Summary
A deficient extinction of memory is particularly important in the regime of fear, where it limits the beneficial outcomes of treatments of anxiety disorders. Fear extinction is thought to involve inhibitory influences of the prefrontal cortex on the amygdala, although the detailed synaptic mechanisms remain unknown. Here we report that neuropeptide S (NPS), a recently discovered transmitter of ascending brainstem neurons, evokes anxiolytic effects and facilitates extinction of conditioned fear responses when administered into the amygdala in mice. An NPS receptor antagonist exerts functionally opposing responses, indicating that endogenous NPS is involved in anxiety behavior and extinction. Cellularly, NPS increases glutamatergic transmission to intercalated GABAergic neurons in the amygdala via presynaptic NPS receptors on connected principal neurons. These results identify mechanisms of NPS in the brain, a key role of intercalated neurons in the amygdala for fear extinction, and a potential pharmacological avenue for treating anxiety disorders.
Introduction
Anxiety disorders are common diseases with a lifetime prevalence of up to 25 % (Kessler et al., 2005). For the development of therapeutic avenues it is of critical importance to identify the neural circuitries and mechanisms of neurotransmission mediating fear acquisition and, perhaps even more clinically important, fear subsidence. One established experimental paradigm to study these processes is Pavlovian fear conditioning, in which cues paired with aversive outcomes come to elicit typical fear responses, and in which the organism learns to predict danger in their environment (LeDoux, 2000). When conditioned cues no longer predict danger, as can be experimentally modelled through repetitive presentations of non-reinforced cues, fear responses decline: a behavioral phenomenon known as extinction (Maren and Quirk, 2004). Substantial evidence indicates that extinction involves new learning which inhibits the expression of conditioned fear rather than erases the fear memory (Maren and Quirk, 2004; Bouton et al., 2006; Myers and Davis, 2007). In fact, fear responses can spontaneously recover with the passage of time, be reinstated by the reinforcer alone or be renewed in a context-dependent manner (Maren and Quirk, 2004). This balance between fear memory consolidation and extinction has important clinical relevance, in that it severely limits the beneficial outcomes of current treatments of anxiety disorders, such as panic and posttraumatic stress disorders. Studies in both animals (Paré et al., 2004; Maren and Quirk, 2004) and humans (Phelps and LeDoux, 2005), have indicated that interactions between the infralimbic region (IL) of the medial prefrontal cortex (mPFC) and the amygdala are critically involved in the consolidation of extinction learning. One intriguing possibility is that the IL exerts an inhibitory control over signal processing in amygdaloid circuits via GABAergic neuronal populations (Paré et al., 2004). Two major populations of GABAergic neurons can be discerned in the amygdala: “local” GABAergic interneurons scattered in the local neuropil, and paracapsular GABAergic intercalated cell masses. The paracapsular intercalated cell masses are organized in two clusters: one cluster (the lateral subdivision, lpara) is located along the external capsule, while a second cluster (the medial subdivision, mpara) is located at the border between the basolateral amygdaloid complex (BLA) and the central amygdaloid nucleus (CeA). The lpara neurons mostly enable feedforward control of signal flow from cortex to the BLA (Marowsky et al., 2005), while the mpara neurons provide a feedforward inhibitory gate for signals between BLA and CeA, and thereby between the major input and output station of the amygdala (Royer et al., 1999). In particular the GABAergic intercalated cells have been suggested prime candidates for mediating mPFC influences during extinction, although the case rests on indirect evidence only (as reviewed by Paré et al., 2004). While formation of new memory represents the prevailing model of fear extinction, it does not rule out the possibility that multiple mechanisms underlie extinction of consolidated memory, as for instance, erasure of conditioned fear through synaptic depotentiation (Myers et al., 2006; Kim et al., 2007). Of the various transmitter systems controlling synaptic interactions within the amygdala, the implication of endocannabioids in extinction of conditioned fear has been well-established (Marsicano et al., 2002; Lutz, 2007), although information about the neuronal targets and synaptic network mechanisms mediating the fear alleviating effects is sparse to date.
In this respect it is of particular interest to note that mRNA for receptors of neuropetide S (NPS), a recently discovered transmitter with anxiolytic-like effects, displays a specific expression pattern within the rat amygdala, with high levels occurring in and around the intercalated cell masses (Xu et al., 2007). NPS is a neuropeptide consisting of 20 amino acids with serine as the amino-terminal residue (Xu et al., 2004), is highly conserved in different vertebrate species, including humans (Reinscheid 2007), originates from a cluster of cells in the brainstem between the locus coeruleus and Barrington´s nucleus, and produces robust anxiolytic effects when administered intracerebroventricularly to mice in various tests of generalized anxiety (Xu et al., 2004).
These findings prompted us to examine the mechanisms of action of NPS in the amygdala in relation to anxious behavior, fear acquisition and extinction. We have combined behavioral studies in mice and electrophysiological in vitro experiments in amygdala slice preparations making use of GAD67-EGFP knock-in mice, a transgenic mouse strain, in which EGFP was used as a reporter gene to tag GAD67-expressing neurons (Tamamaki et al., 2003). The data demonstrate that endogenous NPS in the amygdala acts to reduce general anxiety and to facilitate the extinction of conditioned fear responses, through mechanisms involving a subpopulation of intercalated GABAergic neurons in the amygdala, and thereby pave the way for novel pharmacological avenues in the control of fear acquisition and extinction.
Results
Anxiolytic-like effects of NPS in the amygdala
The first series of experiments aimed at identifying the effects of NPS in the amygdala on anxious behavior. NPS was locally infused in small volumes bilaterally in the amygdala, with histologically verified injection sites centered in the lateral amygdala (LA) and basolateral (BLA) amygdaloid complex (Figure S1). Anxiety was tested 20 minutes after termination of NPS application via the open field and elevated plus maze test. Data are illustrated in Figure 1. NPS-treated mice displayed a reduction in anxious behavior in both the open field test (n = 9; Figure 1A) and the elevated plus maze (n = 7; Figure 1B) when compared to saline-injected controls (n = 6 for each test), as evidenced by the significant increase in visits and distances covered in the centre, and the significant increase and decrease in time spent on open and closed arms, respectively. In order to investigate the possible involvement of endogenous NPS in anxiety behavior, the NPS receptor antagonist SHA 68 (Okamura et al., 2008) was locally injected into the amygdala, using protocols as for NPS. Compared to vehicle-injected controls (Cremophor-PBS; n = 6), SHA 68 produced a significant anxiogenic response in mice tested in the open field (n = 6; Figure 1A). Importantly, locomotor activity and distance covered at the border were not different between the different pharmacological groups, and Cremophor-PBS controls did not differ in any of the tested behavior from saline-injected controls. In separate groups of animals the time course of the effect of NPS on general anxiety was tested in the elevated plus maze. The anxiolytic responses were significant 20 minutes after application of NPS (n = 7), while behavioral activity had returned to control level within 2 hours (n = 4) and 4 hours (n = 4) thereafter (Figure S3). Total locomotor activities were not significantly different between groups at any time. Finally, NPS-treated animals displayed anxiolytic-like behavior in the dark-light test (Figure S4). Overall, these results provide evidence that endogenous NPS in the amygdala, more specifically in the LA/BLA region, induces a reduction in general anxiety, thereby separating anxiolytic from hyperlocomotor effects that have been observed after intracerebroventricular injections (Xu et al., 2004).
Figure 1. Effects of NPS in the LA/BLA on generalized anxiety behavior in the open field and elevated plus maze.
