1128/iai.38.2.462-470.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarafian S. K., Tam M. R., Morse S. A. Gonococcal protein I-specific opsonic IgG in normal human serum. J Infect Dis. 1983 Dec;148(6):1025–1032. doi: 10.1093/infdis/148.6.1025. [DOI] [PubMed] [Google Scholar]
- Schoolnik G. K., Tai J. Y., Gotschlich E. C. A pilus peptide vaccine for the prevention of gonorrhea. Prog Allergy. 1983;33:314–331. [PubMed] [Google Scholar]
- Siddiqui A., Goldberg I. D. Intrageneric transformation of neisseria gonorrhoeae and neisseria perflava to streptomycin resistance and nutritional independence. J Bacteriol. 1975 Dec;124(3):1359–1365. doi: 10.1128/jb.124.3.1359-1365.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonson C., Brener D., DeVoe I. W. Expression of a high-affinity mechanism for acquisition of transferrin iron by Neisseria meningitidis. Infect Immun. 1982 Apr;36(1):107–113. doi: 10.1128/iai.36.1.107-113.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 1;119(1):115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- West S. E., Sparling P. F. Response of Neisseria gonorrhoeae to iron limitation: alterations in expression of membrane proteins without apparent siderophore production. Infect Immun. 1985 Feb;47(2):388–394. doi: 10.1128/iai.47.2.388-394.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiseman G. M., Caird J. D. Composition of the lipopolysaccharide of Neisseria gonorrhoeae. Infect Immun. 1977 May;16(2):550–556. doi: 10.1128/iai.16.2.550-556.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yancey R. J., Finkelstein R. A. Assmilation of iron by pathogenic Neisseria spp. Infect Immun. 1981 May;32(2):592–599. doi: 10.1128/iai.32.2.592-599.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zak K., Diaz J. L., Jackson D., Heckels J. E. Antigenic variation during infection with Neisseria gonorrhoeae: detection of antibodies to surface proteins in sera of patients with gonorrhea. J Infect Dis. 1984 Feb;149(2):166–174. doi: 10.1093/infdis/149.2.166. [DOI] [PubMed] [Google Scholar]
- de StGroth S. F., Scheidegger D. Production of monoclonal antibodies: strategy and tactics. J Immunol Methods. 1980;35(1-2):1–21. doi: 10.1016/0022-1759(80)90146-5. [DOI] [PubMed] [Google Scholar]