Abstract
Recent studies showed that Fyn is a mediator of the LHR-induced activation of the ERK1/2 cascade in MA-10 cells. Since the LHR is a G protein-coupled receptor and the Src family of kinases can be activated by some Gα subunits and by the non-visual arrestins we investigated the role of these signaling molecules in the LHR-provoked activation of Fyn.
Small interfering RNAs (siRNAs) that target two Gα subunits that participate in LHR signaling (Gαs and Gα11) and one that targets arrestin-3 were co-transfected with the hLHR in MA-10 cells. We then determined the effects of these siRNAs on the LHR-provoked activation of Fyn, the phosphorylation of FAK (a prominent Fyn substrate) and the release of EGF-like growth factors (a Fyn-mediated process).
Expression of the siRNA against Gαs decreased the level of Gαs and LHR-stimulated cAMP production by ~50% but did not affect LHR-stimulated Fyn activation or FAK phosphorylation. Likewise, expression of the siRNA against Gα11 decreased the level of Gα11 and LHR-stimulated inositol phosphate production by ~50% but did not affect LHR-stimulated Fyn activation or FAK phosphorylation. Expression of the siRNA against arrestin-3 decreased the level of arrestin-3 and the rate of internalization of hCG by ~50% and it also inhibited the LHR-provoked stimulation of Fyn, the phosphorylation of FAK and the release of EGF-like growth factors.
These results show that, in MA-10 cells, the hLHR activates Fyn through and arrestin-3-dependent pathway and that this pathway is a mediator of the hLHR-provoked release of EGF-like growth factors.
Keywords: gonadotropin receptors, G protein-coupled receptors, Src family kinases, Fyn, arrestins, G proteins
1-INTRODUCTION
Luteinizing hormone (LH) and choriogonadotropin (CG) are homologous glycoprotein hormones that bind to a common G-protein coupled receptor, the LH receptor (LHR). Although the activation of the Gs/cAMP/PKA pathway is still recognized as the most important mediator of the actions of LH/CG it is now well established that the LHR activates other G protein families, interacts with the non-visual arrestins [reviewed in refs. 1, 2] and activates signaling cascades involving tyrosine phosphorylation [3–6]. These less-traditional pathways are important because they appear to co-operate with the classical LHR-dependent signaling pathways in the activation of the ERK1/2 cascade in Leydig [6–10] and granulosa cells [11–16].
There are at least two ways by which the gonadotropin receptors can activate tyrosine kinase cascades. One is by increasing the synthesis and/or release of EGF-like growth factors that subsequently activate the EGFR [9, 14, 17–19] and the second is by direct activation of the Src family of kinases (SFKs) [5, 20].
In a previous report, we showed that the LHR actives Fyn (a member of the SFKs) and that Fyn activation is an important component of the pathway leading to the phosphorylation of ERK1/2 in MA-10 cells. Based on studies done with other G protein-coupled receptors [reviewed in refs. 21–23] we considered four different mechanisms by which the LHR could activate Fyn: (a) a direct interaction of the LHR and Fyn [24, 25]; (b) direct activation of Fyn by the Gα subunits liberated in response to LHR activation [26, 27]; (c) indirect activation by second messenger-dependent kinases [28]; and (d) formation of a ternary complex containing the LHR, a non-visual arrestin and Fyn [22]. In the experiments described herein we used siRNA-mediated approaches to test the role of G proteins and non-visual arrestins on the LHR-provoked activation of Fyn and on Fyn-dependent pathways in MA-10 cells.
2-Materials and Methods
2-1 Plasmid and cells
The expression vector coding for the hLHR-wt has been described [29]. The expression vector for a dominant-negative mutant of human Fyn (i.e., kinase-deficient mutant, K229M) was generously provided by Dr. Marylin Resh of the Memorial Sloan Kettering Cancer Center [30]. Expression vectors for C-terminally tagged forms of the human EGFR (EGFR-GFP) and ERK1 (ERK1-GFP) were donated by Dr. John Koland of this institution. Expression vectors for arrestin-2 and arrestin-3 were donated by Dr. Jeff Benovic of Thomas Jefferson University [31].
The siRNA duplexes targeting mouse Gαs, Gα11 and arrestin-3 were designed using the BLOCK-IT™ RNAi Designer website from Invitrogen (https://rnaidesigner.invitrogen.com/sirna). The sequence of the Gαs siRNA (5’- GCAGAUGAGGAUCCUGCAU-3’) targets the 174 to 194 region of mouse Gαs mRNA. The sequence of the Gα11 siRNA (5’-GGAAGUGGAUCCAUUGCUU-3’) targets the 641 to 661 region of mouse Gα11 mRNA. The sequence of the arrestin-3 siRNA (5’-ACGUCCAUGUCACCAACAA-3’) targets the 656 to 676 region of mouse arrestin-3 mRNA. A siRNA corresponding to the reverse sequence of the Gαs siRNA (5’-UACGUCCUAGGAGUAGACG-3’) was used as a control in all experiments. These synthetic oligonucleotide duplexes were purchased from Invitrogen.
The origin and handling of MA-10 cells were as described earlier [32] with recent modifications [29]. I-10 cells (CCL-83), a clonal strain of Leydig tumor cells that lack LH receptors [9, 33] were purchased from the American Type Culture Collection and maintained using the same growth medium used for MA-10 cells (DMEM-F12 medium supplemented with 15% horse serum, 20 mM Hepes and 50 µg/ml gentamicin, pH 7.4).
2-2 Determination of siRNA silencing efficiency
MA-10 cells were plated on 6 wells plates at a density of 4×105 cells and transfected one day after plating. Transfections were done using 1 µg of mychLHR-wt and 250 pmol of the siRNA of interest using Lipofectamine® as previously described [9, 29]. On the day of the experiment the cells were washed twice with assay medium (DMEM/F12 medium supplemented with 1 mg/ml bovine serum albumin, 20mM HEPES and 50 µg/ml gentamycin, pH 7.4) and incubated in 1 ml of fresh warm assay medium with or without hormones as indicated in the figure legends. For Western blots analysis the medium was aspirated and the cells were lysed with 100 µl of RIPA buffer (150mM NaCl, 50mM Tris, 1mM EDTA, 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS, pH7.4) supplemented with an EDTA-free protease inhibitor cocktail (Roche Applied Bioscience) and clarified by centrifugation. Equal amounts of protein were separated on SDS gels and electrophoretically transferred to PVDF membranes for Western blotting. The membranes were incubated with a 1:1,000 dilution of a rabbit anti-Gαs antibody (Upstate Biotechnology catalog # 06–237) in 1% bovine serum albumin for 1h at room temperature; a rabbit anti-Gµq/11 antibody (Santa Cruz Biotechnology, catalog # sc-392) at a 1:1000 dilution in 1% milk for 1h at room temperature; a rabbit anti-Gαi3/o (Calbiochem, catalog # 371726) at a 1:2000 dilution in 1% bovine serum albumin for 1h at room temperature; or a rabbit anti-arrestin antibody (a gift from Jeffrey Benovic) at a 1:1000 dilution in 1% bovine serum albumin overnight at room temperature. All primary antibody incubations were followed by a constant 1h incubation at room temperature with a 1:3,000 dilution of a secondary antibody covalently coupled to horseradish peroxidase (Bio-Rad laboratories Inc. catalog # 170-6515). All immune complexes in the Western blots were visualized using the Super Signal West Femto Maximum Sensitivity detection system (Pierce Chemical) and exposed to film or captured digitally with a Kodak Digital Imaging system (Eastman Kodak). The quantitative analysis presented was done with the Digital Imaging system. Quantitation of the digital images captured using this system is more accurate because of its wider dynamic range that makes signal saturation less likely. In addition, the software included with this imaging system can readily determine if an image is saturated thus preventing its quantitation.
