Abstract
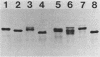
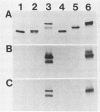
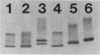
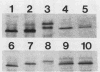
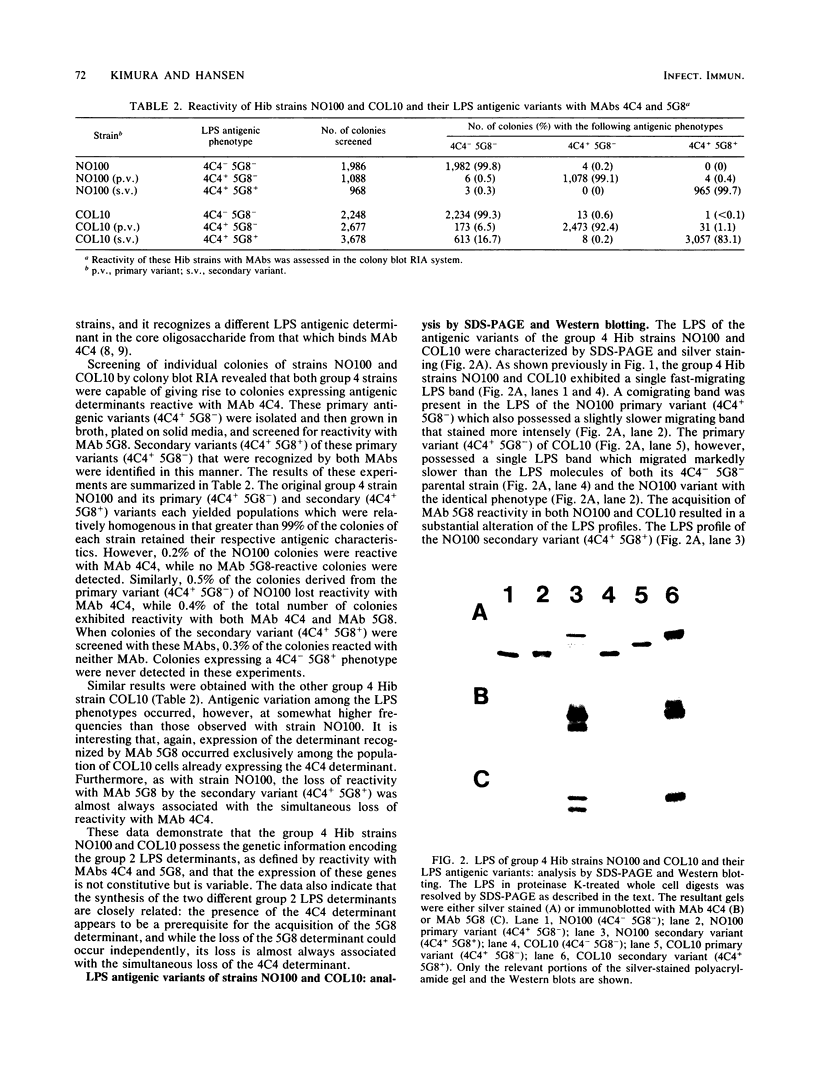
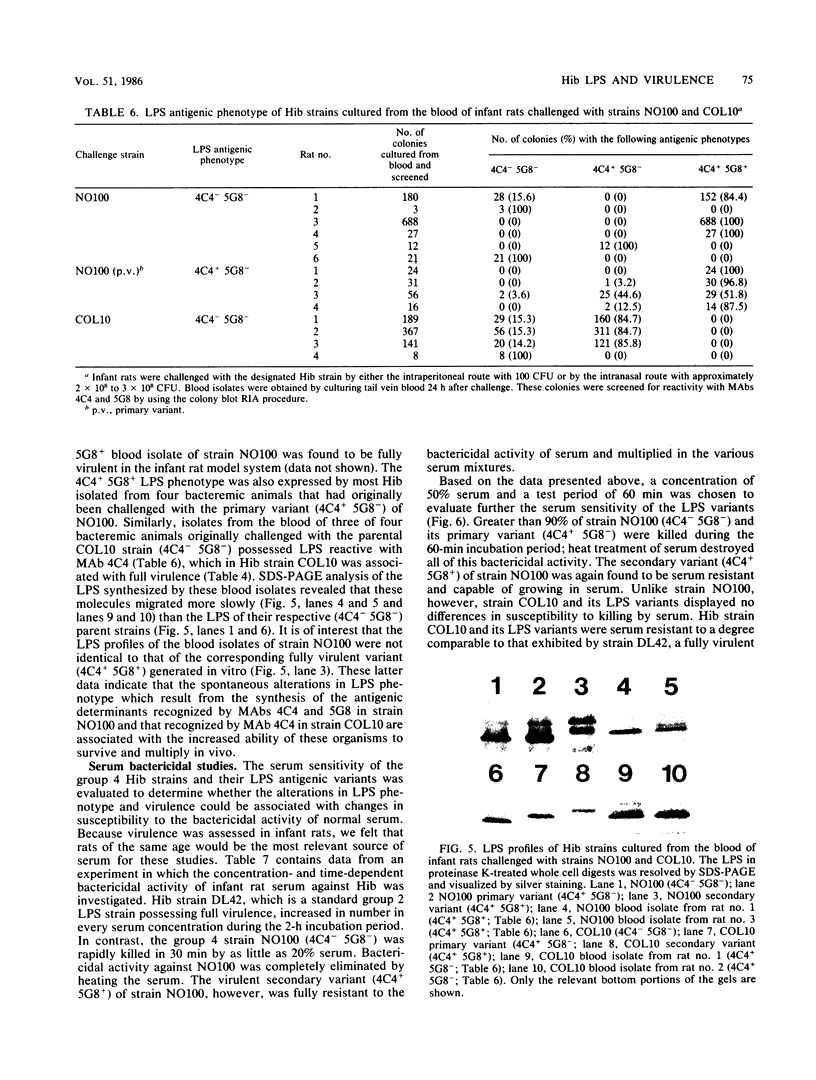
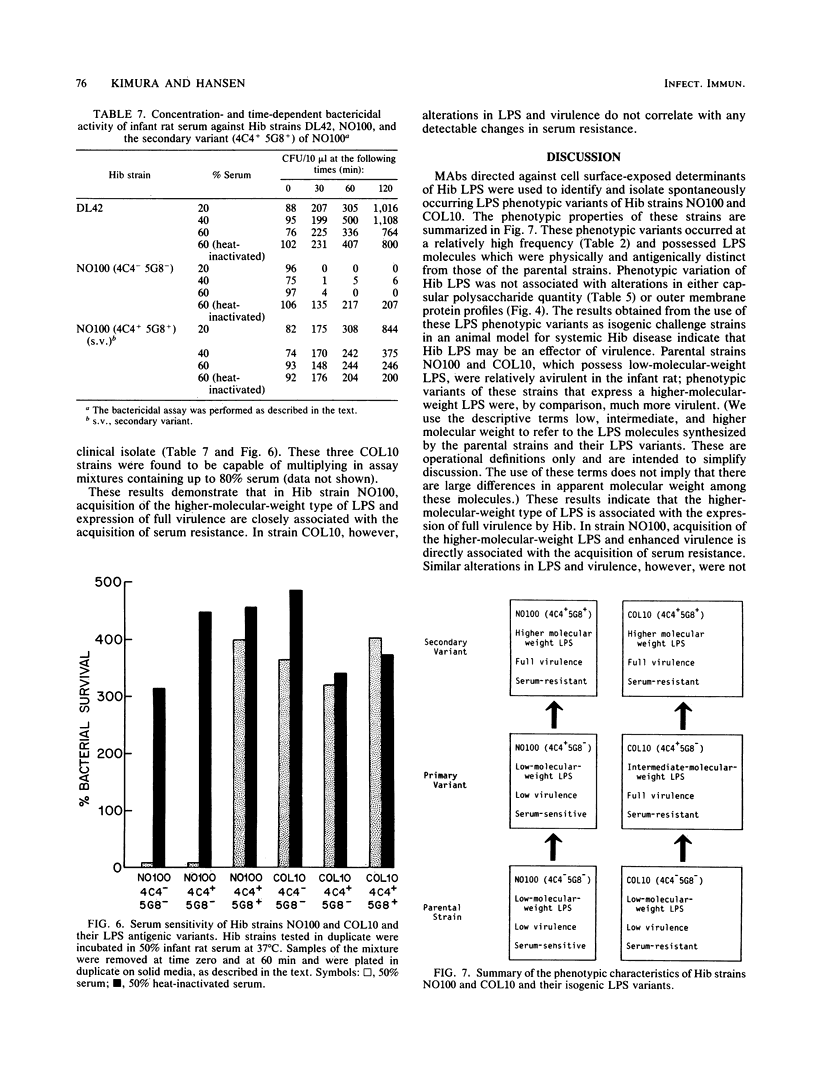
Haemophilus influenzae type b (Hib) strains NO100 and COL10 were found to produce bacteremia in infant rats at a much lower frequency than other Hib strains previously tested. These relatively avirulent strains were the only Hib strains among 200 clinical isolates examined to date which failed to react with two Hib lipopolysaccharide (LPS)-specific monoclonal antibodies (MAbs). LPS analysis by sodium dodecyl sulfate-polyacrylamide gel electrophoresis showed that strains NO100 and COL10 possessed LPS which migrated faster than the LPS of Hib strains that reacted with one of the two or with both of these MAbs. These observations suggested that the relative lack of virulence of strains NO100 and COL10 might be related to their unusual LPS phenotype. To determine whether alteration of LPS structure would affect the virulence of these strains, we identified and isolated isogenic LPS antigenic variants of strains NO100 and COL10 using the LPS-specific MAbs 4C4 and 5G8 in a colony blot radioimmunoassay. Antigenic variation of LPS was found to occur spontaneously in these two strains at a relatively high frequency in terms of both acquisition and loss of MAb reactivity (ca. 0.2 to 16.7%). LPS antigenic variants of strains NO100 and COL10 reactive with both MAbs 4C4 and 5G8 (4C4+ 5G8+) were more virulent in the infant rat model than their respective 4C4- 5G8- parental strains (P less than 0.01). An antigenic variant of COL10 reactive with only MAb 4C4 (4C4+ 5G8-) was also significantly more virulent than its 4C4- 5G8- parent. These LPS antigenic variants with increased virulence synthesized altered LPS molecules which possessed apparent molecular weights higher than those of the LPS of the parental strains. Increased resistance of strain NO100 to the bactericidal activity of normal infant rat serum was associated with changes in LPS structure, while strain COL10 and its LPS variants were all uniformly resistant to serum bactericidal activity. Our results demonstrate that (i) spontaneous antigenic and phenotypic variation of LPS occurs at a relatively high frequency in some strains of Hib; (ii) the higher-molecular-weight type of LPS is associated with the full expression of Hib virulence; (iii) LPS phenotype may not correlate with Hib serum resistance; and (iv) serum resistance of Hib is not an accurate indicator of virulence.