(A) Representative examples of locomotor paths, diagrams of averaged visits of the central zone, time spent in centre, distances in the centre and in the border field, and total locomotor activity of NPS-, SHA 68-, and saline-treated mice in open field. (B) Representative examples of locomotor paths (open arms in vertical direction), and averaged entries into open/closed arms and time spent on open/closed arms of NPS- and saline-treated mice in an elevated plus maze. Note the significantly increased number of visits of the central zone, time spent in centre and distance in centre (A) and of entries into opens arms and time spent on open arms (B) of NPS-treated mice compared to saline controls, while locomotor activity was similar in both groups and in both behavioral tests (data not shown for EPM-test). SHA 68-treated mice showed significantly reduced (anxiogenic-like) responses in the open field. Behavioral data were obtained 20 minutes after bilateral injection of NPS (0.5 µl, 10 µM), SHA 68 (0.5 µl, 10 µM), NaCl and Cremophor-PBS buffer, respectively. Values are mean ± SEM; *, significantly different from the control group (p < 0.05).
NPS in the amygdala facilitates extinction of conditioned fear
The effects of NPS in the amygdala on conditioned fear and extinction were investigated next. Mice were fear-conditioned in an auditory fear conditioning paradigm, and responses to the conditioned (CS+) and neutral (CS−) tone were determined to assess fear memory. The conditioned response generally took a graduated development of different behavioral components, and in accordance with previous observations (Laxmi et al. 2003), we considered freezing as one adequate behavioral response indicating fear in different test situations. Twenty-four hours after fear training, retrieval of conditioned fear was analyzed (R1), followed by fear extinction training through successive presentation of the non-reinforced cues during 5 retrieval sessions (R2–R6). Twenty-four hours later, extinction recall was tested in two successive sessions (E1, E2), followed by renewal of conditioned fear through exposure to the conditioning context (RN). NPS was injected at different time points during the fear training protocol, in order to distinguish between effects on fear acquisition, and extinction. NPS-treated animals were compared with controls that had received saline injections at matching time points. Freezing responses upon CS+ presentation were statistically analyzed using the Mann-Whitney U-test for single test session (R1–R6, E1, E2, RN, compared to saline controls) and the Wilcoxon test for within group differences between the test sessions. Data are illustrated in Figure 2. Saline controls (n = 15, in two different groups) displayed a high level of conditioned freezing responses upon CS+ presentation during the first retrieval session (R1), and a continuous decline during non-reinforced CS+ exposure during subsequent retrieval sessions (R2–R6), indicating fear extinction (Figure 2A and B dashed line). Differences to R1 (Wilcoxon-test) became significant at R4 in the different groups and persisted throughout extinction training (p < 0.05). Consolidation of extinction was observed during recall 24 hours later (E1, E2) (p < 0.05), and re-exposure to the conditioning context resulted in a significant increase in freezing responses to CS+ compared to E2 (p < 0.05), indicating renewal of fear memory (RN). Next, fear training was performed 20 minutes after local infusion of NPS bilaterally into the LA/BLA, at times coinciding with anxiolytic-like effects of the drug. Conditioned fear responses, fear extinction, extinction recall and renewal of fear memory were undistinguishable from those observed in saline-injected control animals (n = 6 each group; Figure 2A). A similar lack of effect of NPS was observed upon application one hour after training during a period of fear memory consolidation (n = 3; data not shown). When NPS was injected 2 hours before fear memory retrieval (Figure 2B, n = 7), animals displayed unaltered freezing responses to CS+ presentations during the first retrieval session (R1), but an acceleration of fear extinction compared to controls (n = 6) upon presentation of the non-reinforced cues during subsequent retrieval sessions with significantly reduced freezing at R3 (p < 0.05). Application of NPS 20 minutes prior to R1 resulted in a significant reduction in freezing responses at R1 (p < 0.05), R2 (p < 0.05), R3 (p < 0.01), and the acute anxiolytic-like action could not be discerned from effects on fear extinction (n = 6; data not shown). A facilitatory effect of NPS on fear extinction became evident during extinction recall, where NPS-treated animals displayed significantly reduced freezing responses compared to saline-treated controls (E1 and E2; p < 0.05), while re-exposure to the conditioning context resulted in a renewed fear response with no differences between groups of animals. Finally, application of NPS after extinction training (20 minutes post R6; n = 5, data not shown) had no significant effect on extinction recall nor renewal of conditioned fear responses. Freezing in response to CS− presentation was at a low level at the various retrieval sessions in all groups (see Laxmi et al., 2003), and a significant effect of NPS was not observed.
Figure 2. Effects of NPS in the LA/BLA during retrieval and extinction of conditioned fear.
Averages of relative freezing duration upon CS+ presentation during retrieval sessions (R1–R6), extinction (E1, E1), and renewal (RN). Diagrams represent effect of bilateral injections into LA/BLA of (A) NPS and NaCl 20 minutes before training, and (B) NPS, SHA 68 and NaCl two hours before retrieval session 1. Data are mean ± S.E.M. Asterisks indicate differences in freezing in a respective session after administration of NPS or SHA 68 compared to saline (*, p < 0.05; **, p < 0.01).
In order to investigate the possible involvement of endogenous NPS in the amygdala in conditioned fear responses, we locally injected the NPS receptor antagonist SHA 68 (Okamura et al., 2008) into the LA/BLA region at times that had been proven relevant with respect to NPS action. Upon injection of SHA 68 at 2 hours before fear memory retrieval (Figure 2B, filled circles, n = 7), animals displayed high freezing responses to CS+ presentations throughout extinction training, with significant differences compared to vehicle-injected controls from R4 to E2 (Mann-Whitney U-test; p < 0.05). Mice injected with the solvent Cremophor-PBS at 2 hours before R1 (n = 3) showed undistinguishable freezing responses from those in saline-injected controls (data not shown). Noteworthy, conditioned fear behavior and extinction were undistinguishable in the three saline-injected groups at the three different time points (20 minutes prior to fear training, 20 minutes and 2 hours prior to R1; n = 20 total) and the Cremophor-PBS-injected controls (n = 3). Behavioral experiments in the different pharmacological groups were performed largely in parallel and included mates of the same litters, thereby minimizing the impact of uncontrolled variables.
NPS enhances synaptic transmission at glutamatergic synapses on paracapsular GABAergic cells
The next series of experiments aimed at identifying the mechanisms of action of NPS in the synaptic network of the LA/BLA complex and paracapsular GABAergic intercalated cells, based upon our behavioral data and the high expression level of NPS receptors in that area of the amygdala (Xu et al., 2007). In slice preparations of the amygdala from GAD67-EGFP mice (Tamamaki et al., 2003), whole-cell recordings were obtained from LA principal neurons (LA PN) and paracapsular GABAergic intercalated cells (para IN). The paracapsular GABAergic intercalated cells were readily discernible in coronal slices as clusters of densely packed EGFP-labelled cells located at the lateral border of the LA along the external capsule (lateral cluster, lpara) and at medial sites facing the CeA (medial cluster, mpara; Figure S5). Furthermore, scattered EGFP-labelled neurons were present within the LA, most likely representing local GABAergic interneurons (LA IN; Figure S5). The different neuronal cell types were separated by their electrophysiological properties in current-clamp recordings.(Figure S5).
