Abstract
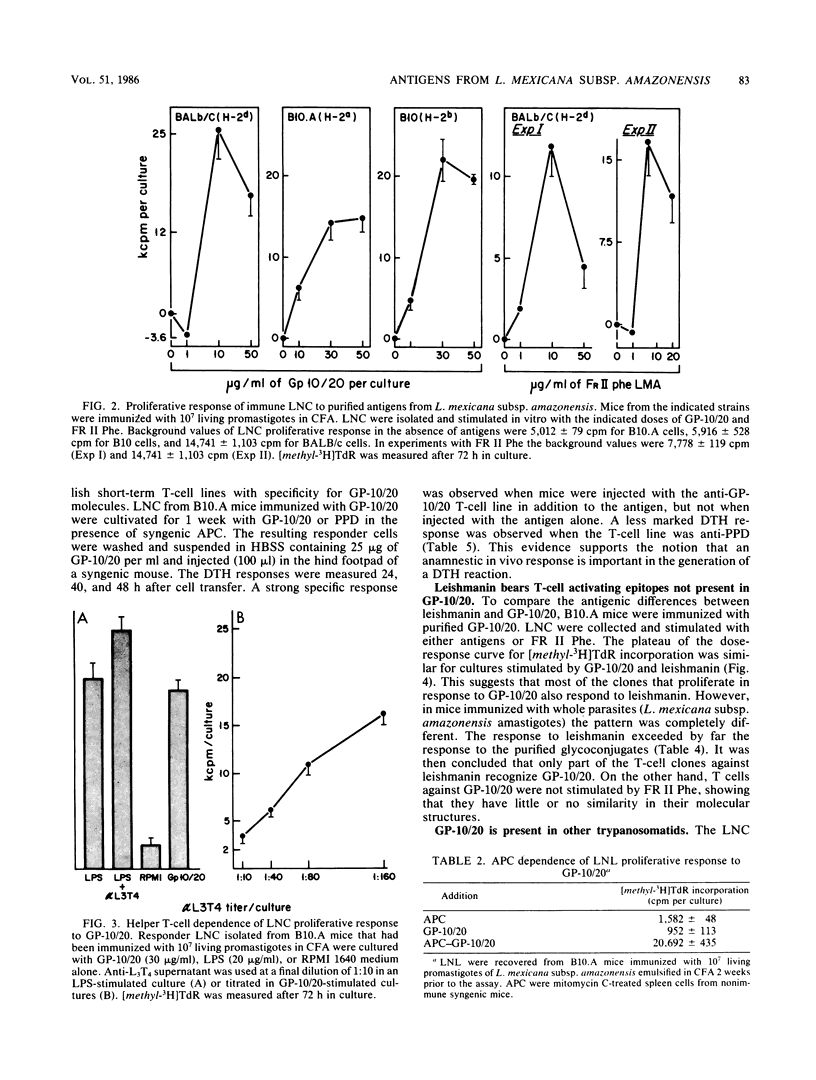
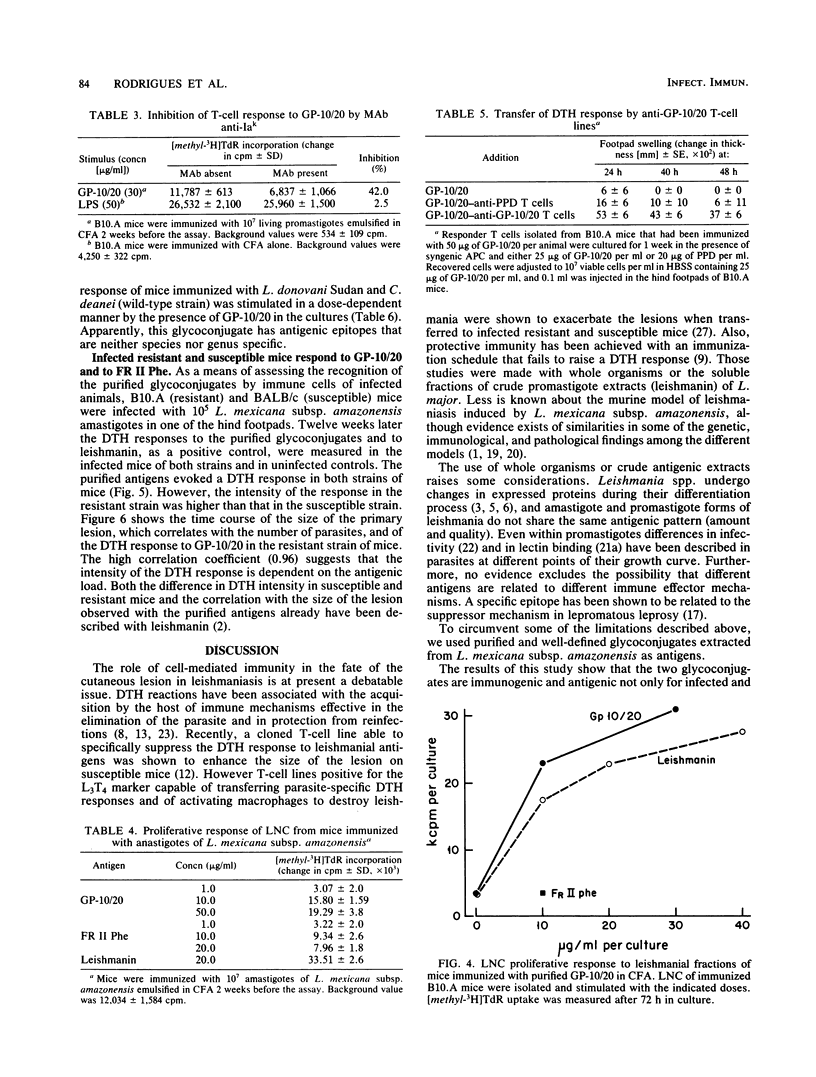
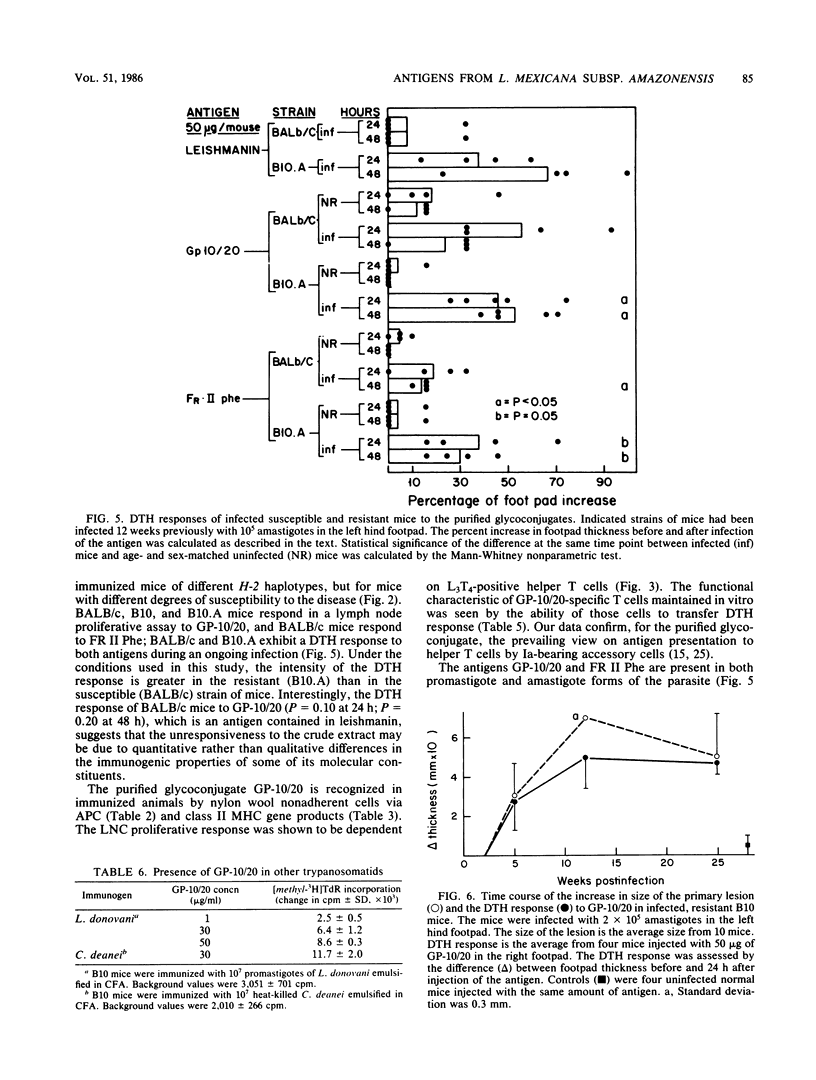
Two defined glycoconjugates (GP-10/20 and FR II Phe) purified from Leishmania mexicana subsp. amazonensis were analyzed with respect to their ability to induce cellular responses in immunized and infected mice. Each glycoconjugate was recognized by specific immune cells, as assessed by the proliferative response of lymph node cells of immunized mice. The response to GP-10/20 depended on helper T cells and antigen-presenting cells and was restricted by a major histocompatibility complex class II gene product. A specific anti-GP-10/20 T-cell line was established, and it was able to transfer a delayed-type hypersensitivity (DTH) response to normal mice. Both antigens were also recognized during an ongoing disease, as assessed by DTH response of infected mice. By this response, it was possible to distinguish susceptible from resistant strains of mice. In the course of the disease in resistant mice a correlation between the size of the primary lesion and the DTH response to GP-10/20 was observed. The presence of the glycoproteins on both promastigote and amastigote forms of the parasite, the antigenic similarities between both fractions, and the distribution of the GP-10/20 antigen in other trypanosomatids were studied. The results showed that both antigens were present on promastigotes and amastigotes. GP-10/20 shared no epitopes with FR II Phe, was included as part of the crude preparation leishmanin, and had some cross-reactive determinants with Leishmania donovani and Crithidia deanei.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arredondo B., Pérez H. Alterations of the immune response associated with chronic experimental leishmaniasis. Infect Immun. 1979 Jul;25(1):16–22. doi: 10.1128/iai.25.1.16-22.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barral A., Petersen E. A., Sacks D. L., Neva F. A. Late metastatic Leishmaniasis in the mouse. A model for mucocutaneous disease. Am J Trop Med Hyg. 1983 Mar;32(2):277–285. doi: 10.4269/ajtmh.1983.32.277. [DOI] [PubMed] [Google Scholar]