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames G. F. Resolution of bacterial proteins by polyacrylamide gel electrophoresis on slabs. Membrane, soluble, and periplasmic fractions. J Biol Chem. 1974 Jan 25;249(2):634–644. [PubMed] [Google Scholar]
- Anderson P., Flesher A., Shaw S., Harding A. L., Smith D. H. Phenotypic and genetic variation in the susceptibility of Haemophilus influenzae type b to antibodies to somatic antigens. J Clin Invest. 1980 Apr;65(4):885–891. doi: 10.1172/JCI109741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrall C. J., Winkelstein J. A., Moxon E. R. Participation of complement in host defense against encapsulated Haemophilus influenzae types a, c, and d. Infect Immun. 1982 Mar;35(3):759–763. doi: 10.1128/iai.35.3.759-763.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryz S. J., Jr, Pitt T. L., Fürer E., Germanier R. Role of lipopolysaccharide in virulence of Pseudomonas aeruginosa. Infect Immun. 1984 May;44(2):508–513. doi: 10.1128/iai.44.2.508-513.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flesher A. R., Insel R. A. Characterization of lipopolysaccharide of Haemophilus influenzae. J Infect Dis. 1978 Dec;138(6):719–730. doi: 10.1093/infdis/138.6.719. [DOI] [PubMed] [Google Scholar]
- Goldman R. C., Joiner K., Leive L. Serum-resistant mutants of Escherichia coli O111 contain increased lipopolysaccharide, lack an O antigen-containing capsule, and cover more of their lipid A core with O antigen. J Bacteriol. 1984 Sep;159(3):877–882. doi: 10.1128/jb.159.3.877-882.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulig P. A., Frisch C. F., Hansen E. J. A set of two monoclonal antibodies specific for the cell surface-exposed 39K major outer membrane protein of Haemophilus influenzae type b defines all strains of this pathogen. Infect Immun. 1983 Nov;42(2):516–524. doi: 10.1128/iai.42.2.516-524.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulig P. A., Hansen E. J. Coprecipitation of lipopolysaccharide and the 39,000-molecular-weight major outer membrane protein of Haemophilus influenzae type b by lipopolysaccharide-directed monoclonal antibody. Infect Immun. 1985 Sep;49(3):819–827. doi: 10.1128/iai.49.3.819-827.