Abstract
Purpose
To compare peak enhancement (PE), determined from dynamic contrast-enhanced (DCE) MRI, and the MR directionally-averaged apparent diffusion coefficient (<D>) in glandular versus stromal prostatic tissues and, with this comparison, to infer if the hypothesis that Gd-DTPA does not enter healthy glands or ducts is plausible.
Materials and Methods
MRI, MR spectroscopic imaging, DCE MRI, and MR diffusion were evaluated in 17 untreated subjects with suspected or proven prostate cancer. PE and <D> were compared in glandular-ductal tissues (normal peripheral zone and glandular benign prostatic hyperplasia (BPH)) and stromal-low ductal tissues (central gland/mixed BPH and stromal BPH).
Results
The glandular-ductal tissues had lower PE (125±6.4 [%baseline]) and higher <D> (1.57±0.15[sec/10−3mm2]) than the stromal-low ductal tissues (PE=132±5.5[%baseline](p<0.0008), <D>=1.18±0.20[sec/10−3mm2] (p<1x10−8)). A statistical model based upon stepwise regression was generated and completely separated the tissue types: Ductal Measure = 448 + 669×<D>[sec/10−3mm2] - 10.7×PE[1/%], R2=1.0 and p<8x10−10.
Conclusions
The very different MR results in the glandular-ductal versus stromal-low ductal tissues suggest that these tissues have different underlying structure. These results support the hypothesis that Gd-DTPA does not enter healthy prostatic glands or ducts. This may explain the higher PE and lower <D> that previously have been reported in prostate cancer versus healthy tissue.
Keywords: DCE MRI, prostate cancer, MR diffusion, ducts, Gadolinium, contrast
1. Introduction
Prostate cancer is the most common invasive cancer diagnosed in men in the United States, affecting approximately one in six men [1]. It is often not deadly because it grows so slowly that it does not escape the prostate. Identifying which prostate cancers will metastasize, leading to increased morbidity and loss of life, is currently a difficult clinical management problem. Current diagnostic methods cannot always discriminate among the different tissues of the prostate, nor can they fully assess a cancer’s aggressiveness.
The prostate contains glands and ducts that change dramatically with cancer. Prostate cancer is diagnosed based on histopathological assessment of biopsy tissue. The Gleason Grading system is commonly used to assess the aggressiveness of the prostate cancer. With this system, the tissue is graded from 1 to 5, with 5 representing the most aggressive cancer, which has been correlated with higher metastatic potential [2]. The Gleason grading system relies on changes in the architectural patterns of the glands and the growth pattern of the tumors. In cancer, the basal cell layer and the basement membrane surrounding the glands and ducts are disrupted. With increasing Gleason grade, the ducts generally become smaller, sparser, and more irregularly shaped. In Gleason grade 5 cancer, the highest and most aggressive grade, there may be no discernable glands. Overall, ductal space is decreased in higher grade cancers [2,3]. Consequently, providing a noninvasive (or minimally invasive, due to the contrast agent injection and the endorectal probe) measure of the healthy ductal volume or glandular versus stromal nature of the tissue may be very important in characterizing prostate cancer aggressiveness.
MR diffusion imaging and dynamic contrast-enhanced MR imaging (DCE MRI) have each shown an ability to differentiate cancer from normal prostatic tissues [4–24]. In particular, DCE MRI has shown higher enhancement and greater washout in prostate cancer versus normal tissues [4–17]. Current understanding of the MR contrast agent, Gadolinium-DTPA (Gd-DTPA) (generic name: gadopentetate dimeglumine), is that it does not penetrate cells but can collect in the extracellular space [25]. The rate of enhancement can be attributed to the amount and permeability of the blood vessels bringing the contrast agent to the tissue. Once the contrast agent is mixed in the vasculature, the peak enhancement in the tissue is due to the size of the extracellular space in which the Gd-DTPA can accumulate (both the interstitium and vascular volume) [26,27]. Thus, the persistent, greater enhancement found in prostate cancer with DCE MRI can be attributed to a larger extracellular space. In contrast, the MR apparent diffusion coefficient and the MR directionally-averaged apparent diffusion coefficient (<D>) have been shown to be lower in prostate cancer than in normal [12, 18–24]. This has been postulated to be due to increased cellularity of cancer, resulting in a smaller extracellular space [28]. A better understanding of the underlying mechanisms of these MR measures will improve their interpretation and increase their value.
A hypothesis that could explain the seemingly conflicting interpretations of these MR measures is that Gd-DTPA cannot reach healthy prostatic ducts. This would imply that DCE MRI would not reflect glandular-ductal volume and would only reflect a subset of the extracellular space. However, diffusion weighted imaging would reflect the glandular-ductal volume in addition to the extracellular space visible to DCE MRI. Additionally, diffusion weighted imaging may be able to reflect some of the intracellular space, which DCE MRI would also not be able to assess. However, at lower b-values (an MR diffusion parameter indicating the strength of the diffusion gradient), such as b=600, the measured diffusion appears to be dominated by the extracellular water [29].
It is difficult to prove whether this hypothesis that Gd-DTPA cannot reach healthy prostatic ducts is true. There is no simple way to measure if Gd-DTPA is indeed in the healthy ducts, even by step-section histopathology. Ejaculate could potentially be tested for Gd-DTPA, but such samples were not available from the subjects in this study to perform this type of analysis. Step section histopathological analysis with MR image correlation could help assess the MR measures in cancer, but is limited by the sampling of the tissue slides (generally less than 1 slide per 3 mm) and the correlation to the images.
It is, however, possible to investigate whether this hypothesis is plausible by using the MR data that has already been acquired. Pathology demonstrates that different noncancerous prostatic tissues have different amounts of glands and ducts [2,3]. Healthy peripheral zone tissue and glandular benign prostatic hyperphasia (BPH) are highly glandular-ductal tissues whereas stromal BPH and central gland tissue, with its commonly found BPH, are more stromal and less ductal [30]. MR imaging, MR spectroscopic imaging and biopsy, when available, can be used to identify these different tissues [31]. Then, the DCE MRI peak enhancement and the MR diffusion behavior (MR <D>) in these tissues can be evaluated to infer if the hypothesis that Gd-DTPA does not enter the glands or ducts is plausible.
In this study, citrate, as measured by MRSI, will be used as an additional indicator of healthy prostatic glands and ducts [32]. It is high, on the order of twice as large, compared to creatine, in the highly glandular-ductal prostatic tissues ( healthy peripheral zone tissue and glandular benign prostatic hyperplasia (BPH)). It is low, less than or similar to creatine, in the stromal-low ductal prostatic tissues ( stromal BPH and central gland / mixed BPH tissue). It is also decreased in peripheral zone cancer as compared to healthy peripheral zone tissue [32]. This decrease may reflect a decrease in healthy glands and ducts but may also reflect changes in citrate metabolism.