Functional levels of Gs and Gq/11 were also determined by measuring cAMP and inositol phosphate accumulation, respectively, in cells incubated with or without hCG [29]. Functional levels of non-visual arrestins were measured using the internalization of 125I-hCG [34].
2-3 Fyn kinase assay
Fyn kinase assays were performed as previously described [5] with some minor modifications as follows. The Fyn immunoprecipitates were incubated in a solution composed of 40 µl of a kinase assay buffer (50mM Tris-HCl pH 7.2, 5mM MgCl2, 5mM MnCl2, 10 µM ATP, 100 µM Na3VO4), 10 µl of a 1 mCi/ml solution of [32P-γ] ATP (Perkin Elmer) and either 10 µl of water or 10 µl of a 600 µM solution of Src substrate (Upstate Biotechnology). After 30 min at 30C, the tubes were centrifuged and duplicate aliquots (20µl) of each reaction were spotted on P81 phosphocellulose paper. These were washed five times with 0.75% phosphoric acid, dried for 20min at room temperature and counted on a liquid scintillation counter. The radioactivity found in the samples incubated without the Src substrate was subtracted from those containing the substrate.
2-4 Bioassay for EGF-like growth factors
The co-culture assay used for detection of EGF-like growth factors has been previously described [9, 19]. Transfections of MA-10 or I-10 cells were done with Lipofectamine® as described elsewhere [9, 29]. Routine transfections of MA-10 cells included the following amounts of plasmids per 100 mm dish: 5 µg of the myc-hLHR and 250 pmol of control or arrestin 3 siRNA. Alternatively 5 µg of the hLHR were co-transfected with 2.5 µg of DN-Fyn plasmid and 2.5 µg of empty vector. I-10 cells (the test cells) were always transfected with a total of 10 µg DNA/100 mm dish consisting of 3.75 µg of ERK1-GFP, 3.75 µg of EGFR-GFP and 2.5 µg of empty vector. The individually transfected MA-10 and I-10 cells were trypsinized one day after transfection and plated simultaneously in 35 mm wells at a density of 1.5 × 106 and 0.5 × 106 cells, respectively. These co-cultures were maintained for 8 hours in growth medium and then for 16–18 hours in assay medium. The medium was replaced with 1 ml of fresh assay medium and the cells were incubated in the absence or presence of hCG at a final concentration of 100 ng/ml for 1h at 37C. Lysates were prepared in 100 µl of RIPA buffer supplemented with an EDTA free protease inhibitor cocktail (Roche Applied Bioscience) 1 mM sodium orthovanadate and 1 mM sodium fluoride.
The lysates were clarified by centrifugation and equal amounts of protein (10–20 µg) from each lysates were resolved on SDS-polyacrylamide gels and transferred electrophoretically to PVDF membranes. The phosphorylation of ERK1-GFP was detected using a 1:5,000 dilution of a phosphoERK1/2 antibody (Santa Cruz Biotechnology, catalog # 7383) in 1% bovine serum albumin overnight at room temperature. After visualization of the phosphorylated proteins with secondary antibodies (see above) the blots were stripped [9, 19] and total ERK1-GFP was detected by incubation of the membranes with a 1:1,000 dilution of a rabbit anti-GFP antibody coupled to horseradish peroxidase (Abcam, catalog # ab6663-1000) in 10% milk overnight at room temperature. This allowed for correction of the phospho-ERK1 signals for the total amount of ERK1 protein present.
2-5 Other methods
Individual cultures of MA-10 cells were also used in some experiments to measure the phosphorylation of endogenous ERK1/2 or FAK. The phosphorylation of endogenous ERK1/2 was measured as described above for the co-cultures, but endogenous ERK1/2 expression was determined using a 1:2,000 dilution of rabbit polyclonal anti-ERK2 (Upstated Biotechnology, catalog # 06–182) in 10% milk overnight at room temperature. PhosphoFAK was detected using a 1:1,000 dilution of a rabbit polyclonal antibody specific for phosphotyrosine 576 (Biosource, catalog # 44–652G or Abcam, catalog # ab4515) in 1% bovine serum albumin overnight at room temperature. Secondary antibody incubations were done as described above.
2-6 Hormones and Supplies
Recombinant hCG was provided by Ares Serono (Randolph, MA). Cell culture media were obtained from Invitrogen. Other cells culture supplies and reagents were obtained from Corning. All other chemical were obtained from commonly used suppliers.
2-7 Statistical analysis
All statistical analyses were conducted using paired t tests or ANOVA followed by a Tukey post-hoc test. In all cases, statistical significance was considered at P < 0.05.
3- RESULTS
3-1 The hLHR does not activate Fyn through a G protein-mediated pathway
We first tested the hypothesis that the LHR-provoked activation of Fyn is G protein-mediated. In MA-10 cells the hLHR activates the Gs, Gi/o and Gq/11 families of G proteins [35] but we concentrated only on Gs, and Gq/11 because the involvement of Gαi or Gαo has already been ruled out [5].
A siRNA to Gαs effectively reduced the expression of Gαs without affecting the expression of Gαq/11 or Gαi3/o (Figure 1A). The siRNA also induced a similar decrease in the functional levels of Gαs as judged by a decrease in the hCG-stimulated cAMP accumulation (Figure 1B). To test for the effect of Gαs reduction on Fyn activation we measured kinase activity in Fyn immunoprecipitates of cell lysates (Figure 1C) or by assessing the phosphorylation of FAK (a Fyn substrate) in intact cells (Figure 1D). Neither was affected by reducing the levels of Gαs.
Figure 1. Gαs siRNA decreases the expression of Gαs and hCG-stimulated cAMP accumulation but does not affect hCG-stimulated Fyn kinase activity or FAK phosphorylation.
MA-10 cells were co-transfected with the hLHR and a control siRNA or the Gαs siRNA as described in Material and Methods.
Panel A. The relevant portion of a representative Western blot of lysates probed with anti-Gαs, anti-Gαq/11, or anti-Gαi3/o antibody are shown. The numbers below each blot show a quantitative assessment (mean ± SEM) of Gα levels from 6–9 independent experiments. The asterisk indicates statistically significant difference (P < 0.05, two-tailed t test) between control and test cells.
Panel B. Cells were incubated with or without 100 ng/ml of hCG for 30min at 37C and used to measure cAMP production. Each bar is the mean ± SEM of 3 independent experiments. Means with different letters are significantly different from each other (p < 0.05, repeated measurements ANOVA with Tukey post test).
Panels C and D. Cells were incubated for 30 min with or without 100 ng/ml hCG, lysed and used to measure kinase activity in Fyn immunoprecipitates (panel C) or the phosphorylation of FAK with an antibody to phosphotyrosine 576 (panel D). Each bar is the mean ± SEM of 3 to 4 independent experiments. Means with different letters are significantly different from each other (p < 0.05, repeated measurements ANOVA with Tukey post test). A representative Western blot of the FAK phosphorylation assay is also shown in panel D.