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulig P. A., McCracken G. H., Jr, Frisch C. F., Johnston K. H., Hansen E. J. Antibody response of infants to cell surface-exposed outer membrane proteins of Haemophilus influenzae type b after systemic Haemophilus disease. Infect Immun. 1982 Jul;37(1):82–88. doi: 10.1128/iai.37.1.82-88.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guymon L. F., Esser M., Shafer W. M. Pyocin-resistant lipopolysaccharide mutans of Neisseria gonorrhoeae: alterations in sensitivity to normal human serum and polymyxin B. Infect Immun. 1982 May;36(2):541–547. doi: 10.1128/iai.36.2.541-547.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen E. J., Frisch C. F., McDade R. L., Jr, Johnston K. H. Identification of immunogenic outer membrane proteins of Haemophilus influenzae type b in the infant rat model system. Infect Immun. 1981 Jun;32(3):1084–1092. doi: 10.1128/iai.32.3.1084-1092.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitchcock P. J., Brown T. M. Morphological heterogeneity among Salmonella lipopolysaccharide chemotypes in silver-stained polyacrylamide gels. J Bacteriol. 1983 Apr;154(1):269–277. doi: 10.1128/jb.154.1.269-277.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inzana T. J., Anderson P. Serum factor-dependent resistance of Haemophilus influenzae type b to antibody to lipopolysaccharide. J Infect Dis. 1985 May;151(5):869–877. doi: 10.1093/infdis/151.5.869. [DOI] [PubMed] [Google Scholar]
- Inzana T. J. Electrophoretic heterogeneity and interstrain variation of the lipopolysaccharide of Haemophilus influenzae. J Infect Dis. 1983 Sep;148(3):492–499. doi: 10.1093/infdis/148.3.492. [DOI] [PubMed] [Google Scholar]
- Inzana T. J., Pichichero M. E. Lipopolysaccharide subtypes of Haemophilus influenzae type b from an outbreak of invasive disease. J Clin Microbiol. 1984 Aug;20(2):145–150. doi: 10.1128/jcm.20.2.145-150.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inzana T. J., Seifert W. E., Jr, Williams R. P. Composition and antigenic activity of the oligosaccharide moiety of Haemophilus influenzae type b lipooligosaccharide. Infect Immun. 1985 May;48(2):324–330. doi: 10.1128/iai.48.2.324-330.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura A., Gulig P. A., McCracken G. H., Jr, Loftus T. A., Hansen E. J. A minor high-molecular-weight outer membrane protein of Haemophilus influenzae type b is a protective antigen. Infect Immun. 1985 Jan;47(1):253–259. doi: 10.1128/iai.47.1.253-259.