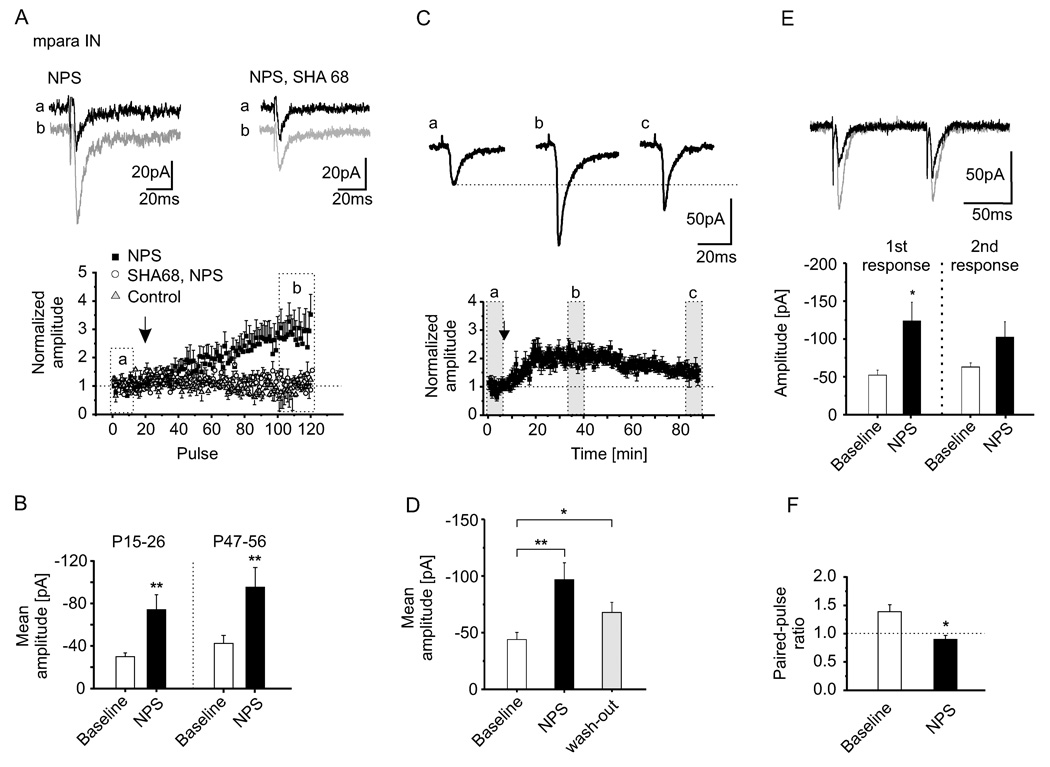
Next, glutamatergic synaptic transmission was evoked in mpara IN by extracellular stimulation within the LA in the presence of 100 µM picrotoxin and 50 µM AP5. Bath-application of NPS (10 µM) resulted in an enhancement of glutamate-receptor mediated EPSCs, as illustrated by the representative traces in Figure 3A. The mean amplitudes of EPSCs in para IN were significantly increased from −30 ± 4 pA to −74 ± 14 pA (n = 13) after NPS application (Figure 3B, n = 13; p < 0.01) in slices obtained from mice at postnatal ages P15–26. In slices from mice of the same developmental state (P47–56) as in behavioral testing, NPS application similarly increased the mean amplitudes of evoked EPSCs in mpara IN from −43 ± 8 pA to −96 ± 19 pA (Figure 3B, n = 12; p < 0.01). The effect of NPS was near maximal at concentrations 10–50 µM (Fig. S6). Previous addition of the NPS receptor antagonist SHA 68 (100 µM; Okamura et al., 2008) abolished responses to NPS in all tested neurons (n = 5; Figure 3A). Plotting the averaged amplitudes, normalized to the mean amplitude of the EPSCs during baseline stimulation, against time demonstrated a gradual increase of the postsynaptic responses after NPS application (Figure 3A). On average, the maximal change of EPSC amplitudes in the presence of NPS was 262 ± 62 % and 220 ± 21 % at P15–26 and P47–56, respectively. The membrane input resistance was not significantly altered by NPS (477 ± 30 MΩ at baseline, 502 ± 25 MΩ in NPS, n = 26, p > 0.5; data not shown). The EPSC amplitudes stayed constant during control stimulation (−32 ± 7 pA at baseline and −32 ± 3 pA at the end of the experiment; change 100 ± 5 % of baseline; n = 3, P15–26; −50 ± 7 pA at baseline and −44 ± 6 pA at the end of the experiment; 82 ± 7 %; n = 6, P47–56) and in the presence of the NPS receptor antagonist SHA 68 (−29 ± 7 pA at baseline and −30 ± 1 pA after NPS application; change 108 ± 5 %; n = 5; P15–26). The EPSCs were maximally increased within 20–40 minutes after application of NPS and upon wash-out returned to near-control values within 70–80 minutes (Figure 3C; n = 12; P47–56). Mean amplitudes were significantly increased by NPS application from −43 ± 8 pA at baseline to −96 ± 19 pA during maximal NPS action and gradually declined to −68 ± 9 pA during wash-out (Figure 3D). The relative change of the EPSC-amplitudes was significantly reduced after wash-out (144 ± 11 %, n = 9) compared to the maximal change (220 ± 21 %, n = 12; p < 0.05). Furthermore, in the same types of mpara IN, GABAA-receptor mediated IPSCs were recorded in the presence of DNQX and AP5, and the effects of NPS were tested (Figure S6). Plotting the normalized amplitudes of the IPSCs revealed no change in responses after NPS application compared to baseline stimulation. The mean amplitudes did not differ significantly (−56 ± 6 pA during baseline stimulation and −62 ± 8 pA after NPS; n = 5; Figure S6). In addition, the effects of NPS on paired-pulse facilitation was examined. Paired-pulse facilitation refers to an increase in a second synaptic response in a double-stimulation protocol, relating to a presynaptically mediated increase in transmitter release. EPSCs in mpara IN were recorded upon intra LA stimulation with a paired-pulse interval of 100 ms in slices from P47–56 mice. EPSCs showed robust paired-pulse facilitation of 1.4 ± 0.1 (−52 ± 7 pA for the first response and −63 ± 5 pA for the second response; n = 11; Figure 3E and 3F). Application of 10 µM NPS significantly (p < 0.05) reduced the paired-pulse ratio to 0.89 ± 0.1 (−124 ± 25 pA for the first response and −102 ± 21 pA for the second response; n = 11; Figure 3E and 3F). During presence of NPS the EPSC-amplitude of the first response was significantly increased compared to baseline-stimulation (p < 0.05). Finally, intrinsic electrotonic or electrogenic properties of LA PN were not different before or in the presence of NPS (Suppl. Data).
Figure 3. Target specific effect of NPS on evoked EPSCs.
(A) Representative current traces of glutamate receptor-mediated EPSCs in mpara IN (in P15–26 animals, n = 13) show an increase in EPSC amplitude in the presence of NPS at 10 µM (b, grey) compared to baseline stimulation (a, black), which is blocked by the NPS receptor antagonist SHA 68 (100 µM). Example traces refer to the time-points (a, b) depicted in the normalized amplitude-plot. At times of near-maximal NPS action, the mean EPSC amplitudes are significantly increased compared to pre-application baseline (B; p < 0.01), whereas amplitudes remain unchanged with no added NPS or in the presence of both NPS and receptor antagonist (see text for further details). (C) Representative current traces of glutamate receptor-mediated EPSCs in mpara IN (in P47–56 animals, n = 12) and normalized amplitudes during baseline stimulation (a), maximal NPS effect (b) and ~70 minutes after NPS application (c, wash-out) show a time-dependent decline of the NPS induced effects on evoked EPSCs. (D) Changes of the mean amplitudes compared to baseline amplitudes during full NPS effect (NPS vs. baseline p < 0.01) and after ~70 minutes of wash-out (wash-out vs. baseline p < 0.05). Comparison of the relative increase of EPSC-amplitudes at timepoints b and c revealed a significant reduction after wash-out (F; p < 0.05). (E) Effects of NPS on paired-pulse facilitation of EPSCs evoked upon intra LA stimulation in mpara IN. Representative current traces of paired-pulse experiments with a paired-pulse interval of 100 ms before (black) and during presence of 10 µM NPS (grey). During NPS, only the first response increased significantly (p < 0.05), and the paired pulse ratio was significantly reduced (p < 0.05; F). Data are mean ± SEM.