If the evaluation of DCE MRI and diffusion in the glandular-ductal tissues versus the stromal-low ductal tissues supports the hypothesis that Gd-DTPA does not reach prostatic ducts, it will help the interpretation of DCE MRI and diffusion MR in the prostate and may lead to a better evaluation of prostate cancer. In future work, the combination of the DCE MRI pharmacokinetically modeled estimate of extracellular extravascular space in the prostate and the MR directionally-averaged apparent diffusion coefficient (<D>) in the prostate will need to be further evaluated. This combination will need to be compared to step section histopathology of the prostate and assessments made in different Gleason grades of cancer to determine if it can provide an assessment of the cancer’s aggressiveness, as higher Gleason grade cancers have less ductal space [2,3].
The aims of this study are to compare peak enhancement, determined from dynamic contrast-enhanced (DCE MRI), with MR directionally-averaged apparent diffusion coefficient measures in glandular versus stromal prostatic tissues and, with this comparison, to infer if the hypothesis that Gd-DTPA does not enter healthy glands or ducts is plausible..
2. Methods
2.1 Subjects
Eighteen patients with no prior treatment were studied using a combined endorectal probe (MedRad or USA Instruments) and a pelvic phased array on a GE 1.5T Signa Imager. Written informed consent was obtained from all subjects following a protocol approved by the Committee on Human Research at this institution. One subject’s data was unusable due to motion artifacts. The following demographics are for the remaining 17 subjects. The subjects’ mean age was 61 years old, ranging from 50 to 75 years old. These subjects tended to have small volumes of cancer. Eight of the 17 had biopsy proven cancer. These subjects had a mean Gleason score of 6 and a median length of cancer found in biopsy cores of approximately 2mm. Five subjects had either a negative biopsy (4) or no biopsy (1) and no evidence of cancer on MRI/MRSI. The remaining 4 had a negative biopsy (2) or no biopsy (2), but had MRI/MRSI regions suspicious of cancer.
2.2 MR Imaging
Anatomic imaging included axial T1-weighted and fast spin-echo T2–weighted images as described previously [33]. The 16x8x8 3DMRSI used a PRESS sequence incorporating dual band spectral spatial pulses [34] to acquire 7x7x7 mm3 voxels (0.34cc) with a TR/TE = 1000 ms/130 ms. The diffusion MRI data were acquired with a single shot FSE based diffusion tensor imaging (DTI) sequence [21, 35, 36] with a 256 x 128 matrix, a 240 mm FOV, 4 mm thick slices, 2 mm interslice gaps, receiver BW = 62.5KHz, b-values = 0 and 600 sec/mm2, 6 gradient directions, along and orthogonal to the main magnetic field and along the three main diagonals, and a TE = 67 ms with typically 7–9 axial slices covering the prostate in 2.5 minutes [21]. Additionally, on a subset of 12 patients, as described previously (4), a three- or four- point multiple-TR (400, 800, 1600, or (usually) 2400 ms) spin-echo sequence was performed to measure the native T1‘s of the tissue.
The dynamic contrast-enhanced imaging was performed after the anatomic and diffusion imaging using a 2D FSPGR sequence (flip angle = 13°). A power injector (Medrad, Inc., Indianola, PA) was used to provide a bolus injection of gadolinium-DTPA (Magnevist contrast agent; Berlex Schering AG, Berlin, Germany) to obtain a dose of 0.1 mL of Gd-DTPA per Kg of body weight, as described previously [4]. Imaging parameters included: TR/TE = 12 ms/ 2 ms, a 256x128 matrix, a 260 mm FOV, five- 5 mm thick slices with 2 mm gaps per stack, 8 sec per stack, and typically 70 time points for the 5 locations per stack, covering most of the prostate, and totaling 350 images over 9 minutes.
2.3 Pharmacokinetic Simulations
While peak enhancement reflects the volume of the extravascular extracellular space, it can also be affected by the native T1 of the tissue, flow into the tissue, and permeability [27]. To assess the magnitude of these effects in the prostate, peak enhancement levels were simulated based upon a two-compartmental, extended Tofts-Kermode model [26]. The Gd concentration in the vasculature (or plasma) was modeled as a biexponential, as described by Tofts and Kermode [25] and given below.
| [1] |
where D = 0.1mmol/Kg of Gd-DTPA, a1 = 3.99 kg/L, m1 = 0.144 1/min, a2 = 4.78 kg/L, m2 = 0.011 1/min.
The concentration of Gd in the tissue was modeled as in the extended Tofts-Kermode model [26]:
| [2] |
where Ktrans represents the transfer of the contrast agent from the vasculature into the extracellular, extravascular space (EES). The transfer back to the blood is given by kep. The blood plasma volume fraction is given by vp and the EES volume fraction is designated ve.
The T1 relaxation time is assumed to follow:
| [3] |
where r1 = 4.5 (mmol/L · sec)-1, appropriate for 1.5T, and T1o is the native T1.
The signals were then modeled as ideal SPGR signals, based on the theoretical SPGR signal equation, and then normalized to baseline, with the T2 term ignored:
| [4] |
where TR = 12 ms, TE = 2 ms and α = 13º were used.
Based on literature values [7,11,12], parameters were set as the following, except where indicated: Ktrans = 0.33 1/min = 0.0055 1/sec, ve = 0.3, kep = Ktrans/ve, and vp = 0.004 [11]. T1o was set to 1500 ms based on measurements from 12 of the subjects from this study.
Simulations were performed as follows. First, the effect of the native T1 was tested with the mean stromal and glandular T1’s (1430ms and 1590ms, based on values measured in a subset of 12 subjects in this study). Next, each parameter of Ktrans, vp and ve were doubled for the stromal tissue versus the glandular tissue, and the effects measured.
2.4 Data Analyses
Motion was assessed and corrected between the imaging sequences and within the DCE sequence. Inter sequence motion was assessed and corrected by manually aligning manually drawn ROIs of the rectum [4]. Motion within the DCE MRI was assessed by plotting lines of intensities versus time and visually assessing shifts in the bright region locations. This motion assessment has been shown elsewhere [37]. The T2-weighted images were corrected for the intensity variation due to the reception profile of the receiver coils [38].
ROIs were drawn on the coil corrected, aligned, T2-weighted images in normal peripheral zone, stromal benign prostatic hyperplasia (BPH), glandular BPH, and central gland / mixed BPH as determined by concordant findings from MRI, MRSI and, when available, biopsy. The normal peripheral zone and glandular BPH were identified as bright regions on the T2-weighted images and as containing high levels of citrate by MRSI whereas stromal BPH was identified as dark regions on the T2-weighted images and as regions with low citrate by MRSI. The ROIs were conservatively drawn, restricted to regions such that the immediate neighboring slices also contained the same tissue type at that location, to reduce partial voluming effects. Central gland / mixed BPH ROIs were drawn avoiding the urethra and any regions of very high T2-weighted intensity, likely due to cysts or a nodule of glandular BPH. Remaining tissue in this central gland / mixed BPH ROI had relatively low citrate levels by MRSI. ROIs were drawn 5mm thick, matching the DCE MRI slices. Additionally, in the eight patients with biopsy confirmed prostate cancer, ROIs were drawn in the region of cancerous peripheral zone as determined by concordant findings from MRI, MRSI, and biopsy. In one patient, an ROI was also drawn in a focus of prostatic intraepithelial neoplasia (PIN), detected by pathological review of sextant biopsies. This region was hypointense on the T2-weighted images and had lower citrate than surrounding peripheral zone tissue, but not as low as to meet the criteria for cancer. Examples of the manually drawn ROIs are given in Figure 1 and Figure 2.