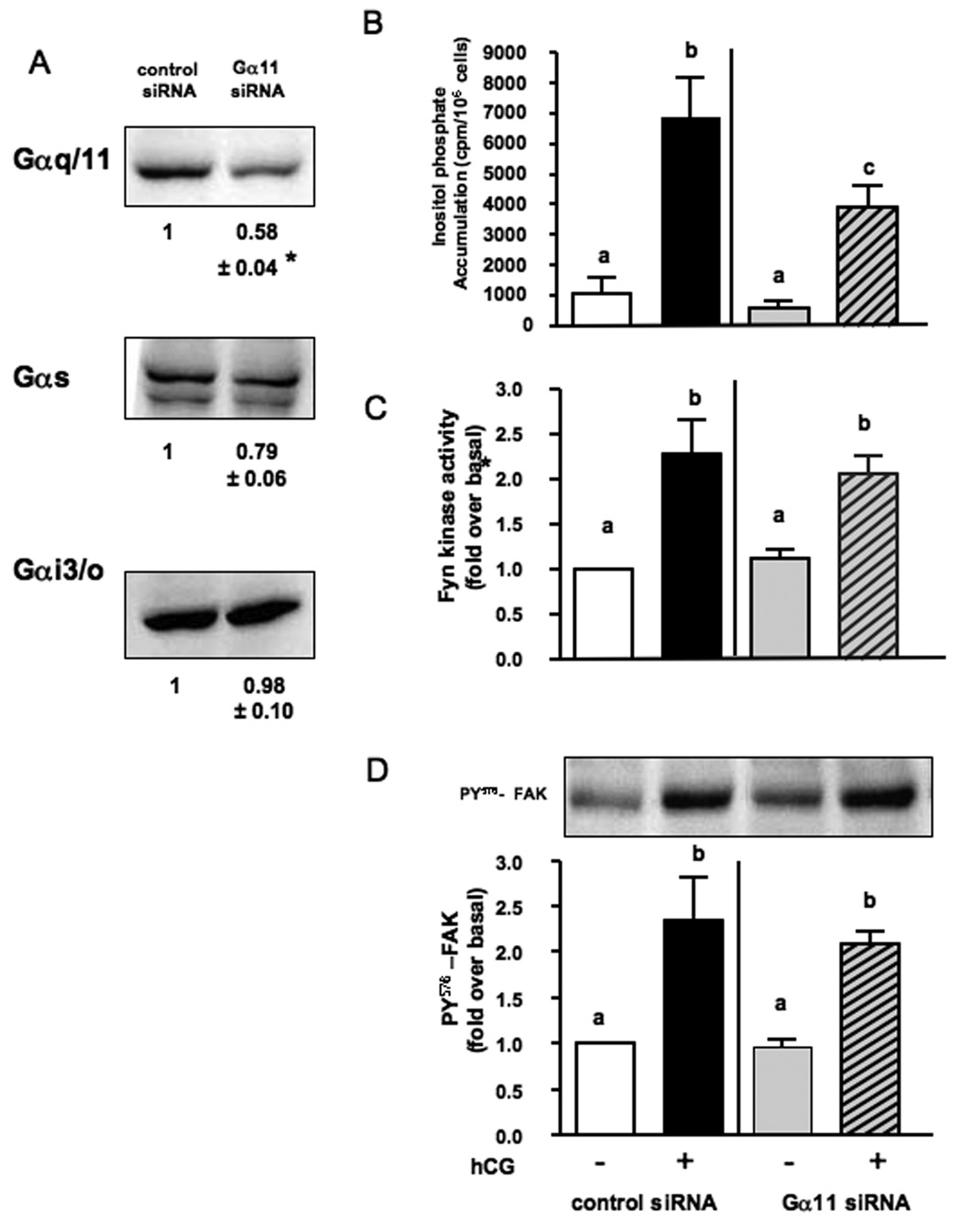
Using a similar strategy we next examined the involvement of Gα11. We did not examine the involvement of Gαq because it is not detectable in MA-10 cells [35]. Figure 2A shows that the Gα11 siRNA decreased the expression of Gα11 without affecting the expression of Gαs or Gαi3/o. A similar reduction in the levels of functional Gα11 was detected by measuring hCG-stimulated inositol phosphate accumulation (Figure 2B). The hCG-induced activation of Fyn (Figure 2C) and the phosphorylation of FAK (Figure 2D) were not affected, however.
Figure 2. Gα11 siRNA decreases the expression of Gα11 and hCG-stimulated inositol phosphate accumulation but does not affect hCG-stimulated Fyn kinase activity or FAK phosphorylation.
MA-10 cells were co-transfected with the hLHR and a control siRNA or the Gα11 siRNA as described in Material and Methods.
Panel A. The relevant portion of a representative Western blot of lysates probed with, anti-Gαq/11, anti-Gαs or anti-Gαi3/o antibody are shown. The numbers below each blot show a quantitative assessment (mean ± SEM) of Gα levels from 6 independent experiments. The asterisk indicates statistically significant difference (P < 0.05, two-tailed t test) between control and test cells.
Panel B. Cells were incubated with or without 1000 ng/ml of hCG for 60min at 37C and used to measure inositol phosphate accumulation. Each bar is the mean ± SEM of 4 independent experiments. Means with different letters are significantly different from each other (p < 0.05, repeated measurements ANOVA with Tukey post test).
Panel C and D. Cells were incubated for 30 min with or without 100 ng/ml hCG, lysed and used to measure kinase activity in Fyn immunoprecipitates (panel C) or the phosphorylation of FAK using an antibody to phosphotyrosine 576 (panel D). Each bar is the mean ± SEM of 4–5 independent experiments. Means with different letters are significantly different from each other (p < 0.05, repeated measurements ANOVA with Tukey post test). A representative Western blot of the FAK phosphorylation assay is also shown in panel D.