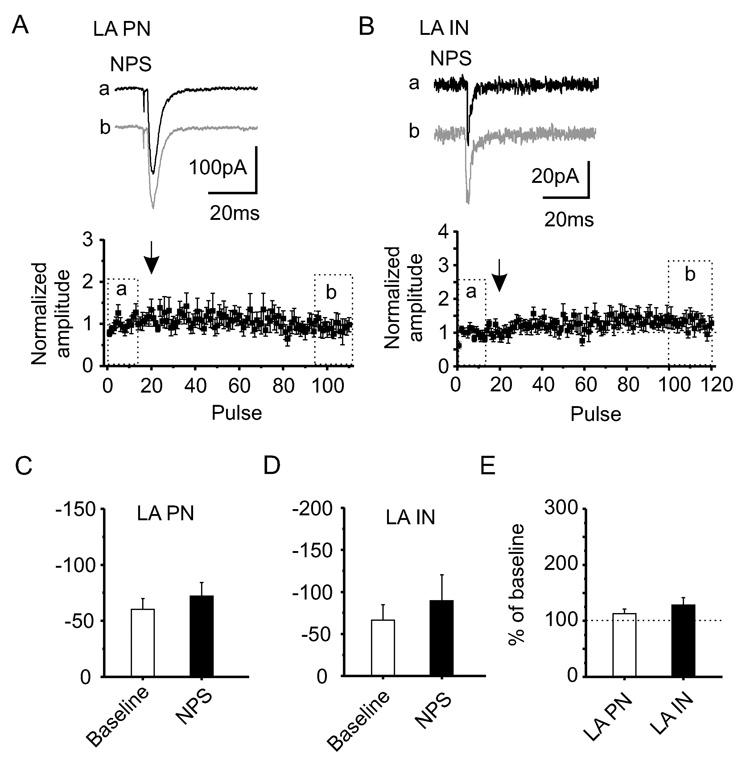
Effects of NPS on glutamatergic synaptic responses in the other major types of neurons in this region of the amygdala, principal neurons (LA PN) and local interneurons (LA IN), were investigated next. The stimulation protocol and placement of the stimulation electrodes were as described before. Neither for LA PN nor for LA IN could an NPS-induced increase of EPSCs be detected, as illustrated by representative current traces and plots of normalized amplitudes (Figures 4A and 4B). The EPSC-amplitudes were not significantly different before or in the presence of NPS compared to baseline values (−60 ± 10 pA during baseline stimulation and −72 ± 12 pA after NPS application, n = 6 for LA PN; −66 ± 18 pA during baseline stimulation and −89 ± 31 pA after NPS application, n = 8 for LA IN; Figure 4C and 4D). The relative changes of the EPSC-amplitudes in LA PN were 113 ± 8 % and in LA IN 128 ± 13 % (n = 6 and n = 8, respectively; Figure 4E). The observed changes in LA PN and LA IN were significantly smaller than those detected in mpara IN (p < 0.01).
Figure 4. Lack of NPS modulatory influence in LA PN and LA IN.
Principal neurons (A) and local interneurons (B) within the LA display no NPS-induced increase of EPSC amplitudes as shown by representative current traces and normalized amplitude-plot. (C, D and E) EPSC amplitudes are not significantly increased in presence of NPS compared to baseline-stimulation in LA PN (n = 6) or LA IN (n = 8). Data are mean ± SEM.
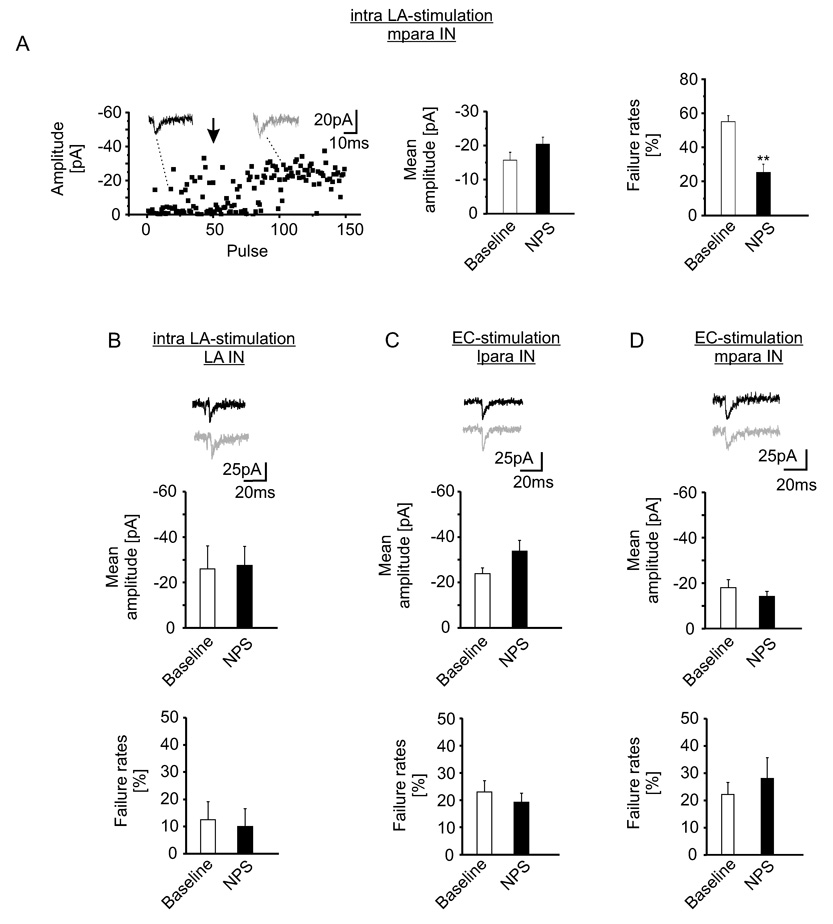
These data suggest that NPS positively modulates transmission at glutamatergic, but not at GABAergic synapses, on mpara IN. Furthermore, glutamatergic synapses projecting on principal neurons or local interneurons within the LA seem to be unaffected. One caveat of the electrical stimulation is that polysynaptic components might contaminate glutamatergic EPSCs in the different types of neurons. Polysynaptic responses were particularly evident in lpara IN, which led us to exclude these types of neurons from the foregoing analysis. Therefore, in the next experimental line, stimulation strength was reduced to evoke putative monosynaptic responses in the major types of neurons, and investigate the effects of NPS. Stimulation electrodes were positioned within the LA for recording EPSCs in LA IN, and in the external capsule for recording EPSCs in lpara IN; in view of the synaptic input to mpara IN from both cortical fibers and LA/BLA, stimulation electrodes were positioned in the external capsule and the LA neuropil in two series of experiments (Szinyei et al., 2000; Marowsky et al., 2005; Geracitano et al., 2007). The monosynaptic nature of evoked responses were indicated by high failure-rates (55 ± 4 %, n = 18; 13 ± 7 %, n = 5; 23 ± 4 %, n = 13; for mpara IN, LA IN and lpara IN, respectively; and 22 ± 5 %, n = 7 for mpara IN during EC-stimulation, see Figure 5), constant latencies (3.5 ± 0.2 ms, n = 18; 3.7 ± 0.2 ms, n = 5; 4.2 ± 0.3 ms, n = 13; for mpara IN, LA IN and lpara IN, respectively; and 4.6 ± 0.5 ms, n = 7; for mpara IN during EC-stimulation), and typical rise-times (10–90 %) (0.9 ± 0.1 ms, n = 10; 0.9 ± 0.2 ms, n = 5; and 1.0 ± 0.1 ms, n = 18; for lpara IN, LA IN and mpara IN, respectively; and 1.1 ± 0.3 ms, n = 7; for mpara IN during EC-stimulation). Therefore, the EPSCs are referred to as monosynaptic in the following. In mpara IN, NPS modulated monosynaptic EPSCs evoked upon stimulation of the local LA neuropil, but left cortically evoked EPSCs unaltered. During presence of NPS, EPSC-success amplitudes upon stimulation of the local LA neuropil showed only a slight, not significant increase (−16 ± 2 pA during baseline stimulation and −20 ± 2 pA after NPS application, n = 18; Figure 5A). In the same neurons, NPS reduced the failure-rates significantly (25 ± 5 %) compared to baseline (control: 55 ± 4 %, n = 18; p < 0.01; Figure 5A). By contrast, monosynaptic EPSCs upon stimulation of the external capsule in mpara IN (n = 7; Figure 5D) were not changed after addition of NPS in terms of amplitude (−17 ± 4 pA versus −14 ± 2 pA) and failure rates (22 ± 5 % versus 28 ± 8 %. Furthermore, NPS was not found to modify properties of monosynaptic EPSCs in LA IN or lpara IN. In LA IN (Figure 5B), neither EPSC-success amplitudes (−26 ± 10 pA and −28 ± 8 pA for baseline stimulation and during NPS, respectively; n = 5) nor failure-rates (13 ± 7 % and 10 ± 6 %) changed significantly. In lpara IN (Figure 5C), amplitudes of EPSCs amounted to −24 ± 3 pA during baseline stimulation, and non-significantly increased to −33 ± 5 pA after NPS application (n = 13). Failure-rates remained unaltered in the presence of NPS compared to baseline (23 ± 4 % and 19 ± 3 %, respectively; n = 13). These data confirmed the specific effect of NPS on glutamatergic synaptic transmission to mpara IN within the amygdala. Furthermore, the reduction in failure-rate hinted at a presynaptic location of the mediating receptors.