- Chang K. P., Fong D. Antigenic changes during intracellular differentiation of Leishmania mexicana in cultured macrophages. Infect Immun. 1982 Apr;36(1):430–431. doi: 10.1128/iai.36.1.430-431.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong D., Chang K. P. Tubulin biosynthesis in the developmental cycle of a parasitic protozoan, Leishmania mexicana: changes during differentiation of motile and nonmotile stages. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7624–7628. doi: 10.1073/pnas.78.12.7624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handman E., Jarvis H. M., Mitchell G. F. Leishmania major: identification of stage-specific antigens and antigens shared by promastigotes and amastigotes. Parasite Immunol. 1984 May;6(3):223–233. doi: 10.1111/j.1365-3024.1984.tb00795.x. [DOI] [PubMed] [Google Scholar]
- Howard J. G., Hale C., Chan-Liew W. L. Immunological regulation of experimental cutaneous leishmaniasis. 1. Immunogenetic aspects of susceptibility to Leishmania tropica in mice. Parasite Immunol. 1980 Winter;2(4):303–314. doi: 10.1111/j.1365-3024.1980.tb00061.x. [DOI] [PubMed] [Google Scholar]
- Howard J. G., Hale C., Liew F. Y. Immunological regulation of experimental cutaneous leishmaniasis. IV. Prophylactic effect of sublethal irradiation as a result of abrogation of suppressor T cell generation in mice genetically susceptible to Leishmania tropica. J Exp Med. 1981 Mar 1;153(3):557–568. doi: 10.1084/jem.153.3.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard J. G., Nicklin S., Hale C., Liew F. Y. Prophylactic immunization against experimental leishmaniasis: I. Protection induced in mice genetically vulnerable to fatal Leishmania tropica infection. J Immunol. 1982 Nov;129(5):2206–2212. [PubMed] [Google Scholar]
- Julius M. H., Simpson E., Herzenberg L. A. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973 Oct;3(10):645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Liew F. Y., Hale C., Howard J. G. Immunologic regulation of experimental cutaneous leishmaniasis. V. Characterization of effector and specific suppressor T cells. J Immunol. 1982 Apr;128(4):1917–1922. [PubMed] [Google Scholar]
- Liew F. Y. Specific suppression of responses to Leishmania tropica by a cloned T-cell line. Nature. 1983 Oct 13;305(5935):630–632. doi: 10.1038/305630a0. [DOI] [PubMed] [Google Scholar]