3-2 Arrestin3 is involved in the activation of Fyn by the hLHR
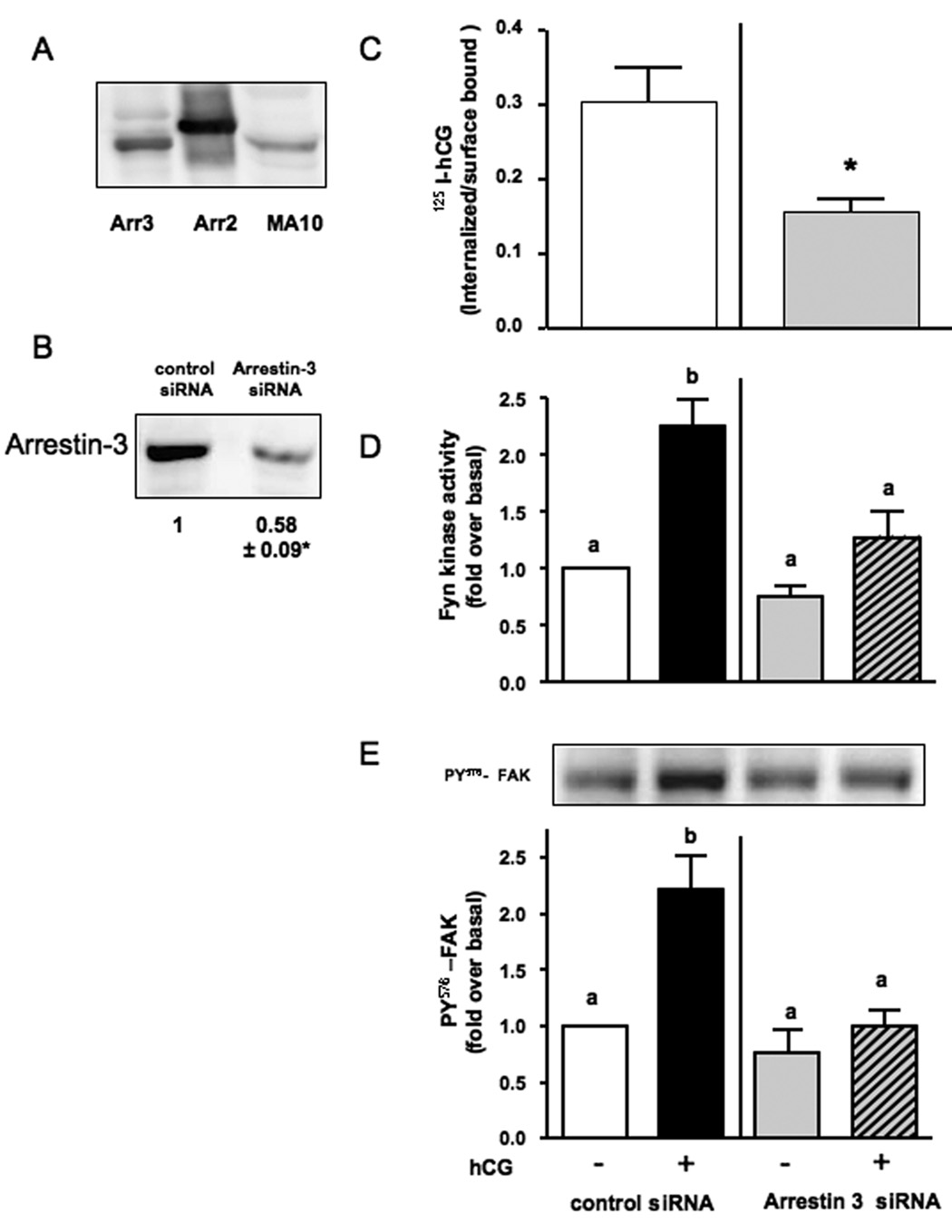
The role of the non-visual arrestins was also examined by using siRNA-mediated silencing. Here we focused only on arrestin-3 because our initial studies showed that arrestin-3 is more prominent than arrestin-2 in MA-10 cells (Figure 3A).
Figure 3. Arrestin-3 siRNA decreases the expression of arrestin-3, the internalization of hCG and the hCG-stimulated Fyn kinase activity and FAK phosphorylation.
MA-10 cells were co-transfected with the hLHR and a control siRNA or the arrestin-3 siRNA as described in Material and Methods.
Panel A. The relevant portion of a representative Western blot of lysates of 293T cells transfected with arrestin-3 or arrestin-2 and of lysates of untransfected MA-10 cells probed with an anti-arrestin antibody is shown.
Panel B. The relevant portion of a representative Western blot of lysates of MA-10 cells transfected with the control or arrestin-3 siRNA and probed with an anti-arrestin antibody is shown. The numbers below the blots show a quantitative assessment of arrestin-3 levels (mean ± SEM) from 4 independent experiments. The asterisk indicates a statistically significant difference (P < 0.05, two-tailed t test) between the control- and arrestin-3-siRNA-transfected cells.
Panel C. Cells were incubated with 100 ng/ml of 125I-hCG for 10 min at 37C and used to measure the internalized and surface-bound 125I-hCG. Each bar is the mean ± SEM of 3 independent experiments. The asterisk indicates a statistically significant difference (P < 0.05, two-tailed t test) between the control- and arrestin 3-siRNA transfected cells.
Panels D and E. Cells were incubated for 30 min with or without 100 ng/ml hCG, lysed and used to measure kinase activity in Fyn immunoprecipitates (panel D) or the phosphorylation of FAK using an antibody to phosphotyrosine 576 (panel E). Each bar is the mean ± SEM of 4–5 independent experiments. Means with different letters are significantly different from each other (p < 0.05, repeated measurements ANOVA with Tukey post test). A representative Western blot of the FAK phosphorylation assay is also shown in panel E.
The siRNA against arrestin-3 decreased the level of arrestin-3 protein compared to that of MA10 cells transfected with the control siRNA (Figure 3B). This reduction was accompanied by a similar reduction in the internalization of 125I-hCG, which is a non-visual arrestin-mediated process [36, 37]. Likewise, the data presented in Figures 3C and 3D show that transfection with arrestin-3 siRNA completely inhibits the hCG-induced activation of Fyn and the phosphorylation of FAK.
3-3 Arrestin3 is necessary for the hLHR-induced accumulation of EGF-like growth factors in MA-10 cells
MA-10 cells produce EGF-like growth factors in response to LHR stimulation using Fyn as an intermediate, and this pathway contributes to the LHR-provoked activation of the ERK1/2 cascade [9].
The production of EGF-like growth factors by MA-10 cells can be readily measured in a co-culture system in which these cells are transfected with the hLHR and co-cultured with a test cell line (I-10 cells) that does not express the LHR but express recombinant GFP-tagged forms of the EGFR and ERK1 [9, 19]. Since the test cells are unresponsive to hCG [9] any hCG-induced phosphorylation of the EGFR-GFP or ERK1-GFP measured in the co-cultures must originate from extracellular EGF-like growth factors that are produced by the actions of hCG on the MA-10 cells. In the present studies we concentrated on measuring the phosphorylation of ERK1-GFP as an index of extracellular EGF-like growth factor activity because this signal is more robust than that of the phosphorylated EGFR-GFP [9]. Moreover, the phosphorylation of ERK1-GFP is clearly an EGFR-mediated event because, when added to the co-cultures, hCG increases the phosphorylation of ERK1-GFP in the test cells only if they are first transfected with the EGFR (data not shown).