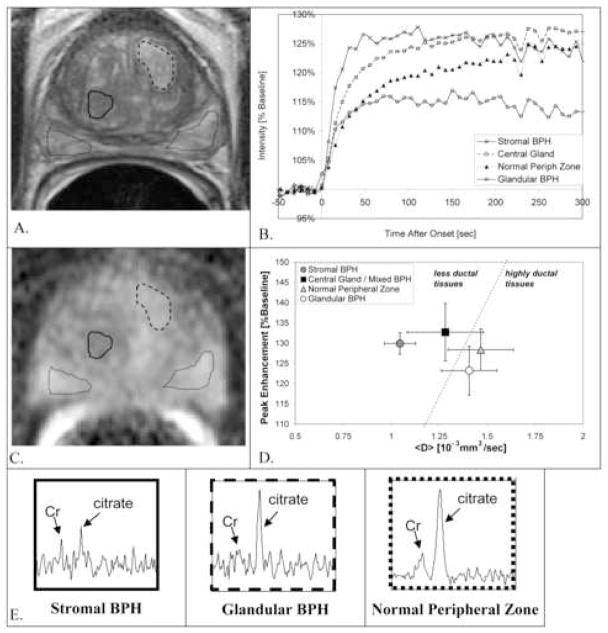
Figure 1.
Different MR patterns in manually drawn ROIs in: stromal BPH (solid line), central gland/mixed BPH (ROI on different slice), glandular BPH (dashed line), and normal peripheral zone (dotted lines). A. T2-weighted MRI. B. DCE MRI enhancement versus time, C. MR <D> image and D. Peak Enhancement plotted versus MR <D>. E. MR spectra from voxels in the ROIs shown in A. and C.
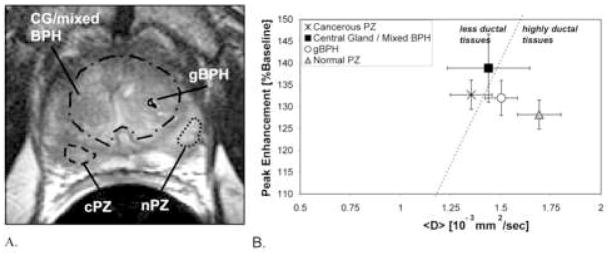
Figure 2.
Peripheral zone cancer (Gleason Score=6) shows lower <D> and higher PE than normal peripheral zone. Manually drawn ROIs are shown in cancerous peripheral zone (dashed line), normal peripheral zone (dotted line), glandular BPH (solid line), and central gland/mixed BPH (with the glandular BPH ROI excluded) (dotted and dashed line). A. T2-weighted MRI. B. Peak Enhancement plotted versus MR <D>.
Peak intensities were determined as the maximum intensity between the injection point and the end of the exam. The peak enhancement (PE) values were then determined by normalizing the peak intensity values to the baseline intensity (average intensity of timepoints 3–10). The MR diffusion was averaged across the six different directions to obtain the MR directionally-averaged apparent diffusion coefficient (<D>). Both PE and <D> were calculated for all the ROIs. This encompassed 5 mm thick slices for the PE and 4 mm thick slices for the <D>. MR diffusion fractional anisotropy was not evaluated as part of this study. Native T1 values were generated for the ROIs of 12 subjects by fitting the data from the three- or four- point, multiple-TR, spin-echo sequence to the following formula:
| [5] |
where S is the signal intensity, M is a gain factor, TR is the repetition time of the spin echo sequence (400, 800, 1600, or 2400 ms) and T1 is the native T1.
The MRSI voxels were interpolated to 7 x 3.5 x 3.5 mm3 and the data were automatically phase, frequency and baseline corrected using algorithms well-established at our institution [39, 40]. The peak at 2.6 ppm was attributed to citrate. As data were acquired with an endorectal probe coil, sensitivity decreased across the prostate [38]. For this study, no attempts were made to absolutely quantify the citrate for quantitative comparison across subjects. Therefore, the citrate levels in the ROI regions were simply visually categorized as “high” or “low”. This assessment was made based upon comparisons to creatine within the same voxel, with citrate greater than creatine (usually on the order of twice as large) being labeled “high” and citrate lower than or similar to creatine being labeled “low”.
Statistical analyses were performed using JMP V5.1 software (SAS Institute, Cary, NC, USA). Logistic stepwise regression was used to build a statistical model of noncancerous tissue ductal status (highly glandular-ductal tissue versus stromal-low ductal tissue) based upon the parameters DCE MRI peak enhancement and MR diffusion <D>. Forward selection of parameters was used with a probability to enter or leave the model set as 0.1. Regression estimates were based on fitting a linear model to a multilevel logistic response function using an iterative maximum likelihood fit. For additional confirmation of Gd-DTPA behavior in prostate tissues, measurements made in regions of biopsy confirmed cancer and in the one case of PIN were compared to measurements in corresponding normal tissues for those patients.
3. Results
3.1 Pharmacokinetic Simulations
Simulations were performed as described, with the results listed in Table 1. First, the effect of the native T1 was tested with the mean stromal and glandular T1’s (1430 ms and 1590 ms, respectively). These resulted in the relative enhancement of stromal being 96% of the glandular tissues, whereas the study measured an opposite trend, with stromal tissues having 106% the PE of glandular tissues. Therefore, native T1 differences are likely not the source of the PE differences observed.
Table 1.
Simulated peak enhancement, assessing effects of change in T1o, Ktrans, vp and ve.
| Glandular Value | Modified Value | PE - Glandular [%baseline] | PE – Modified [%baseline] | PE - Modified [% PE - Glandular] | |
|---|---|---|---|---|---|
| T1o mean | 1590 ms | 1430 ms (stromal) | 135% | 130% | 96% |
| Ktrans | 0.33 [1/min] | 0.66 [1/min] | 132% | 134% | 102% |
| vp | 0.004 | 0.008 | 132% | 133% | 101% |
| ve | 0.3 | 0.6 | 132% | 142% | 108% |
Formulas and further explanation of values are given in the text.
Next, each parameter of Ktrans, vp and ve were doubled for the stromal tissue versus the glandular tissue, and the effects measured. As shown in Table 1, doubling the ve has a large effect (8% higher) on the peak enhancement whereas doubling the Ktrans or vp has negligible effect (1% or less). Given measurements in central gland tissue and in cancer, stromal tissue would be expected to have lower parameter values than these doubled values whereas cancer may have effects in this range [7,11,12].