Figure 5. NPS reduces failure-rates of glutamatergic transmission to mpara IN.
(A) Failures and amplitudes of putative monosynaptic responses in mpapra IN upon intra LA-stimulation before and after NPS application (NPS was added after 50 baseline-stimulation pulses). Note unaltered success-amplitude and reduction in failure-rate in the example current traces (baseline: black; NPS: grey; arrow denotes application of NPS) and quantified mean amplitude and failure-rates (p < 0.01; n = 18; recordings pooled from mpara IN at P 15–26 and P47–56). (B) EPSC success-amplitudes in LA IN during intra LA-stimulation stayed constant after NPS application. NPS also left failure-rates in LA IN unaffected (n = 5). Recordings of monosynaptic EPSCs in lpara (C, n = 13) or mpara IN (D, n = 7) during stimulation of the external capsule revealed no significant alterations in EPSC success-amplitude or failure-rates upon NPS application. Data are mean ± SEM.
NPS modulates glutamatergic transmission to paracapsular GABAergic neurons through receptors located in presynaptic principal neurons
In order to distinguish between pre- and postsynaptic mechanisms underlying the observed NPS-mediated increase in glutamatergic EPSCs, spontaneous miniature EPSCs (mEPSCs) were recorded in mpara IN, LA PN and lpara IN in the presence of TTX. Results are illustrated in Figure 6. Analyses of the recorded mEPSCs revealed no significant changes of mEPSC amplitudes after NPS application compared to control traces in mpara IN (−19 ± 1 pA in baseline recordings and −18 ± 1 pA after NPS; data averaged from observations in n = 18 cells), LA PN (−18 ± 1 pA in baseline recordings and −18 ± 1 pA after NPS, n = 18) and lpara IN (−21 ± 1 pA in baseline recordings and −19 ± 0.8 pA after NPS, n = 14) (Figures 6A and 6B). By comparison, the frequency of mEPSCs in mpara IN increased upon NPS application from 1.4 ± 0.3 Hz during baseline recordings to 2.6 ± 0.6 Hz after NPS (n = 18; Figure 6C). In LA PN and lpara IN, mEPSCs were not significantly different before or during NPS action (for LA IN 1.2 ± 0.4 Hz baseline, 1.3 ± 0.5 Hz after NPS; n = 18; for lpara IN 2.9 ± 1 Hz baseline and 2.9 ± 1 Hz NPS, n = 14; Figure 6C). Because of variations in frequencies between individual recordings, the relative increase of mEPSC-frequencies was calculated (Figure 6D). NPS significantly increased mEPSC-frequencies in mpara IN to 170 ± 13 % of baseline frequencies (p < 0.01) compared to unaltered frequencies in LA PN (117 ± 12 %) and lpara IN (99 ± 3 %). Furthermore, high-pressure somatic application of 200 µM glutamate in ACSF (in the presence of 100 µM picrotoxin, 50 µM AP5 and 1 µM TTX) to mpara IN evoked robust excitatory responses (−153 ± 32 pA; n = 12), which showed no significant change after NPS application (−176 ± 51 pA, n = 12; 118 ± 16 % of baseline; Figure S6) compared to controls. Similar results were obtained in LA PN, in which responses to exogenous glutamate were not modulated through NPS (−107 ± 17 pA to −115 ± 24 pA; 119 ± 24 % of baseline; n = 10, Figure S6). These findings support the notion that NPS acts via a mechanism that resides on glutamatergic terminals presynaptic to mpara IN.
Figure 6. Presynaptic site of NPS modulation.
(A) Example traces of mEPSCs recorded in mpara IN (i), LA PN (ii), and lpara IN (iii) during baseline conditions (upper traces) and in the presence of NPS (lower traces). (B) mEPSC-amplitudes are not changed by the application of NPS in mpara IN (n = 18), LA PN (n = 18), or lpara IN (n = 14). (C) While the mean mEPSC-frequencies in LA PN and lpara IN stayed constant, the mean mEPSC-frequency in mpara IN is increased in presence of NPS. (D) The relative increase of mEPSC-frequencies in mpara IN in the presence of NPS compared to LA PN and lpara IN is significant (p < 0.01). Data are mean ± SEM.