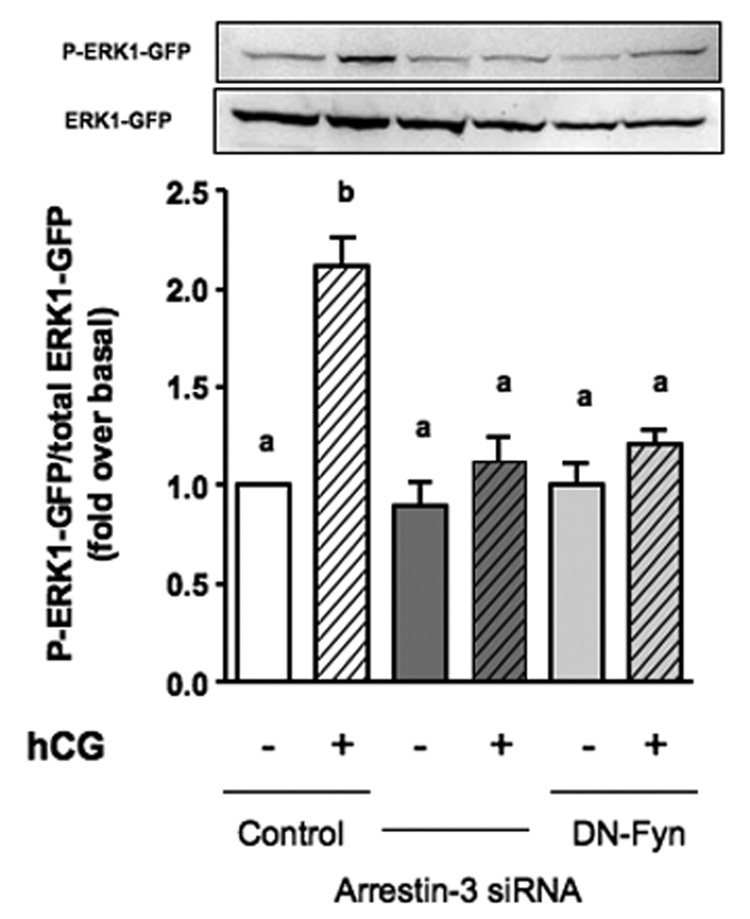
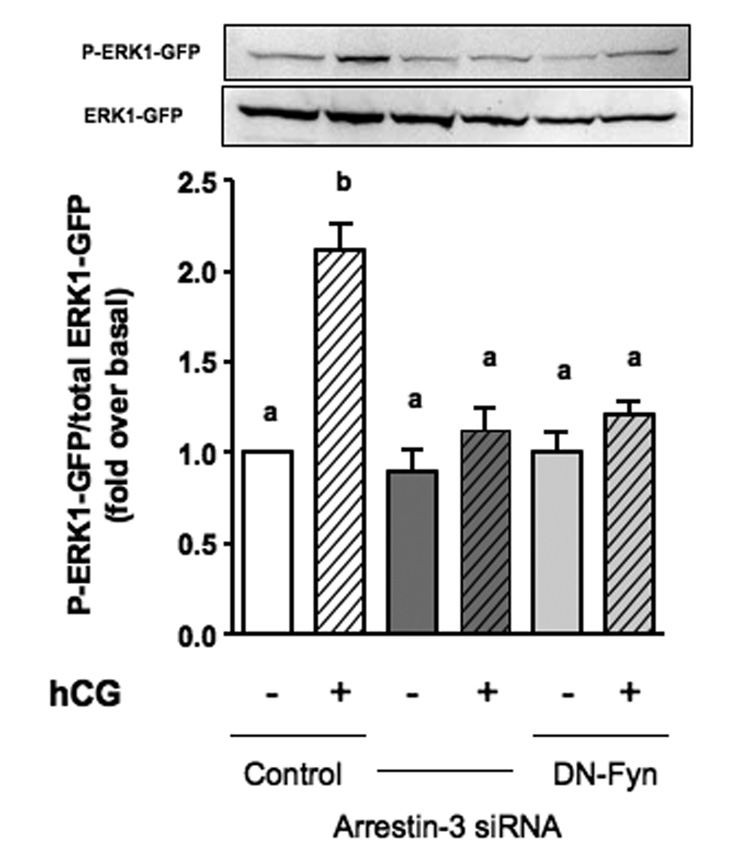
Using this co-culture assay we previously showed that a dominant-negative mutant of Fyn (DN-Fyn) blocks the hCG-provoked accumulation of EGF-like growth factors [9]. Since we have shown here that an arrestin-3 siRNA prevents the LHR-provoked activation of Fyn in MA-10 cells we predicted that transfection of MA-10 cells with this siRNA would also block the hCG-provoked accumulation of EGF-like growth factors. The data presented in Figure 4 show that this prediction was correct. In fact, the arrestin-3 siRNA is as effective as the DN-Fyn construct in inhibiting the accumulation of EGF-like growth factors and the subsequent phosphorylation of ERK1-GFP in test cells.
Figure 4. Silencing of arrestin-3 inhibits EGF-like growth factor accumulation.
MA-10 cells were co-transfected with the hLHR and a control siRNA, the arrestin 3 siRNA or a dominant negative Fyn construct (DN-Fyn) and co-cultured with test cells expressing ERK1-GFP and EGFR-GFP. The co-cultures were incubated without or with hCG (100 ng/ml) for 1h at 37 °C and the phosphorylated and total ERK1-GPF were measured in Western blots of the lysates. Each bar represents the mean ± SEM of 6 independent experiments. Means with different letters are significantly different from each other (p < 0.05, repeated measurements ANOVA with Tukey post test).
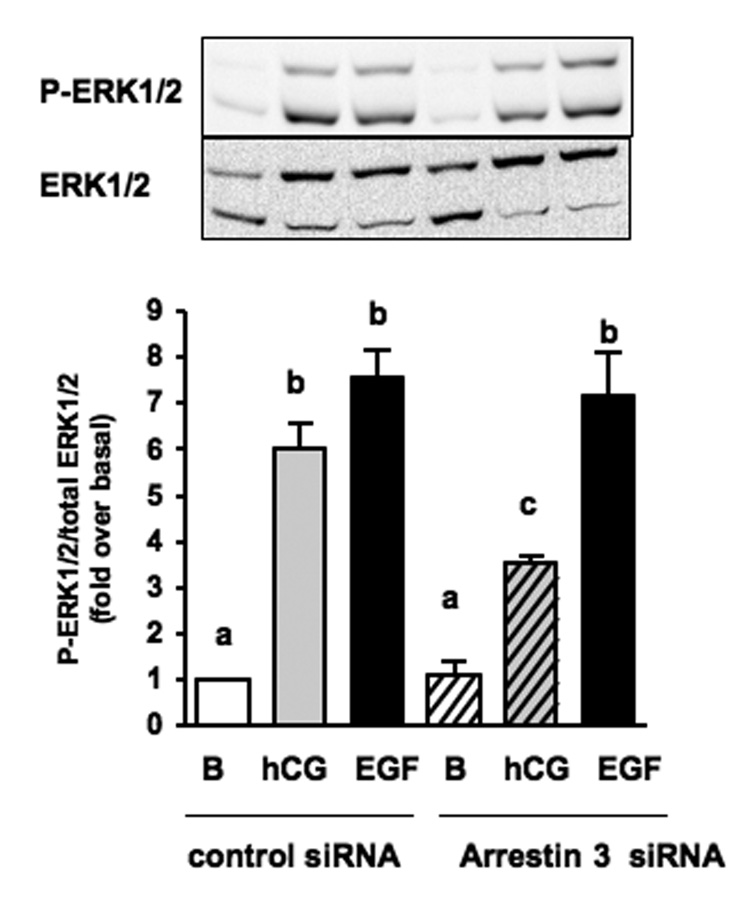
3-4. Silencing the expression of arrestin-3 inhibits the hCG-provoked phosphorylation of ERK1/2 in MA-10 cells
In the last set of experiments we analyzed the effects of the arrestin-3 siRNA on the hCG- and EGF-induced phosphorylation of endogenous ERK1/2 in MA-10 cells.