An additional consideration is that the stromal and glandular tissues do have different peak times (62 vs 74 sec, respectively, measured at 90% of the peak enhancement). This could lead to the peak enhancements being different, as the arterial input function is different at these two times. However, the modeled plasma concentration (from Eq. 1) is only 1% lower at the later timepoint, so this should not be a significant contributor to the peak enhancement.
In summary, for the typical values of tissue parameters found in the prostate, ve is the primary contributor to changes in PE. Meanwhile, both Ktrans and vp are shown to have a much smaller effect on PE. Additionally, the higher PE found in stromal tissues versus glandular tissues is not due to their native T1’s, as the simulations demonstrated that lower T1’s (as found in stromal tissues vs. glandular tissues) caused a lower PE in contrast to the measured higher PE found in stromal versus glandular tissues.
3.2 Comparisons Across Subjects
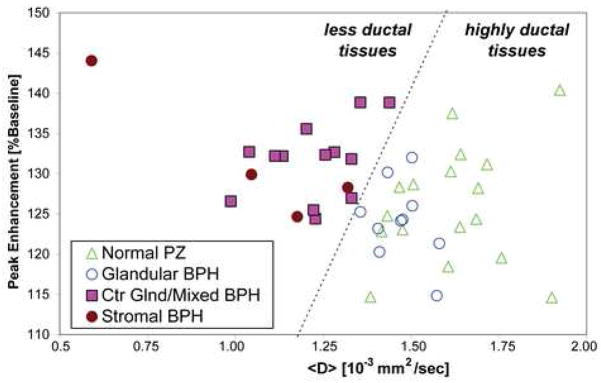
Different DCE MRI and MR <D> patterns are seen in the different tissues, as shown in Figure 1, Figure 2, and Table 2. In Figure 1 the different MR measures for one subject are shown for glandular-ductal tissues (normal peripheral zone and glandular BPH) and stromal-low ductal tissues (stromal BPH and central gland/mixed BPH). Note, the MRSI data shows differences in citrate level between the glandular-ductal tissues and the stromal-low ductal tissues (Figure 1-E.). Figure 2 shows the MR results in another subject demonstrating the different MR measures in peripheral zone cancer. The peak enhancement (PE) values as compared to the <D> values for the different tissues in all subjects are shown in Figure 3. The stromal-low ductal tissues (central gland and stromal BPH) were completely separated from the glandular-ductal tissues (normal peripheral zone and glandular BPH tissues), with the stromal-low ductal tissues having higher PE and lower <D> than the glandular-ductal tissues. Stepwise regression found a model yielding R2 = 1.0 and p< 0.0001, completely separating the tissues, based on the 43 samples in the 17 subjects:
Table 2.
Mean ± std dev. of MR Dav, DCE MRI Peak Enhancement and the Ductal Measure Statistical Model in the glandular-ductal tissues and the stromal-low ductal tissues.
| Tissue (number) | Dav [10−3 mm2/sec] | PE | Ductal Measure: −448 + 669Dav – 10.7PE |
|---|---|---|---|
| Glandular: Normal PZ (17) | 1.616±0.158 | 126±7.2 | 181 ± 115 |
| Glandular: Glandular BPH (9) | 1.472±0.0768 | 124±5.2 | 104 ± 87 |
|
| |||
| Total Glandular-Ductal Tissues (26) | 1.566±0.151 | 125±6.4 | 154 ± 111 |
|
| |||
| Stromal: Central Gland / Mixed BPH (13) | 1.225±0.130 | 132±4.7 | −141 ± 81.0 |
| Stromal: Stromal BPH(4) | 1.033±0.316 | 132±8.5 | −270 ± 298 |
|
| |||
| Total Stromal Low-Ductal Tissues (17) | 1.180±0.196* | 132±5.5** | −171 ± 157*** |
|
| |||
| Cancerous PZ (7) | 1.460±0.101 | 123±5 | 105 ± 92 |
| Cancerous Central Gland (1) | 0.938 | 125 | −260 |
| PIN (1) | 1.252 | 126 | −67 |
PE = peak enhancement, PZ = peripheral zone, BPH = benign prostatic hyperplasia, PIN = prostatic intraepithelial neoplasia
p<0.00000001,
p<0.0008,
p<0.0000000008, t-tests versus glandular-ductal tissues
Figure 3.
Peak Enhancement & MR directionally-averaged apparent diffusion coefficient, <D>, separate highly glandular-ductal tissues from stromal-low ductal tissues. PZ=peripheral zone, BPH=benign prostatic hyperplasia, Ctr Glnd=central gland. The line shows separation of tissue types.
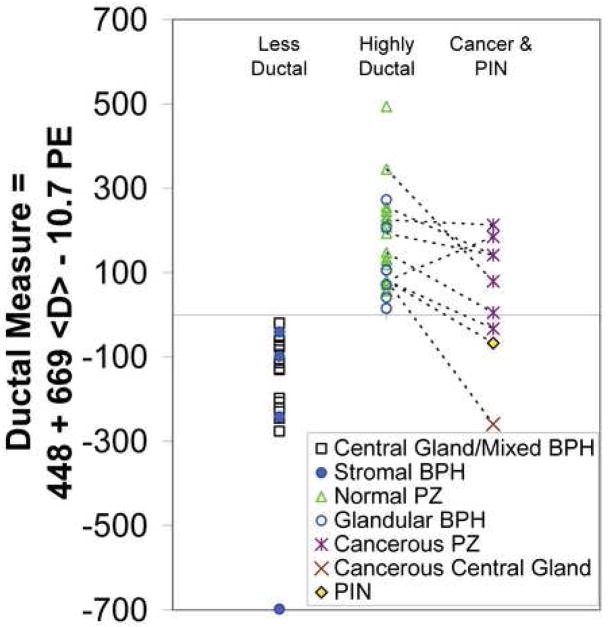
Ductal Measure = 448 + 669 × <D>[sec/10−3mm2] - 10.7 × PE [1/%baseline] This Ductal Measure is unitless and is positive for the highly glandular-ductal tissues and negative for the stromal-low ductal tissues, as shown in Figure 4.
Figure 4.
Plot of the Ductal Measure in the stromal-low ductal, the highly glandular-ductal, and cancerous and PIN tissues. PZ = peripheral zone, BPH = benign prostatic hyperplasia, PIN = prostatic intraepithelial neoplasia. Dotted lines connect normal peripheral zone tissue and cancer or PIN in the same subjects. The one subject with a higher Ductal Measure in cancer versus normal peripheral zone tissue had only a 1 mm focus of cancer by biopsy. Thus, this high Ductal Measure may be in part due to partial voluming with normal peripheral zone tissue.