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDade R. L., Jr, Johnston K. H. Characterization of serologically dominant outer membrane proteins of Neisseria gonorrhoeae. J Bacteriol. 1980 Mar;141(3):1183–1191. doi: 10.1128/jb.141.3.1183-1191.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medearis D. N., Jr, Camitta B. M., Heath E. C. Cell wall composition and virulence in Escherichia coli. J Exp Med. 1968 Sep 1;128(3):399–414. doi: 10.1084/jem.128.3.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer T. F., Mlawer N., So M. Pilus expression in Neisseria gonorrhoeae involves chromosomal rearrangement. Cell. 1982 Aug;30(1):45–52. doi: 10.1016/0092-8674(82)90010-1. [DOI] [PubMed] [Google Scholar]
- Morse S. A., Apicella M. A. Isolation of a lipopolysaccharide mutant of Neisseria gonorrhoeae: an analysis of the antigenic and biologic difference. J Infect Dis. 1982 Feb;145(2):206–216. doi: 10.1093/infdis/145.2.206. [DOI] [PubMed] [Google Scholar]
- Moxon E. R., Glode M. P., Sutton A., Robbins J. B. The infant rat as a model of bacterial meningitis. J Infect Dis. 1977 Aug;136 (Suppl):S186–S190. doi: 10.1093/infdis/136.supplement.s186. [DOI] [PubMed] [Google Scholar]
- Moxon E. R., Smith A. L., Averill D. R., Smith D. H. Haemophilus influenzae meningitis in infant rats after intranasal inoculation. J Infect Dis. 1974 Feb;129(2):154–162. doi: 10.1093/infdis/129.2.154. [DOI] [PubMed] [Google Scholar]
- Moxon E. R., Vaughn K. A. The type b capsular polysaccharide as a virulence determinant of Haemophilus influenzae: studies using clinical isolates and laboratory transformants. J Infect Dis. 1981 Apr;143(4):517–524. doi: 10.1093/infdis/143.4.517. [DOI] [PubMed] [Google Scholar]
- Munford R. S., Hall C. L., Rick P. D. Size heterogeneity of Salmonella typhimurium lipopolysaccharides in outer membranes and culture supernatant membrane fragments. J Bacteriol. 1980 Nov;144(2):630–640. doi: 10.1128/jb.144.2.630-640.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano M., Saito K. Chemical components in the cell wall of Salmonella typhimurium affecting its virulence and immunogenicity in mice. Nature. 1969 Jun 14;222(5198):1085–1086. doi: 10.1038/2221085a0. [DOI] [PubMed] [Google Scholar]
- Palva E. T., Mäkelä P. H. Lipopolysaccharide heterogeneity in Salmonella typhimurium analyzed by sodium dodecyl sulfate polyacrylamide gel electrophoresis. Eur J Biochem. 1980;107(1):137–143. doi: 10.1111/j.1432-1033.1980.tb04634.x. [DOI] [PubMed] [Google Scholar]