For these experiments MA-10 cells that were co-transfected with the hLHR plus the arrestin-3 siRNA or with the hLHR plus a control siRNA. The cells were then stimulated with a maximally effective concentration of hCG (100 ng/ml) or a concentration of EGF (1 ng/ml) chosen to match the magnitude of the phospho ERK1/2 response elicited by hCG. The data presented in Figure 5 show that the arrestin-3 siRNA reduced the hCG-induced phosphorylation of ERK1/2 but had no effect on the EGF-induced ERK1/2 phosphorylation. These results are similar to those obtained when MA-10 cells are transfected with DN-Fyn [6]. We also note that whereas the arrestin-3 siRNA completely inhibited the hCG-induced phosphorylation of ERK1-GFP in the co-cultures (Figure 4), it was only partially effective on the hCG-induced phosphorylation of the endogenous ERK1/2 in MA-10 cells (Figure 5).
Figure 5. Silencing of arrestin-3 impairs the hCG-induced phosphorylation of endogenous ERK1/2 in MA-10 cells.
MA-10 cells were co-transfected with the hLHR and a control siRNA or the hLHR and a siRNA that targets arrestin-3 as indicated. The cells were then incubated with buffer only (B), hCG (100 ng/ml×15 min) or EGF (1 ng/ml×5 min) prior to measuring the phospho- and total ERK1/2. The Western blots are representative of one experiment. The bar graph shows the mean ± SEM of 4 independent experiments. Means with different letters are significantly different from each other (p < 0.05, repeated measurements ANOVA with Tukey post test).
4- DISCUSSION
The experiments described here were designed to investigate the roles of G proteins and non-visual arrestins on the LHR-induced activation of Fyn in MA-10 cells.
We first tested the hypothesis that the LHR activates Fyn using a G protein-mediated pathway. This could take place by a direct interaction of a Gα subunit with Fyn [26, 27] or by the activation of second messenger-dependent tyrosine kinases that phosphorylate and activate Fyn [28].
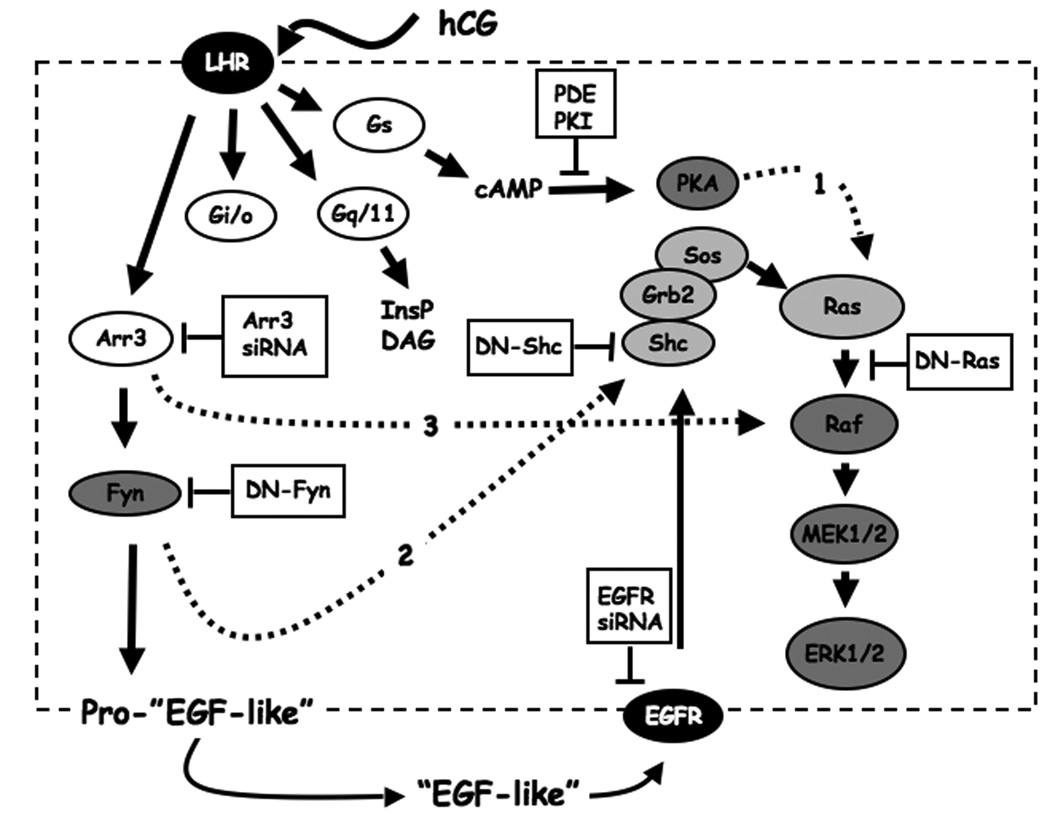
Three G protein families, Gs, Gq/11 and Gi/o were considered as candidates because they are activated by the LHR in MA-10 cells [Figure 6 and ref. 35]. Since the potential involvement of the Gi/o family, has already been excluded in previous experiments [5] we concentrated on Gαs and Gα11. In addition, since others have shown that Gαs binds to and activate SFKs but Gαq does not [26, 27], it appeared to us that Gαs was a better candidate than Gα11.
Figure 6. A model showing the intracellular and intercellular pathways that are involved in the hCG-provoked phosphorylation of ERK1/2 in MA-10 cells.
Human CG binds to the LHR and activates the Gs and Gq/11, families of G proteins leading to an increase in cAMP, inositol phosphates (InsP) and diacylgylcerol (DAG) as shown. The hCG-engaged LHR also activates the Gi/o family but the functional consequence of this activation is not known. Cyclic AMP, acting through PKA, activates Ras and the ERK1/2 cascade by mechanisms that remain to be investigated (dashed line #1). This pathway has been previously documented by the finding that overexpression of a cAMP phosphodiesterase (PDE), a PKA inhibitor (PKI) or a dominant-negative Ras (DN-Ras) inhibit the hCG-induced ERK1/2 response [6, 7, 9]. In addition, PKA-selective analogs of cAMP activate Ras and stimulate ERK1/2 phosphorylation whereas cAMP analogs that are selective for cAMP-dependent guanine nucleotide exchange factors do not [8].
The binding of hCG to the LHR also results in the formation of an LHR/arrestin-3 complex [37] and this complex is required for Fyn activation as shown herein. The co-culture experiments suggest that the involvement of Fyn as a mediator of ERK1/2 phosphorylation involves activation of the proteolysis of the precursor form of an EGF-like growth factor (Pro-“EGF-like”) leading to the release of soluble, diffusible forms of this factor (EGF-like). This “EGF-like growth factor” stimulates the EGFR in an autocrine fashion leading to the activation of Ras by the classical pathway involving Shc, Grb2 and Sos. This pathway was documented by the ability of an arrestin-3 siRNA, Dn-Fyn, an EGFR-siRNA, DNShc and DN-Ras to inhibit the hCG-induced ERK1/2 response [this paper and refs. 6, 7, 8, 9].
Fyn could also directly phosphorylate Shc as shown by dashed arrow #2 and Arr3 could also more directly participate in the activation of the Raf-MEK-ERK cascade by forming scaffolds as shown by dashed arrow # 3 (see Discussion).
The results presented here show that siRNAs can be used to reduce the expression of Gα11 and Gαs by ~50%. These reductions are functionally significant because they are accompanied by a comparable reduction in the LHR-induced activation of adenylate cyclase and phospholipase C, the classical effectors of Gαs and Gα11. Since reducing the expression of Gα11 and Gαs does not affect the LHR-induced activation of Fyn, however, we can conclude that Fyn is not a direct effector of Gα11 or Gαs.