3.3 Comparisons of Different Tissues Within Subjects
When compared within patients with both central gland / mixed BPH and normal peripheral zone measures, the central gland / mixed BPH ROI had a higher peak (mean ± standard deviation = 104 ± 5% of normal peripheral zone, p<0.02 (paired t-test)) in 12/13 cases and a lower <D> (mean ± standard deviation = 75 ± 11% of normal peripheral zone, p<0.000003, paired t-test) in 13/13 cases versus normal peripheral zone.
Seven subjects had both healthy and cancerous peripheral zone measures. Six of these 7 peripheral zone cancers had lower Ductal Measures than their counterpart normal peripheral zone tissue ROIs, as shown in Figure 4. In other words, peripheral zone cancer was a less glandular-ductal tissue in 6/7 cases (p=0.05, one-tailed, paired t-test). The one central gland cancer showed a negative Ductal Measure, corresponding to a less glandular-ductal tissue. The one case of prostatic intraepithelial neoplasia also yielded a negative Ductal Measure, which was a less glandular-ductal tissue measure than all the peripheral zone cancers. Both the central gland cancer and the region of PIN demonstrated lower Ductal Measures than their corresponding (same subject) normal peripheral zone tissue (see Figure 4).
4. Discussion
4.1 Pharmacokinetic Simulations
The pharmacokinetic simulations demonstrated that, for the typical values of prostate tissue, ve is the primary contributor tot changes in PE. Meanwhile, both Ktrans, and vp are shown to have a much smaller effect on PE. Finally, the native T1 has an inverse effect in stromal versus glandular tissue, as compared to the measured values of PE in these tissues. Thus, the higher PE found in stromal-low ductal tissues and reported in cancer likely indicates these tissues have a larger extracellular space (ve + vp) in which Gd-DTPA can accumulate versus noncancerous, highly glandular-ductal tissues.
4.2 Comparison of Glandular and Stromal Tissues
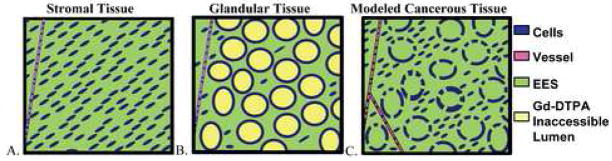
In this study, the stromal-low ductal tissues (central gland tissue and stromal BPH) had lower <D> and higher PE as compared to the highly glandular-ductal tissues (normal peripheral zone and glandular BPH). Combining these two MR measures completely separated the two tissue types. This suggests they have very different underlying structure. Prostate tissue can be modeled as having four compartments: 1) intracellular space, 2) intravascular extracellular space (blood plasma), 3) extravascular extracellular space (interstitium) and 4), glandular-luminal extracellular space. See Figure 5. Stromal-low ductal tissues have primarily the first three (see Figure 5A), whereas highly glandular-ductal tissues have all four (see Figure 5B). Another difference between these tissues, demonstrated by pathology, is that the stromal tissue in the central gland is more dense than in the peripheral zone, i.e. that the extravascular extracellular space surrounding the stromal cells is smaller [30]. These structural differences between stromal-low ductal tissues and highly glandular-ductal tissues can help us interpret the MR differences observed.
Figure 5.
Cartoons demonstrating prostate tissues. A. Stromal Tissue. B. Glandular Tissue. C. Cancerous tissue representative of a Gleason Grade 3 cancer in which cancerous glands have lost their basal cell layer/basement membrane and are infiltrating the stroma. The different compartments in the prostate tissues are: (1) Intracellular space (Blue/ dark grey), (2) Intravascular extracellular space (blood plasma) (pink / medium grey). (3) Extravascular, extracellular space (green / light grey). (4) Glandular-luminal extracellular space (yellow / off-white) – theorized to be inaccessible to Gd-DTPA.
4.3 Hypothesized Gd-DTPA Behavior
In addition to the striking differences observed between stromal and glandular prostate tissues, there are many MR studies showing differences between cancer and normal prostate tissues. There are many DCE MRI studies of the prostate showing a higher peak enhancement (PE) in cancer than in normal tissue [4–12]. Additionally, many MR diffusion studies of prostate cancer have shown a lower apparent diffusion coefficient (ADC) or <D> in prostate cancer versus normal tissue [12, 18–24]. These results would appear to imply that in stromal tissues and cancer: 1) there is more extracellular space (as Gd-DTPA can’t enter the cells) and that 2) there is less extracellular space for water to diffuse (as MR diffusion appears to be dominated by extracellular water when using a b value of 600 sec/mm2, [29]).
The presence of glands and ducts in the normal, glandular prostate offer a likely explanation for these seemingly contradictory interpretations of the peak enhancement increase and <D> decrease in cancer and stromal tissues. In normal, glandular tissues, these glands and ducts are surrounded by an epithelial cell layer, a basal cell layer and a basement membrane. The contrast agent likely cannot penetrate into a gland or duct, as it would need to pass through these layers. Therefore, in normal, glandular tissues, DCE MRI would only reflect a subset of the extracellular space, excluding glands and ducts (see the extracellular intravascular space and the extracellular extravascular space (EES) in Figure 5), leading to a moderate peak enhancement. However, the glands and ducts are large and would contribute to the diffusion measurements, leading to relatively high <D> values for these tissues. The lower <D> observed in cancer may be due to increased cellularity of the cancer, as has been reported in brain tumors [41], and has been suggested in prostate cancer [12]. Cancer is in part described by pathology with the breakdown of the basal cell layer and basement membrane [3,42], implying that Gd-DTPA would be expected to enter such ducts. Thus, the DCE MRI would yield a higher peak enhancement in cancer. Such an explanation of Gd-DTPA behavior in cancer has not been evaluated. However, Liney did suggest that the contrast medium may not reach the citrate-containing lumen in BPH [13]. One study has reported evaluating both DCE MRI and MR diffusion in prostate cancer versus normal prostate tissue. However, this study did not find a significant difference between vees in prostate cancer versus normal peripheral zone cancer nor did they investigate the differences between glandular and stromal prostate tissues [12].
4.4 T1 Effect
The DCE MRI measure of peak enhancement is not just a reflection of space in which Gd-DTPA can accumulate, but also a reflection of the native T1 of the tissue. Thus, the native T1’s of the tissue could be confounding the peak enhancements and interpretation of the data in this study. As a 2D sequence with a poor uniformity slice profile was used for the DCE MRI data, there was an inability to establish native T1’s for all of the ROIs. However, a separate T1 measurement sequence was performed on 12 of these patients, as reported elsewhere [4]. The T1’s found were variable. The average T1 in the highly glandular-ductal tissues (1590 ms) was not significantly different than the T1 of the stromal-low ductal tissues (1430 ms). Median T1’s of these tissues had the opposite trend, 1540 ms vs. 1650 ms [4]. Additionally, T1-weighted images provide poor discrimination of prostatic tissues [43]. Additionally, other researchers have not seen significant differences among normal, BPH and cancerous prostate tissue T1 values [11,44]. Thus the effect of T1 on peak enhancements is likely small. As the acquisition sequence used for DCE MRI in this study does not provide a simple relationship between signal intensity and T1, pharmacokinetic modeling was not performed, which could eliminate or reduce the effect of the native T1. To confirm that the native T1s of the stromal and glandular tissues are not a major cause of the peak enhancements measured, the peak enhancement was simulated for the mean T1 values in stromal and glandular BPH, as described in the Pharmacokinetic Simulations Sections.