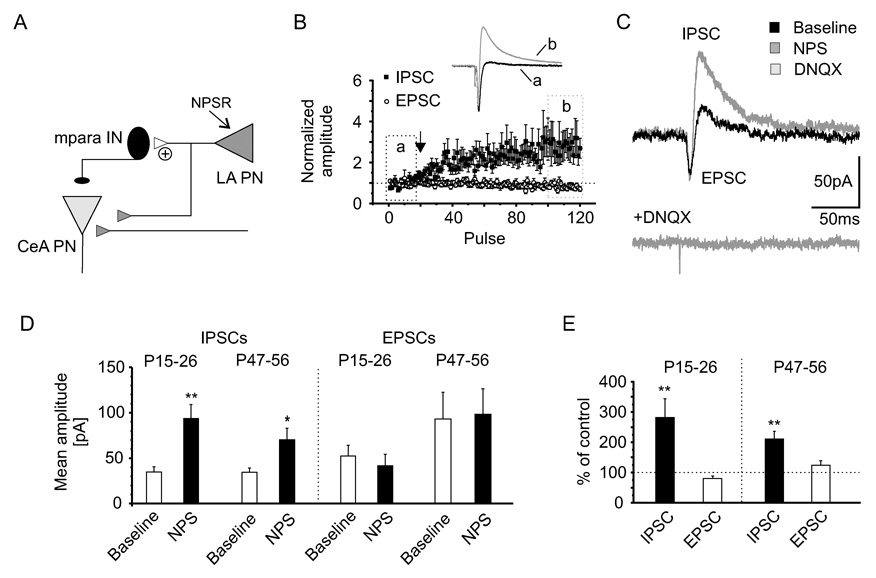
Determination of the exact cellular location of the mediating receptors is hampered by the lack of specific antibodies against the NPS receptor. High densities of neurons expressing NPSR mRNA were observed in the LA and BLA subdivisions of the amygdala while only scattered signals were found in medial, basomedial and anterior cortical amygdaloid nuclei (Figure 7A and 7B). Notably and in contrast to the rat brain, no expression of NPSR was detected in intercalated amygdaloid nuclei of the mouse. In order to obtain more detailed information on NPSR expression sites in amygdaloid neurons, we used two alternative approaches. In one, NPSR mRNA was detected in single neurons, which had been classified as mpara IN, lpara IN or LA PN based upon morphological and electrophysiological criteria, through relative quantitative real-time RT-PCR. The cytoplasm of single lpara IN (n = 41), mpara IN (n = 21), and LA PN (n = 45) was collected individually and subsequently pooled according to cell type (lpara IN, n = 20 + 21; mpara IN, n = 21; LA PN, n = 12 + 16 + 17). The cDNA of each pool was pre-amplified, and subsequent RT-PCR detected the reference gene hypoxanthine-guanine phosphoribosyltransferase (HPRT) in all cell types. By contrast, the NPS receptor transcript was only detected in LA PN (2−ΔC(t) = 0.259 ± 0.143) with no detectable signal in lpara IN or mpara IN (Figure 7C). Results presented thus far allow for a working hypothesis that NPS receptors are expressed in LA PN, activation of which results in an increase in glutamatergic synaptic transmission to mpara IN. The GABAergic neurons of the mpara cell masses have been found to project to the CeA (Paré and Smith, 1993) and to be contacted by axon collaterals of principal neurons in LA/BLA, thereby gating impulse traffic from cortical inputs via LA/BLA to the principal output neurons in the CeA (Royer et al.,1999, Paré et al., 2003). If NPS receptors are located at glutamatergic connections from LA PN to mpara, and activation of these receptors induces an increase in glutamatergic excitation, it can be expected that NPS leads to an increase in both monosynaptic excitatory responses in mpara and disynaptic inhibitory responses in CeA principal neurons (PN), while monosynaptic responses in CeA should not or to a lesser degree be modulated (Figure 8A). In a further attempt to identify the anatomical site of NPS receptor modulation within this circuit, we experimentally tested this hypothesis. Whole-cell recordings were performed at a holding potential of −50 mV in principal neurones of the CeA next to the medial paracapsular intercalated GABAergic clusters. Local stimulation within the LA gave rise to biphasic postsynaptic responses (monosynaptic EPSCs inwardly directed, and disynaptic IPSCs outwardly directed) in CeA PN (Figure 8B). Both current components of the biphasic response could be blocked by the addition of DNQX (n = 4), verifying the disynaptic nature of the IPSC (Figure 8C). EPSC- and IPSC-amplitudes were monitored during 120 stimulation pulses (inter-stimulus interval 20 s) and were normalized to the first 20 baseline responses. Bath application of NPS significantly increased the amplitudes of the inhibitory response component, shifting the mean amplitudes from 35 ± 6 pA during baseline stimulation to 94 ± 15 pA (p < 0.01; n = 17; P15–26) during near-maximal action of NPS, whereas the monosynaptic EPSC component was only slightly reduced from −52 ± 12 pA to −42 ± 13 pA in the same neurons (Figure 8D). The presence of NPS significantly increased the IPSC amplitudes to 282 ± 62 % (p < 0.01), whereas EPSC amplitudes were not affected (80 ± 8 % of control values; Figure 8E). Effects of NPS in neurons at P15–26 were not different from those at P47–56 (Figure 8D,E), in that the mean IPSC amplitudes were significantly increased from 35 ± 5 pA during baseline stimulation to 71 ± 13 pA (relative change of 209 ± 25 %; n = 7; P47–56) after NPS application, whereas the mean amplitudes of the EPSCs was unaffected (−93 ± 30 pA baseline and −98 ± 28 pA NPS; 122 ± 15 %; n = 7; Figure 8D and 8E).
Figure 7. NPS receptor expression in the amygdala.
(nA) In-situ hybridization of a coronal mouse brain section showing strong expression of NPSR mRNA in the lateral and basolateral parts of the amygdaloid complex. (B) Adjoining section stained with cresyl violet as a reference. Abbreviations: opt: optic tract; BLA: basolateral amygdaloid complex, anterior part; BMA: basomedial amygdaloid nucleus, anterior part; MeAV: medial amygdaloid nucleus, anteroventral part; ACo: anterior cortical amygdaloid nucleus; ic: internal capsule; DI: dysgranular insular cortex; GI: granular insular cortex; AIP: agranular insular cortex, posterior part; LaDL: lateral amygdaloid nucleus, dorsolateral part; DEn: dorsal endopiriform nucleus; scale bar: 100 µm. (C) Relative quantitative real-time RT-PCR of pre-amplified cDNA prepared from cytoplasm of single lateral (lpara IN) and medial (mpara IN) paracapsular interneurons, and LA principal neurons (LA PN). Transcript levels of NPSR were normalized to the reference gene HPRT. See text for statistical details.
Figure 8. NPS enforces feedforward inhibition on principal neurons in the central nucleus of the amygdala (CeA).
(A) Schematic representation of synaptic interconnections from principal neurons in the LA (LA PN) via mpara IN to principal neurons in the CeA (CeA PN). Increase in excitatory synaptic transmission by an NPS-dependent presynaptic mechanism in LA PN should result in an increase in feedforward inhibition via mpara IN on CeA PN. (B) Recording of biphasic (EPSC monosynaptic, IPSC disynaptic) postsynaptic currents in CeA PN at a holding-potential of −50 mV during intra LA baseline-stimulation (a, black trace) and in presence of NPS (b, grey trace). Application of NPS specifically enhanced the disynaptic inhibitory current component (IPSC), but leaves the excitatory current component (EPSC) almost unaffected, as indicated by the normalized amplitudes. (C) The disynaptic nature of the IPSC was verified by blocking both current components (IPSC and EPSC) with DNQX after NPS application. (D) NPS selectively increased the amplitudes of the disynaptic IPSC in P15–26 (n = 17) and P47–56 mice (n = 7), whereas the amplitudes of the EPSCs were unaffected at both ages. (E) The relative change of the IPSC-amplitude is highly significant (p < 0.01) compared to the EPSC-amplitude. Data are mean ± SEM.
Discussion
The present study aimed at identifying the mechanisms of action of NPS in neuronal circuits of the mouse amygdala, in relation to anxious behavior, conditioned fear and fear extinction. Three main findings were obtained: First, local injection of NPS into the basolateral amygdaloid complex resulted in an anxiolytic-like effect, reflected by an acute reduction in general anxiety and an accelerated extinction of conditioned fear, while injection of the NPS receptor antagonist SHA 68 exerted functionally opposing effects. Second, NPS increased glutamatergic synaptic transmission to intercalated GABAergic neurons of the medial paracapsular cluster, through a presynaptic effect mostly involving principal neurons in the LA. Third, NPS receptors were shown to be densely expressed in the mouse BLA complex, and, at the single cell level, in LA principal neurons but not paracapsular GABAergic neurons. The following discussion examines the possibility that these effects are functionally interrelated.