The lack of effect of Gαs silencing on Fyn activities is in agreement with the finding that overexpression of a constitutively active form of Gαs or addition of cAMP analogs to MA-10 cells do not readily reproduce the functional effects of Fyn activation [5]. Likewise, overexpression of a cAMP phosphodiesterase or an inhibitor of PKA has little effect on LHR-activated Fyn-mediated events [9]. When considered together these various experimental approaches rule out Gαs and cAMP-dependent kinases as mediators of the activation of Fyn.
Our results also show that silencing Gα11 expression is not associated with a reduction in the LHR-provoked Fyn activation. On the other hand, gain-of-function approaches such as overexpression of constitutively active forms of Gαq or Gα11, engagement of other Gq/11-coupled receptors, or pharmacological activation of protein kinase C, robustly stimulate Fyn-dependent events in MA-10 cells [5]. These seemingly divergent results could be explained by the knowledge that SFKs are activated by Ca+2-dependent tyrosine kinases [28] but are not effectors of the Gαq/11 family [26]. Transfection of constitutively active form of Gαq or Gα11 into MA-10 cells does not substantially increase the amount of Gαq or Gα11 expressed but results in a 20- to 30-fold increase in inositol phosphate accumulation [5]. Thus, the increase in Fyn activities previously reported in MA-10 cells expressing constitutively active Gαq or Gα11 [5] is more likely due to the massive increase in inositol phosphate accumulation rather than to an increase in a direct interaction of the overexpressed Gα subunits with Fyn. Similarly, if there is no direct interaction of Fyn and Gα11 then the lack of effect of the ~50% reduction in Gα11 expression on Fyn activation reported here is also expected. Conversely, if Fyn is activated by Ca+2- or diacylgycerol-dependent kinases or phosphatases one would expect to detect an increase in Fyn activation in the cells expressing constitutively active Gαq or Gα11 because of the elevation in second messenger levels, but the ~50% reduction in the levels of inositol phosphates caused by Gα11 silencing reported here may not be enough to dampen the activation of Fyn because second messengers are usually generated in excess of what is needed to elicit a maximal biological response [38]. Based on this discussion we conclude that the LHR-induced activation of Fyn is not directly mediated by Gα11. The potential involvement of Ca+2- or diacylgycerol-dependent kinases or phosphatases cannot be formally excluded by these experiments but the results obtained with arrestin-3 silencing (discussed below) argue against this possibility.
Some G protein-coupled receptors activate SFKs via the formation of ternary complexes containing the receptor, arrestin-2 or -3 and a SFK [22]. Here we show that the expression of arrestin-3 can also be reduced by ~50% with a siRNA and that this degree of silencing is accompanied by a comparable reduction in the internalization of hCG, a process that requires the physical association of arrestin-3 and the LHR [36, 37]. Importantly, silencing of arrestin-3 also results in a loss of the LHR-provoked activation of Fyn and two Fyn-mediated events, the phosphorylation of FAK and the accumulation of EGF-like growth factors (Figure 6). Clearly then arrestin-3 is an essential mediator of the activation of Fyn. We presume that the involvement of arrestin-3 on the LHR-provoked activation of Fyn involves the formation of a ternary complex containing these three molecules but we have not formally explored this possibility because of the low abundancy of the molecules involved. In most cases the existence of these ternary complexes has been documented only in heterologous cells overexpressing the different components involved [22]. Lastly, we also note that since disruption of the association of the LHR and arrestin-3 has little or no effect on second messenger generation [36] these results argue against the involvement of second messengers as important mediators of the activation of Fyn by the LHR (also see above discussion).
The LHR-provoked phosphorylation of ERK1/2 in Leydig cells is complex and we have proposed that it involves intracellular and intercellular pathways (Figure 6). The intracellular pathway is independent of Fyn, but is mediated by cAMP/PKA and it requires Ras activation [7]. The mechanisms by which cAMP/PKA activates Ras are unclear are unclear, however, as indicated by dashed arrow #1 in Figure 6. The intercellular pathway is independent of cAMP/PKA, and is mediated by Fyn as shown in Figure 6. Studies with pharmacological inhibitors, DN-Fyn, and siRNAs suggest that Fyn is involved in stimulating the release of EGF-like growth factors that bind to and phosphorylate the EGFR [this paper and refs. 6, 8, 9]. This LHR/Fyn-dependent transactivation of the EGFR results in the phosphorylation of Shc and the activation of Ras and the ERK1/2 cascade as shown in Figure 6 [6, 8, 9]. Fyn could also directly phosphorylate Shc [39] and thus could activate the Ras-Raf-MEK-ERK1/2 cascade through an intracellular pathway as shown by the dashed arrow #2 in Figure 6. We do not yet know if this pathway is operational. The extracellular pathway is, however, clearly functional as shown here and elsewhere [6, 8, 9]
Here we show that silencing arrestin-3 is more effective in inhibiting the hCG-induced transphosphorylation of ERK1/2 in test cells than the phosphorylation of ERK1/2 in MA-10 cells. These findings are in full agreement with our model because the hCG-induced transphosphorylation of ERK1/2 in test cells is entirely dependent on the release of EGF-like growth factors whereas the hCG-stimulated phosphorylation of ERK1/2 in MA-10 cells is dependent on the autocrine actions of extracellular EGF-like growth factors and on the intracellular PKA-dependent pathway (Figure 6).
In addition to binding SKFs, the non-visual arrestins bind various members of the MAP kinase cascades [reviewed in ref. 40]. It is therefore possible that the LHRbound arrestin-3 also serves as a scaffold for these other molecules that more directly participate in the activation of ERK1/2. If this is the case arrestin-3 could also participate in the intracellular pathways describe above that lead to the activation of ERK/12 in MA-10 cells. This pathway is shown by dashed arrow #3 in Figure 6.
In summary, our studies show that the LHR activates Fyn using arrestin-3 as an intermediate. These findings are important because they document, for the first time, that arrestin-3 is involved in LHR signaling and because they contribute to our understanding of the mechanisms involved in the LHR-induced activation of Fyn and the ERK1/2 cascade.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This work was supported by a grant from the National Cancer Institute (CA-40629) to MA.