4.5 MR Diffusion
MR diffusion measures the movement of water. As such, it can measure water in any location. However, the timings and sizes of the diffusion gradients in the pulse sequence determine the distances water needs to move to be measured as a signal drop due to diffusion, with larger b-values being sensitive to shorter movement distances. Studies with different b-values have demonstrated the presence of two water diffusion components with different apparent diffusion coefficients when scanning at large b-values (such as b = 1000) mm2/sec), but only a single component at lower b-values (such as b = 600 mm2/sec). This has been suggested to indicate that the higher b-values measured both intra- and extracellular water whereas the lower b-value only measured extracellular water [29]. Therefore, the <D> measured in this study (from b = 0 and b = 600 mm2/sec) likely reflects a measurement of extracellular water, with lower values indicating smaller extracellular space. Thus, the lower <D> in stromal/low ductal tissues and cancer likely indicates a smaller extracellular space.
4.6 Measurements in Cancer
Cancer typically (6/7 patients) yielded lower Ductal Measures than corresponding normal peripheral zone tissue for the patients (see Figure 4). The subject with a higher Ductal Measure in cancer than in normal peripheral zone tissue had only a 1mm focus of cancer by biopsy. There was variability in the <D>, peak enhancement, and Ductal Measures across the patients. As higher grade cancers have less intact ducts and smaller ducts, the Ductal Measure parameter may yield clinically relevant information about the cancer’s aggressiveness. In this study, cancers were primarily Gleason Score 3+3 and were only confirmed by biopsy. The subjects had received no treatment and had only small volumes of cancer (median size identified in biopsy cores ~ 2 mm/patient). The extent of partial voluming of cancer and normal tissue is not known, but undoubtedly exists and contributes to the variability observed in this study. The methods described here will need to be investigated in a broader range of Gleason Score cancers and compared to step section histopathology of the excised prostate gland to evaluate how well DCE MRI with MR <D> can characterize prostate cancer and if it can indicate differences in ductal volumes.
4.7 Observation of PIN
Prostatic intraepithelial neoplasia (PIN) represents dysplastic cellular proliferation within prostatic ducts and acini [42]. The one case of PIN measured in this study shows similar peak enhancement as normal peripheral zone tissue but decreased <D>, consistent with the facts that the ducts in PIN are generally intact (very high grade PIN may have some basal cell layer disruption) and that these intact ducts have less fluid [42]. It must be noted that this case of PIN was only confirmed by biopsy, not by aligned step-section histopathology, so it is possible that the measured location does not match the actual location of PIN. However, this measurement is only given as anecdotal, additional example of the MR behavior observed in this study.
4.8 Variability in Measurements
There was a spread of values, both peak enhancement and <D>, for each tissue type in this study. In addition to actual individual variation, this may be due in part to partial volume inclusions of adjacent tissues. The central gland measurements likely contained BPH, primarily of stromal or mixed tissue types, as it is pervasive in this elderly male population. Nodules of pure glandular BPH were avoided, due to their bright appearance on the T2-weighted images. The remaining mixture of central gland tissue, stromal BPH and some mixed BPH is likely primarily low in prostatic ducts, so this, albeit very mixed, tissue was still used for comparing highly glandular-ductal to stromal-low ductal tissues. Some of the spread of values may be due to this heterogeneous tissue. There may also be variability within the normal peripheral zone ROIs due to the potential presence of undetected, microscopic foci of cancer. Additionally, the spread of values varied among the tissue types, which may be due to: 1) different sizes of the ROIs in the different tissue types, 2) different heterogeneity within the tissue types, 3) different subjects used to determine the averaged values, due to varying presence of some tissue types, and / or 4) errors in the motion correction. Additionally, it is possible that the ROIs were mislabeled as an accurate correlation to a step-section histopathological assessment of the tissues was not available for these subjects. However, MRI and MRSI are good at discriminating tissues, so this is likely not common. The motion correction algorithm had limitations; visual assessments were made and only in-plane motion correction shifts were determined and applied. Rotations and out-of-plane motion on the scale of the slice thickness were not observed, however. Additionally, no interpolation was applied to correct for shifts less than the voxel size. While some errors in ROI homogeneity may exist, the results obtained are very striking and persist, even with potential partial volume averaging and/or mislabeling of tissues.
5. Conclusion
In conclusion, DCE MRI PE and <D> are different between highly glandular-ductal tissues and stromal-low ductal tissues and a statistical model combination of <D> and PE provides excellent separation of these tissues. This suggests that these tissues may have very different underlying structure. The extracellular space in the prostate can be modeled as Gd-DTPA accessible and Gd-DTPA inaccessible. As <D> at b = 600 is primarily affected by the extracellular spaces and would thus reflect both spaces, the decrease in <D> observed in stromal low ductal tissues and reported in cancer versus glandular tissues indicates a lower extracellular space (and higher intracellular space). As PE in the prostate is primarily affected by extracellular space, it would be expected to be lower in these tissues. As PE is in fact higher in stromal-low ductal tissues versus highly glandular-ductal tissues, the noncancerous, highly glandular-ductal tissues appear to contain Gd-DTPA inaccessible space – likely the basement membrane surrounded glands and ducts. The higher PE observed in cancer suggests some cancerous glands may be Gd-DTPA accessible. These results indicate that Gd-DTPA likely does not enter healthy prostatic glands or ducts, but may enter cancerous glands and ducts. This provides important information for interpreting DCE MRI results in the prostate. The combination of DCE MRI peak enhancement and MR <D> can discriminate highly glandular-ductal tissues from stromal-low ductal prostatic tissues and is a promising measure for characterizing prostatic tissues and thus, may aid in the identification and assessment of the aggressiveness of prostate cancer.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57(1):43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 2.Gleason D. Histologic Grading of Prostatic Carcinoma. In: Bostwick D, editor. Pathology of the Prostate. New York: Churchill Livingstone; 1990. [Google Scholar]