- Louis J. A., Zubler R. H., Coutinho S. G., Lima G., Behin R., Mauel J., Engers H. D. The in vitro generation and functional analysis of murine T cell populations and clones specific for a protozoan parasite, Leishmania tropica. Immunol Rev. 1982;61:215–243. doi: 10.1111/j.1600-065x.1982.tb00378.x. [DOI] [PubMed] [Google Scholar]
- Matzinger P. A one-receptor view of T-cell behaviour. Nature. 1981 Aug 6;292(5823):497–501. doi: 10.1038/292497a0. [DOI] [PubMed] [Google Scholar]
- Mehra V., Brennan P. J., Rada E., Convit J., Bloom B. R. Lymphocyte suppression in leprosy induced by unique M. leprae glycolipid. Nature. 1984 Mar 8;308(5955):194–196. doi: 10.1038/308194a0. [DOI] [PubMed] [Google Scholar]
- Mendonça-Previato L., Gorin P. A., Braga A. F., Scharfstein J., Previato J. O. Chemical structure and antigenic aspects of complexes obtained from epimastigotes of Trypanosoma cruzi. Biochemistry. 1983 Oct 11;22(21):4980–4987. doi: 10.1021/bi00290a016. [DOI] [PubMed] [Google Scholar]
- Pratt D. M., David J. R. Monoclonal antibodies that distinguish between New World species of Leishmania. Nature. 1981 Jun 18;291(5816):581–583. doi: 10.1038/291581a0. [DOI] [PubMed] [Google Scholar]
- Previato J. O., Mendonça-Previato L., Lewanczuk R. Z., Travassos L. R., Gorin P. A. Crithidia spp.: structural comparison of polysaccharides for taxonomic significance. Exp Parasitol. 1982 Apr;53(2):170–178. doi: 10.1016/0014-4894(82)90058-3. [DOI] [PubMed] [Google Scholar]
- Previato J. O., Xavier M. T., Brazil R. P., Gorin P. A., Mendonça-Previato L. Formation of (1----2)-linked beta-D-mannopyranan by Leishmania mexicana amazonensis: relationship with certain Crithidia and Herpetomonas species. J Parasitol. 1984 Jun;70(3):449–450. [PubMed] [Google Scholar]
- Pérez H., Labrador F., Torrealba J. W. Variations in the response of five strains of mice to Leishmania mexicana. Int J Parasitol. 1979 Feb;9(1):27–32. doi: 10.1016/0020-7519(79)90062-6. [DOI] [PubMed] [Google Scholar]
- Sacks D. L., Hieny S., Sher A. Identification of cell surface carbohydrate and antigenic changes between noninfective and infective developmental stages of Leishmania major promastigotes. J Immunol. 1985 Jul;135(1):564–569. [PubMed] [Google Scholar]