References
- 1.Ascoli M, Fanelli F, Segaloff DL. Endocr. Rev. 2002;23:141–174. doi: 10.1210/edrv.23.2.0462. [DOI] [PubMed] [Google Scholar]
- 2.Dufau ML, Tsai-Morris CH. In: The Leydig cell in health and disease. Payne AH, Hardy MP, editors. Totowa, NJ: Humana Press; 2007. pp. 227–252. [Google Scholar]
- 3.Carvalho CRO, Carvalheira JBC, Lima MHM, Zimmerman SF, Caperuto LC, Amanso A, Gasparetti AL, Meneghetti V, Zimmerman LF, Velloso LA, Saad MJA. Endocrinology. 2003;144:638–647. doi: 10.1210/en.2002-220706. [DOI] [PubMed] [Google Scholar]
- 4.Conti M, Hsieh M, Park JY, Su YQ. Mol. Endocrinol. 2005;20:715–723. doi: 10.1210/me.2005-0185. [DOI] [PubMed] [Google Scholar]
- 5.Mizutani T, Shiraishi K, Welsh T, Ascoli M. Mol. Endocrinol. 2006;20:619–630. doi: 10.1210/me.2005-0277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shiraishi K, Ascoli M. Endocrinology. 2006;147:3419–3427. doi: 10.1210/en.2005-1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hirakawa T, Ascoli M. Mol. Endocrinol. 2003;17:2189–2200. doi: 10.1210/me.2003-0205. [DOI] [PubMed] [Google Scholar]
- 8.Shiraishi K, Ascoli M. Endocrinology. 2007;148:3214–3225. doi: 10.1210/en.2007-0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shiraishi K, Ascoli M. Exp. Cell Res. 2008;314:25–37. doi: 10.1016/j.yexcr.2007.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martinelle N, Holst M, Soder O, Svechnikov K. Endocrinology. 2004;145:4629–4634. doi: 10.1210/en.2004-0496. [DOI] [PubMed] [Google Scholar]
- 11.Salvador LM, Maizels E, Hales DB, Miyamoto E, Yamamoto H, Hunzicker-Dunn M. Endocrinology. 2002;143:2986–2994. doi: 10.1210/endo.143.8.8976. [DOI] [PubMed] [Google Scholar]
- 12.Maizels ET, Mukherjee A, Sithanandam G, Peters CA, Cottom J, Mayo KE, Hunzicker-Dunn M. Mol. Endocrinol. 2001;15:716–733. doi: 10.1210/mend.15.5.0634. [DOI] [PubMed] [Google Scholar]
- 13.Seger R, Hanoch T, Rosenberg R, Dantes A, Merz WE, Strauss JF, III, Amsterdam A. J. Biol. Chem. 2001;276:13957–13964. doi: 10.1074/jbc.M006852200. [DOI] [PubMed] [Google Scholar]
- 14.Panigone S, Hsieh M, Fu M, Persani L, Conti M. Mol. Endocrinol. 2008;22:915–923. doi: 10.1210/me.2007-0246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Su Y-Q, Nyegaard M, Overgaard MT, Qiao J, Giudice LC. Biol. Reprod. 2006;75:859–867. doi: 10.1095/biolreprod.106.052613. [DOI] [PubMed] [Google Scholar]
- 16.Andric N, Ascoli M. Mol. Endocrinol. 2006;20:3308–3320. doi: 10.1210/me.2006-0241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hsieh M, Lee D, Panigone S, Horner K, Chen R, Theologis A, Lee DC, Threadgill DW, Conti M. Mol. Cell. Biol. 2007;27:1914–1924. doi: 10.1128/MCB.01919-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park J-Y, Su Y-Q, Ariga M, Law E, Jin SLC, Conti M. Science. 2004;303:682–684. doi: 10.1126/science.1092463. [DOI] [PubMed] [Google Scholar]
- 19.Andric N, Ascoli M. Mol. Cell. Endocrinol. 2008;285:62–72. doi: 10.1016/j.mce.2008.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wayne CM, Fan H-Y, Cheng X, Richards JS. Mol. Endocrinol. 2007;21:1940–1957. doi: 10.1210/me.2007-0020. [DOI] [PubMed] [Google Scholar]
- 21.Luttrell DK, Luttrell LM. Oncogene. 2004;23:7969–7978. doi: 10.1038/sj.onc.1208162. [DOI] [PubMed] [Google Scholar]
- 22.Lefkowitz RJ, Shenoy SK. Science. 2005;308:512–517. doi: 10.1126/science.1109237. [DOI] [PubMed] [Google Scholar]
- 23.Gavi S, Shumay E, Wang H, Malbon CC. Trends Endocrinol. Metab. 2006;17 doi: 10.1016/j.tem.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 24.Cao W, Luttrell LM, Medvedev AV, Pierce KL, Daniel KW, Dixon TM, Lefkowitz RJ, Collins S. J. Biol. Chem. 2000;275:38131–38134. doi: 10.1074/jbc.C000592200. [DOI] [PubMed] [Google Scholar]
- 25.Fan G-f, Shumay E, Malbon CC, Wang H-y. J. Biol. Chem. 2001;276:13240–13247. doi: 10.1074/jbc.M011578200. [DOI] [PubMed] [Google Scholar]
- 26.Ma Y-C, Huang J, Ali s, Lowry W, Huang X-Y. Cell. 2000;102:635–646. doi: 10.1016/s0092-8674(00)00086-6. [DOI] [PubMed] [Google Scholar]
- 27.Gu C, Ma Y-C, Benjamin J, Littman D, Chao MV, Huang X-Y. J. Biol. Chem. 2000;275:20726–20733. doi: 10.1074/jbc.M000152200. [DOI] [PubMed] [Google Scholar]
- 28.Dikic I, Tokiwa G, Lev S, Courtneidge SA, Schlessinger J. Nature. 1996;383:547–550. doi: 10.1038/383547a0. [DOI] [PubMed] [Google Scholar]
- 29.Hirakawa T, Galet C, Ascoli M. Endocrinology. 2002;143:1026–1035. doi: 10.1210/endo.143.3.8702. [DOI] [PubMed] [Google Scholar]
- 30.Resh MD. International Journal of Biochemistry and Cell Biology. 1998;30:1159–1162. doi: 10.1016/s1357-2725(98)00089-2. [DOI] [PubMed] [Google Scholar]
- 31.Krupnick JG, Santini F, Gagnon AW, Keen JH, Benovic JL. J. Biol. Chem. 1997;272:32507–32512. doi: 10.1074/jbc.272.51.32507. [DOI] [PubMed] [Google Scholar]
- 32.Ascoli M. Endocrinology. 1981;108:88–95. doi: 10.1210/endo-108-1-88. [DOI] [PubMed] [Google Scholar]
- 33.Shin S-I. Endocrinology. 1967;81:440–448. doi: 10.1210/endo-81-3-440. [DOI] [PubMed] [Google Scholar]
- 34.Nakamura K, Liu X, Ascoli M. J. Biol. Chem. 2000;275:241–247. doi: 10.1074/jbc.275.1.241. [DOI] [PubMed] [Google Scholar]
- 35.Hirakawa T, Ascoli M. Endocrinology. 2003;144:3872–3878. doi: 10.1210/en.2003-0365. [DOI] [PubMed] [Google Scholar]
- 36.Bhaskaran RS, Min L, Krishnamurthy H, Ascoli M. Biochemistry. 2003;42:13950–13959. doi: 10.1021/bi034907w. [DOI] [PubMed] [Google Scholar]
- 37.Min L, Galet C, Ascoli M. J. Biol. Chem. 2002;277:702–710. doi: 10.1074/jbc.M106082200. [DOI] [PubMed] [Google Scholar]
- 38.Strickland S, Loeb JN. Proc. Natl. Acad. Sci. USA. 1981;78:1366–1370. doi: 10.1073/pnas.78.3.1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van der Geer P, Wiley S, Gish GD, Pawson T. Curr. Biol. 1996;6:1435–1444. doi: 10.1016/s0960-9822(96)00748-8. [DOI] [PubMed] [Google Scholar]
- 40.DeWire SM, Ahn S, Lefkowitz RJ, Shenoy SK. Annu. Rev. Physiol. 2007;69:483–510. doi: 10.1146/annurev.physiol.69.022405.154749. [DOI] [PubMed] [Google Scholar]