- Peppler M. S. Two physically and serologically distinct lipopolysaccharide profiles in strains of Bordetella pertussis and their phenotype variants. Infect Immun. 1984 Jan;43(1):224–232. doi: 10.1128/iai.43.1.224-232.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts M., Stull T. L., Smith A. L. Comparative virulence of Haemophilus influenzae with a type b or type d capsule. Infect Immun. 1981 May;32(2):518–524. doi: 10.1128/iai.32.2.518-524.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider H., Hale T. L., Zollinger W. D., Seid R. C., Jr, Hammack C. A., Griffiss J. M. Heterogeneity of molecular size and antigenic expression within lipooligosaccharides of individual strains of Neisseria gonorrhoeae and Neisseria meningitidis. Infect Immun. 1984 Sep;45(3):544–549. doi: 10.1128/iai.45.3.544-549.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A. L., Smith D. H., Averill D. R., Jr, Marino J., Moxon E. R. Production of Haemophilus influenzae b meningitis in infant rats by intraperitoneal inoculation. Infect Immun. 1973 Aug;8(2):278–290. doi: 10.1128/iai.8.2.278-290.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton A., Schneerson R., Kendall-Morris S., Robbins J. B. Differential complement resistance mediates virulence of Haemophilus influenzae type b. Infect Immun. 1982 Jan;35(1):95–104. doi: 10.1128/iai.35.1.95-104.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C. M., Boykins R., Frasch C. E. Heterogeneity and variation among Neisseria meningitidis lipopolysaccharides. J Bacteriol. 1983 Aug;155(2):498–504. doi: 10.1128/jb.155.2.498-504.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 1;119(1):115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- Zieg J., Silverman M., Hilmen M., Simon M. Recombinational switch for gene expression. Science. 1977 Apr 8;196(4286):170–172. doi: 10.1126/science.322276. [DOI] [PubMed] [Google Scholar]
- Zwahlen A., Winkelstein J. A., Moxon E. R. Surface determinants of Haemophilus influenzae pathogenicity: comparative virulence of capsular transformants in normal and complement-depleted rats. J Infect Dis. 1983 Sep;148(3):385–394. doi: 10.1093/infdis/148.3.385. [DOI] [PubMed] [Google Scholar]
- van Alphen L., Riemens T., Poolman J., Hopman C., Zanen H. C. Homogeneity of cell envelope protein subtypes, lipopolysaccharide serotypes, and biotypes among Haemophilus influenzae type b from patients with meningitis in The Netherlands. J Infect Dis. 1983 Jul;148(1):75–81. doi: 10.1093/infdis/148.1.75. [DOI] [PubMed] [Google Scholar]