Sites of NPS modulation in neuronal circuits of the amygdala
By virtue of their location, paracapsular intercalated cells are ideally suited to control signal flow in the amygdala in a feedforward inhibitory manner. While neurons in both lpara and mpara clusters share a GABAergic nature and are densely innervated by excitatory cortical input fibers (reviewed by Paré et al., 2004), they contact different major targets in the amygdaloid neuronal network. Neurons in the lpara cluster project to the BLA complex and generate feedforward IPSPs in BLA principal neurons (Marowsky et al., 2005), while mpara neurons are connected to mediate topographically organized feedforward inhibition to principal neurons in the CeA nucleus (Royer et al., 1999). Furthermore, stimulation of the BLA complex evokes short-latency EPSPs in mpara neurons, indicating monosynaptic input from the respective principal neurons (Royer et al., 1999). Another population of GABAergic neurons is represented by local interneurons, comprising around 25% of the neuronal population, which also receive excitatory cortical inputs and mediate feedforward (and feedback) inhibition to the principal neurons within the local neuropil (Szinyei et al., 2000). We have shown in the present study that stimulation of NPS receptors resulted in an increase in glutamatergic synaptic transmission to paracapsular intercalated neurons. The following line of evidence suggests that synaptic connections between LA principal neurons and mpara IN are specifically involved, and that NPS receptors are located at presynaptic sites of these connections. (a) NPS increases the frequency, but not the amplitude, of mEPSCs in mpara IN, but not lpara IN or principal neurons. (b) NPS decreases the failure rate with no effect on amplitude of putative monosynaptic EPSCs evoked by LA stimulation in mpara IN. (c) Putative monosynaptic EPSCs evoked in mpara IN by stimulation within the LA are modulated by NPS, whereas putative monosynaptic EPSCs evoked in the same type of neurons by stimulation of the external capsule are not modulated by NPS. (d) Putative monosynaptic EPSCs evoked by stimulation of the external capsule or within the LA in lpara IN or local IN in LA, respectively, are not affected by NPS. (e) Upon paired stimulation within the LA, NPS increases the amplitude of EPSCs in mpara IN in response to the first stimulus and decreases the paired-pulse ratio, indicating an NPS-mediated increase in initially low release probability (Debanne et al., 1996). (f) Semi-quantitative PCR from single, physiologically identified types of neurons shows that NPS receptors are expressed in LA principal neurons, but not in mpara or lpara interneurons.
Target-specific modulation of presynaptic release has been described in various brain regions, including glutamatergic transmission to subsets of GABAergic neurons (Ferraguti et al., 2005). The mechanisms underlying the target specific action of NPS in the amygdala observed in the present study remain unknown, but may relate to various subtypes of LA/BLA principal neurons (Sosulina et al., 2006) or synapse-specific expression of NPSR, as has recently been reported for presynaptic NMDA receptors in rat somatosensory cortex (Brasier and Feldman, 2008). It is important to note that different sites of stimulation were used in the present study, in order to activate the major glutamatergic synaptic connections to the different types of neurons of interest. This approach allows us to draw conclusions on their involvement in NPS modulation, but does not, of course, provide an exclusive or complete profile. For instance, microstimulation within the LA may have activated cortical fibres en passant, adding to the recorded EPSCs in mpara IN. However, no NPS receptor expression has been observed in the mouse infralimbic prefrontal cortex by in situ hybridization (Xu and Reinscheid; unpublished observations), thereby voting against the possibility that the major cortical input relating to fear extinction is involved in NPS action. Taken together, available data strongly suggest that NPS mediates an increase in glutamatergic synaptic transmission to mpara IN through NPS receptors located at presynaptic sites in connected principal neurons in the LA. While this scenario is not an exclusive or complete one, it nevertheless represents the most parsimonious interpretation of our data from single cell electrophysiological, RNA expression and anatomical experiments.
Action of NPS in amygdaloid circuits in relation to fear behavior
The behavioral consequences of NPS in the amygdala were investigated in the present study through bilateral application of NPS and the NPS receptor antagonist SHA 68 aimed at the basolateral amygdaloid complex. The exact distribution of the drug could not be assessed under the present experimental conditions, but the locality of the injection site in LA/BLA and the restricted diffusion of NPS were verified through histological reconstruction of the position of the needle injector tip and fluorescently labelled Cy3-NPS, respectively. NPS, under these conditions, exerted anxiolytic-like effects in three different tests of anxiety, namely the open field, the elevated plus maze, and the dark-light test, which were not associated with an increase in locomotor activity and thereby differed from results obtained after intracerebroventricular application of NPS (Xu et al., 2004). These findings support the notion that NPS affected a local neuronal network in the present study and limit the possibility that arousal-like effects (Xu et al., 2004) confounded the interpretation of the behavioral data. The anxiolytic effects of NPS were observed within 20 minutes after application, and fully declined after two hours, indicating an acute effect on the expression of fear. It is important to note that a similar time course of action was also observed with respect to arousal-like hyperlocomotion after intracerebroventricular application of NPS (Xu et al., 2004) and with respect to the increase in glutamatergic transmission to mpara IN in vitro (present study). That endogenous NPS in the amygdala is involved in fear behavior is indicated by the anxiogenic-like effect obtained upon application of the NPS receptor antagonist SHA 68.
Another series of experiments sought to determine whether NPS is involved in the acquisition or extinction of fear memory by delivering NPS at defined time points in relation to training or testing in a Pavlovian fear conditioning paradigm. To distinguish between possible effects on acquisition and expression of fear, two groups of animals received NPS immediately (20 minutes) before fear training or immediately (20 minutes) before testing conditioned responses to the CS during retrieval sessions 24 hours after training. Pre-training application of NPS had no effect on conditioned fear responses as compared to vehicle-injected controls, whereas pre-testing application resulted in a decrease in conditioned fear responses, indicating an influence of NPS on fear expression rather than fear learning. The finding that NPS applied one hour after training yielded conditioned fear responses indistinguishable from controls is in line with these conclusions. Furthermore, the lack of effect of NPS upon both pre- and post-training injections on conditioned fear responses is suggestive of a lack of influence on fear memory consolidation. It should be kept in mind, however, that the results in the present study were obtained upon local injection of NPS into the BLA complex and the use of auditory cued fear conditioning paradigms. Additional studies involving other forms of fear memory and extended neuronal circuits, as for instance, contextual fear and hippocampal networks (see Maren and Quirk, 2004), are needed to unravel additional potential sites and mechanisms of NPS influence on conditioned fear behavior. One consistent observation made in the present study was that application of NPS immediately (20 minutes) before the first retrieval session resulted in decreased fear responses throughout successive retrieval trials used for extinction training. In addition, tests of consolidated extinction performed 24 hours after extinction learning revealed reduced fear responsiveness. To further distinguish between effects on expression of fear vs. extinction of fear, NPS was applied 2 hours before the first retrieval session. Conditioned fear responses during the first retrieval trial were not significantly different from control, while extinction learning followed a faster time course compared to controls. Application of the NPS receptor antagonist SHA 68 at the same time point before retrieval had no significant effect on the expression of the first conditioned response, but resulted in a significant impairment of extinction learning and recall. Thus, NPS in the amygdala engages cellular processes that facilitate fear extinction through an action in addition to that on fear expression.
How might this dual effect of NPS be mediated? One likely route involves mobilization of intracellular Ca2+ upon activation of NPS receptors (Reinscheid et al., 2005). NPS receptors are located at glutamatergic synapses from LA principal neurons to mpara IN, and receptor activation results in an increase in glutamate release (as discussed above). The major projection site of mpara IN is the CeA (Royer et al., 1999; Likhtik et al., 2005), and the increase in glutamatergic transmission to this population of GABAergic neurons will impose an additional inhibitory influence on the CeA, the major output station of the amygdala for fear expression of behaviour. It is interesting to note that IPSCs in the CeA are almost exclusively carried by α2-subunit-containing GABAA receptors (Marowsky et al., 2004). Given the importance of α2-subunit-containing GABAA receptors in anxiolysis (Rudolph et al., 2001), an NPS-mediated increase in afferent activation of these GABAergic mechanisms via the mpara IN may help to explain the strong anxiolytic-like effect observed upon local injection of NPS in the present study. Importantly, NPS receptors are positively coupled to the cAMP/PKA system (Reinscheid et al., 2005). A result of NPS receptor-activated cAMP/PKA is phosphorylation of MAPK (Reinscheid et al., 2005), potentially giving rise to long term effects involving nuclear regulation of protein synthesis. In fact, fear extinction is sensitive to modulation of kinase activity in the BLA complex, including the MAPK-ERK pathway (Lu et al., 2001; Herry et al., 2006), and requires de novo protein synthesis in the BLA (Yang and Lu, 2005). NPS may thus represent a transmitter system supporting a link to these processes in principal neurons in the LA, thereby enabling a lasting increase in efficacy of synaptic connections to GABAergic mpapra IN and a modulation of fear extinction on a long term scale. In line with this is the previous finding that synaptic long term potentiation of BLA inputs to mpara IN involves presynaptic mechanisms (Royer and Paré, 2002). Many neurons in the LA/BLA remain CS responsive during extinction (Repa et al., 2001), and will mediate via NPS-potentiated transmission to mpara IN an increased inhibitory input to the CeA, thereby facilitating fear extinction on a long term scale.