- Sacks D. L., Perkins P. V. Identification of an infective stage of Leishmania promastigotes. Science. 1984 Mar 30;223(4643):1417–1419. doi: 10.1126/science.6701528. [DOI] [PubMed] [Google Scholar]
- Sacks D. L., Scott P. A., Asofsky R., Sher F. A. Cutaneous leishmaniasis in anti-IgM-treated mice: enhanced resistance due to functional depletion of a B cell-dependent T cell involved in the suppressor pathway. J Immunol. 1984 Apr;132(4):2072–2077. [PubMed] [Google Scholar]
- Scharfstein J., Rodrigues M. M., Alves C. A., de Souza W., Previato J. O., Mendonça-Previato L. Trypanosoma cruzi: description of a highly purified surface antigen defined by human antibodies. J Immunol. 1983 Aug;131(2):972–976. [PubMed] [Google Scholar]
- Schwartz R. H., Yano A., Paul W. E. Interaction between antigen-presenting cells and primed T lymphocytes: an assessment of Ir gene expression in the antigen-presenting cell. Immunol Rev. 1978;40:153–180. doi: 10.1111/j.1600-065x.1978.tb00405.x. [DOI] [PubMed] [Google Scholar]
- Sheppard H. W., Scott P. A., Dwyer D. M. Recognition of Leishmania donovani antigens by murine T lymphocyte lines and clones. Species cross-reactivity, functional correlates of cell-mediated immunity, and antigen characterization. J Immunol. 1983 Sep;131(3):1496–1503. [PubMed] [Google Scholar]
- Titus R. G., Lima G. C., Engers H. D., Louis J. A. Exacerbation of murine cutaneous leishmaniasis by adoptive transfer of parasite-specific helper T cell populations capable of mediating Leishmania major-specific delayed-type hypersensitivity. J Immunol. 1984 Sep;133(3):1594–1600. [PubMed] [Google Scholar]
- Wilde D. B., Marrack P., Kappler J., Dialynas D. P., Fitch F. W. Evidence implicating L3T4 in class II MHC antigen reactivity; monoclonal antibody GK1.5 (anti-L3T4a) blocks class II MHC antigen-specific proliferation, release of lymphokines, and binding by cloned murine helper T lymphocyte lines. J Immunol. 1983 Nov;131(5):2178–2183. [PubMed] [Google Scholar]
- de Ibarra A. A., Howard J. G., Snary D. Monoclonal antibodies to Leishmania tropica major: specificities and antigen location. Parasitology. 1982 Dec;85(Pt 3):523–531. doi: 10.1017/s0031182000056304. [DOI] [PubMed] [Google Scholar]