- 3.Fuchs M, Brawer M, Rennels M, Nagle R. The relationship of basement membrane to histologic grade of human prostatic carcinoma. Mod Pathol. 1989;2:105–11. [PubMed] [Google Scholar]
- 4.Noworolski SM, Henry RG, Vigneron DB, Kurhanewicz J. Dynamic contrast-enhanced MRI in normal and abnormal prostate tissues as defined by biopsy, MRI, and 3D MRSI. Magn Reson Med. 2005;53(2):249–255. doi: 10.1002/mrm.20374. [DOI] [PubMed] [Google Scholar]
- 5.Engelbrecht MR, Huisman HJ, Laheij RJ, Jager GJ, van Leenders GJ, Hulsbergen-Van De Kaa CA, de la Rosette JJ, Blickman JG, Barentsz JO. Discrimination of prostate cancer from normal peripheral zone and central gland tissue by using dynamic contrast-enhanced MR imaging. Radiology. 2003;229:248–54. doi: 10.1148/radiol.2291020200. [DOI] [PubMed] [Google Scholar]
- 6.Rouviere O, Raudrant A, Ecochard R, Colin-Pangaud C, Pasquiou C, Bouvier R, Marechal JM, Lyonnet D. Characterization of time-enhancement curves of benign and malignant prostate tissue at dynamic MR imaging. Eur Radiol. 2003;13:931–42. doi: 10.1007/s00330-002-1617-6. [DOI] [PubMed] [Google Scholar]
- 7.Padhani AR, Gapinski CJ, Macvicar DA, Parker GJ, Suckling J, Revell PB, Leach MO, Dearnaley DP, Husband JE. Dynamic contrast enhanced MRI of prostate cancer: correlation with morphology and tumour stage, histological grade and PSA. Clin Radiol. 2000;55(2):99–109. doi: 10.1053/crad.1999.0327. [DOI] [PubMed] [Google Scholar]
- 8.Futterer JJ, Scheenen TW, Huisman HJ, Klomp DW, van Dorsten FA, Hulsbergen-van de Kaa CA, Witjes JA, Heerschap A, Barentsz JO. Initial experience of 3 tesla endorectal coil magnetic resonance imaging and 1H-spectroscopic imaging of the prostate. Invest Radiol. 2004;39(11):671–680. doi: 10.1097/00004424-200411000-00005. [DOI] [PubMed] [Google Scholar]
- 9.Ito H, Kamoi K, Yokoyama K, Yamada K, Nishimura T. Visualization of prostate cancer using dynamic contrast-enhanced MRI: comparison with transrectal power Doppler ultrasound. Br J Radiol. 2003;76(909):617–624. doi: 10.1259/bjr/52526261. [DOI] [PubMed] [Google Scholar]
- 10.Preziosi P, Orlacchio A, Di Giambattista G, Di Renzi P, Bortolotti L, Fabiano A, Cruciani E, Pasqualetti P. Enhancement patterns of prostate cancer in dynamic MRI. Eur Radiol. 2003;13(5):925–930. doi: 10.1007/s00330-002-1703-9. [DOI] [PubMed] [Google Scholar]
- 11.Buckley DL, Roberts C, Parker GJ, Logue JP, Hutchinson CE. Prostate cancer: evaluation of vascular characteristics with dynamic contrast-enhanced T1-weighted MR imaging--initial experience. Radiology. 2004;233(3):709–715. doi: 10.1148/radiol.2333032098. [DOI] [PubMed] [Google Scholar]
- 12.Kozlowski P, Chang SD, Jones EC, Berean KW, Chen H, Goldenberg SL. Combined diffusion-weighted and dynamic contrast-enhanced MRI for prostate cancer diagnosis--correlation with biopsy and histopathology. J Magn Reson Imaging. 2006;24(1):108–113. doi: 10.1002/jmri.20626. [DOI] [PubMed] [Google Scholar]
- 13.Liney GP, Turnbull LW, Knowles AJ. In vivo magnetic resonance spectroscopy and dynamic contrast enhanced imaging of the prostate gland. NMR Biomed. 1999;12:39–44. doi: 10.1002/(sici)1099-1492(199902)12:1<39::aid-nbm543>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 14.Barentsz JO, Engelbrecht M, Jager GJ, Witjes JA, de LaRosette J, van Der Sanden BP, Huisman HJ, Heerschap A. Fast dynamic gadolinium-enhanced MR imaging of urinary bladder and prostate cancer. Journal of Magnetic Resonance Imaging. 1999;10(3):295–304. doi: 10.1002/(sici)1522-2586(199909)10:3<295::aid-jmri10>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 15.Jager GJ, Ruijter ET, van de Kaa CA, de la Rosette JJ, Oosterhof GO, Thornbury JR, Barentsz JO. Local staging of prostate cancer with endorectal MR imaging: correlation with histopathology. Ajr American Journal of Roentgenology. 1996;166(4):845–852. doi: 10.2214/ajr.166.4.8610561. [DOI] [PubMed] [Google Scholar]
- 16.Jager GJ, Ruijter ET, van de Kaa CA, de la Rosette JJ, Oosterhof GO, Thornbury JR, Ruijs SH, Barentsz JO. Dynamic TurboFLASH subtraction technique for contrast-enhanced MR imaging of the prostate: correlation with histopathologic results. Radiology. 1997;203(3):645–652. doi: 10.1148/radiology.203.3.9169683. [DOI] [PubMed] [Google Scholar]
- 17.Turnbull LW, Buckley DL, Turnbull LS, Liney GP, Knowles AJ. Differentiation of prostatic carcinoma and benign prostatic hyperplasia: correlation between dynamic Gd-DTPA-enhanced MR imaging and histopathology. J Magn Reson Imaging. 1999;9:311–6. doi: 10.1002/(sici)1522-2586(199902)9:2<311::aid-jmri24>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 18.Issa B. In vivo measurement of the apparent diffusion coefficient in normal and malignant prostatic tissues using echo-planar imaging. J Magn Reson Imaging. 2002;16:196–200. doi: 10.1002/jmri.10139. [DOI] [PubMed] [Google Scholar]
- 19.Sato C, Naganawa S, Nakamura T, Kumada H, Miura S, Takizawa O, Ishigaki T. Differentiation of noncancerous tissue and cancer lesions by apparent diffusion coefficient values in transition and peripheral zones of the prostate. J Magn Reson Imaging. 2005;21:258–62. doi: 10.1002/jmri.20251. [DOI] [PubMed] [Google Scholar]
- 20.Hosseinzadeh K, Schwarz SD. Endorectal diffusion-weighted imaging in prostate cancer to differentiate malignant and benign peripheral zone tissue. J Magn Reson Imaging. 2004;20:654–61. doi: 10.1002/jmri.20159. [DOI] [PubMed] [Google Scholar]
- 21.Vigneron D, Xu D, Chen A, Swanson M, Kurhanewicz J. Diffusion Tensor Imaging of the Prostate using Single-Shot Fast Spin Echo. Proc Intl Soc Magn Reson Med. 2002:457. [Google Scholar]
- 22.Pickles MD, Gibbs P, Sreenivas M, Turnbull LW. Diffusion-weighted imaging of normal and malignant prostate tissue at 3.0T. J Magn Reson Imaging. 2006;23(2):130–134. doi: 10.1002/jmri.20477. [DOI] [PubMed] [Google Scholar]
- 23.Manenti G, Squillaci E, Di Roma M, Carlani M, Mancino S, Simonetti G. In vivo measurement of the apparent diffusion coefficient in normal and malignant prostatic tissue using thin-slice echo-planar imaging. Radiol Med (Torino) 2006;111(8):1124–1133. doi: 10.1007/s11547-006-0110-8. [DOI] [PubMed] [Google Scholar]
- 24.Desouza NM, Reinsberg SA, Scurr ED, Brewster JM, Payne GS. Magnetic resonance imaging in prostate cancer: value of apparent diffusion coefficients for identifying malignant nodules. Br J Radiol. 2007;80(950):90–5. doi: 10.1259/bjr/24232319. [DOI] [PubMed] [Google Scholar]
- 25.Tofts PS, Kermode AG. Measurement of the blood-brain barrier permeability and leakage space using dynamic MR imaging. 1. Fundamental concepts. Magn Reson Med. 1991;17(2):357–367. doi: 10.1002/mrm.1910170208. [DOI] [PubMed] [Google Scholar]
- 26.Tofts PS. Modeling tracer kinetics in dynamic Gd-DTPA MR imaging. J Magn Reson Imaging. 1997;7(1):91–101. doi: 10.1002/jmri.1880070113. [DOI] [PubMed] [Google Scholar]
- 27.Tofts PS, Brix G, Buckley DL, Evelhoch JL, Henderson E, Knopp MV, Larsson HB, Lee TY, Mayr NA, Parker GJ, Port RE, Taylor J, Weisskoff RM. Estimating kinetic parameters from dynamic contrast-enhanced T(1)-weighted MRI of a diffusable tracer: standardized quantities and symbols. J Magn Reson Imaging. 1999;10(3):223–232. doi: 10.1002/(sici)1522-2586(199909)10:3<223::aid-jmri2>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 28.Norris DG. The effects of microscopic tissue parameters on the diffusion weighted magnetic resonance imaging experiment. NMR Biomed. 2001;14:77–93. doi: 10.1002/nbm.682. [DOI] [PubMed] [Google Scholar]
- 29.Mulkern RV, Barnes AS, Haker SJ, Hung YP, Rybicki FJ, Maier SE, Tempany CM. Biexponential characterization of prostate tissue water diffusion decay curves over an extended b-factor range. Magn Reson Imaging. 2006;24(5):563–568. doi: 10.1016/j.mri.2005.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McNeal J, Bostwick D. Anatomy of the Prostate: Implications for Disease. In: Bostwick D, editor. Pathology of the Prostate. Volume 15, Contemporary Issues in Surgical Pathology. New York: Churchill Livingstone; 1990. [Google Scholar]
- 31.Grossfeld G, Coakley F. Benign Prostatic Hyperplasia: Clinical Overview and Value of Diagnostic Imaging. In: Hricak H, Carroll P, editors. The Prostate Gland: A Clinically Relevant Approach to Imaging. Volume 38, The Radiologic Clinics of North America. Philadelphia: WB Saunders Company; 2000. [DOI] [PubMed] [Google Scholar]
- 32.Kurhanewicz J, Vigneron DB, Nelson SJ, Hricak H, MacDonald JM, Konety B, Narayan P. Citrate as an in vivo marker to discriminate prostate cancer from benign prostatic hyperplasia and normal prostate peripheral zone: detection via localized proton spectroscopy. Urology. 1995;45:459–66. doi: 10.1016/S0090-4295(99)80016-8. [DOI] [PubMed] [Google Scholar]
- 33.Kurhanewicz J, Swanson M, Nelson SJ, Vigneron D. Combined Magnetic Resonance Imaging and Spectroscopic Imaging Approach to Molecular Imaging of Prostate Cancer. J Magn Reson Imaging. 2002;16:451–463. doi: 10.1002/jmri.10172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schricker AA, Pauly JM, Kurhanewicz J, Swanson MG, Vigneron DB. Dualband Spectral-Spatial RF Pulses for Prostate MR Spectroscopic Imaging. Magn Reson in Med. 2001;46:1079–1087. doi: 10.1002/mrm.1302. [DOI] [PubMed] [Google Scholar]
- 35.Alsop DC. Phase insensitive preparation of single-shot RARE: application to diffusion imaging in humans. Magn Reson Med. 1997;38(4):527–533. doi: 10.1002/mrm.1910380404. [DOI] [PubMed] [Google Scholar]
- 36.Xu D, Henry RG, Mukherjee P, Carvajal L, Miller SP, Barkovich AJ, Vigneron DB. Single-shot fast spin-echo diffusion tensor imaging of the brain and spine with head and phased array coils at 1.5 T and 3.0 T. Magn Reson Imaging. 2004;22(6):751–759. doi: 10.1016/j.mri.2004.01.075. [DOI] [PubMed] [Google Scholar]
- 37.Noworolski SM, Fischbein NJ, Kaplan MJ, Lu Y, Nelson SJ, Carvajal L, Henry RG. Challenges in Dynamic Contrast-Enhanced MR Imaging of Cervical Lymph Nodes to Detect Metastatic Disease. J Magn Reson Imaging. 2003;17:455–462. doi: 10.1002/jmri.10280. [DOI] [PubMed] [Google Scholar]
- 38.Noworolski SM. High Spatial Resolution Magnetic Resonance Imaging and Spectroscopy Using Surface Coils [Ph.D.] San Francisco and Berkeley: The University of California at San Francisco and the University of California at Berkeley; 1999. p. 326. [Google Scholar]
- 39.Nelson S, Brown T. A new method for automatic quantifcation of 1-D spectra with low signal to noise ratio. J Magn Reson Imaging. 1987;84:95–109. [Google Scholar]
- 40.Nelson SJ. Analysis of volume MRI and MR spectroscopic imaging data for the evaluation of patients with brain tumors. Magn Reson Med. 2001;46(2):228–239. doi: 10.1002/mrm.1183. [DOI] [PubMed] [Google Scholar]
- 41.Lyng H, Haraldseth O, Rofsttad EK. Measurement of cell density and necrotic fraction in human melanoma xenografts by diffusion weighted magnetic resonance imaging. Magn Reson Med. 2000;43:828–836. doi: 10.1002/1522-2594(200006)43:6<828::aid-mrm8>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 42.Bostwick DG, Srigley JR. Premalignant Lesions. In: Bostwick D, editor. Pathology of the Prostate. New York: Churchill Livingstone; 1990. [Google Scholar]
- 43.Hricak H. The prostate gland. In: Hricak H, Carrington B, editors. MRI of the pelvis. London: Martin Dunitz; 1991. pp. 249–311. [Google Scholar]
- 44.Kjaer L, Thomsen C, Iversen P, Henriksen O. In vivo estimation of relaxation processes in benign hyperplasia and carcinoma of the prostate gland by magnetic resonance imaging. Magn Reson Imaging. 1987;5(1):23–30. doi: 10.1016/0730-725x(87)90480-2. [DOI] [PubMed] [Google Scholar]