Collectively, our findings imply that endogenous NPS has a dual function to acutely attenuate anxiety-like responses and later facilitate extinction of aversive memories. Such dual effects could be therapeutically beneficial to treat conditions like post-traumatic stress disorder or chronic anxiety disorders.
Experimental procedures
All experiments were carried out in accordance with the European Committees Council Directive (86/609/EEC) and US federal regulations and guidelines for experimentation on animals. Protocols were approved by the Bezirksregierung Münster (AZ 50.0835.1.0, G 53/2005) and the local Institutional Animal Care and Use Committee (IACUC) of the University of California, Irvine.
Behavioral testing in vivo
Mice (C57BL/6J; 8–12 weeks old) were implanted with a 26 gauge stainless-steel guide cannula bilaterally in the LA/BLA (stereotaxic coordinates: 1.8 mm anterior, 3.7 mm lateral, and 3.2 mm dorso-ventral from brain surface) under deep pentobarbital anesthesia (75 mg/kg i.p.). Animals were allowed to recover from surgery for at least 4 days. Local drug infusion was performed under anesthesia through forene inhalation (isofluran, 1-Chloro-2,2,2-trifluoroethyl-difluoromethylether; induction: 2.5%, maintenance: 1.5%; in O2; flow rate 1 l/min). Using a 10 µl Hamilton microliter syringe, the following solutions were infused with a 33 gauge beveled needle injector (0.1 µl/min, 0.5 µl each side): SHA 68 (10 µM, in 10% Cremophor-PBS buffer), NPS (10 µM), and, as vehicle controls, saline, 10% Cremophor-PBS buffer. Drugs were applied (i) at different times (20 minutes, 2 hours, 4 hours) before tests of general anxiety, (ii) before (20 minutes) or after (one hour) fear conditioning, and (iii) at different times before (20 minutes, 2 hours) retrieval 1 as well as immediately after retrieval 6 (Suppl. Data, Figure S2). One individual animal underwent only one type of test of general anxiety or underwent the fear training/retrieval protocol, only one substance was tested in an individual animal, drug effects were compared with vehicle controls using the same injection protocols, and the behavioral testing was performed by the experimenter blind to pharmacological treatment of mice. At the end of the experiments, locations of the infusion cannula were histologically verified in frozen frontal sections of 40 µm, stained with cresyl violet. Furthermore in individual animals (n = 9), the diffusion of NPS solution was verified using microinjection of Cy3-NPS (Phoenix Pharmaceuticals, Inc., Belmont, USA) and verification of injection/diffusion sites in 30 µm coronal cryosections under a fluorescent microscope, followed by histological verification through cresyl violet (Figure S1). General anxiety was tested using the open field test, plus maze test, and dark-light test (Suppl. Data). Fear conditioning was performed using a fear training apparatus (TSE, Bad Homburg, Germany). On day 1, animals were adapted through two presentations of 6 CS− (2.5 kHz tone, 85 dB, stimulus duration 10 s, inter-stimulus interval 20 s; inter-trial interval 6 h). On the next day, fear conditioning was performed through two exposures of 3 randomly presented CS+ (10 kHz tone, 85 dB, stimulus duration 10 s, randomized inter-stimulus interval 10–30 s; inter-trial interval 6 hours), each of which was co-terminated with a US (scrambled foot shock of 0.4 mA, duration 1 s). Twenty-four hours later (day 3), single animals were transferred to the retrieval environment (novel context) and habituated over a period of 30 min, before being exposed to 6 retrieval sessions (R1–R6) for extinction training (inter-trial interval 30 min), each consisting of a set of 4 CS− and (40 s later) a set of 4 CS+ (stimulus duration 10 s, inter-stimulus interval 20 s). After 24 hours (day 4), recall of extinction was tested by exposing the animal to one set of 4 CS− and 40 s later to a set of 4 CS+ (stimulus duration 10 s, inter-stimulus interval 20 s). Extinction recall was tested twice (E1, E2; interval 30 minutes). For renewal of extinct fear (RN), mice were returned to the initial shock context and received a set of 4 CS− and 40 s later a set of 4 CS+. The conditioning protocol is illustrated in Figure S2.
Analysis of behavioral data
For off-line evaluation of conditioned freezing behavior (immobilization except for respiratory movements) a time line version of Wintrack was used (see Laxmi et al., 2003). Freezing time was calculated as percentage during total CS+ presentations within a recording session (R1 to R6, E1, E2, RN). Data are presented as mean with standard error of the mean (± SEM). Mann-Whitney U-test or Wilcoxon test were used, as applicable.
In situ hybridization for NPS receptors
A rat NPSR probe was used to hybridize coronal mouse brain sections at low stringency. Procedures and materials were essentially as described before (Xu et al., 2007). For the final stringent wash, sections were incubated for 30 min in 0.3 × SSC/1 mM DTT at 60°C.
Electrophysiological recordings in amygdala slices in vitro
GAD67-EGFP mice (P15–P26 or P47–P56) were anesthetized with forene (isofluran, 1-Chloro-2,2,2-trifluoroethyl-difluoromethylether) and killed by decapitation. Coronal slices containing the amygdala were prepared and whole-cell patch-clamp recordings were performed as described previously (Suppl. Data; Szinyei et al., 2000, 2003). Specifically, neurons were approached under visual control by differential interference contrast infrared videomicroscopy (B/W-camera CF8/1, Kappa, Gleichen, Germany). EGFP-GAD67 expressing neurons were identified by fluorescent microscopy (Axioskop 2 FS plus, Zeiss, Germany). Neurons with resting membrane potential positive to −60 mV were rejected from analysis. Extracellular stimuli (100 µsec duration, 200 to 600 µA) were delivered through a bipolar stainless steel electrode placed in the LA or external capsule (Szinyei et al., 2000, 2003). Picrotoxin (100µM), CGP55845 (10µM) and D-(−)-2-Amino-5-phosphonopentanoic acid (AP5, 50µM) or 6,7-Dinitroquinoxaline-2,3-dione (DNQX, 10µM) and AP5 (50µM) were added to the bathing solution as required to isolate AMPA- or GABAA -receptor mediated current components (toxins purchased from Tocris Cookson Ltd). NPS stock-solution (NPS in phosphate-buffered saline and 0.1% bovine serum albumin) was diluted to a concentration of 1 mM and bath applied (final concentration of 10 µM; for dose-response see Figure S6). The NPS receptor antagonist SHA 68 was solved in dimethyl sulfoxide (DMSO) and was applied at a final concentration of 100µM prior to addition of NPS (final concentration of DMSO < 0.2 %). Glutamate (200 µM, Biotrend) was locally applied in close vicinity (15–20 µm) of a recorded cell in small volumes (approx. 10–50 pl, 8 times at 5 min intervals) using pressure pulses (Picospritzer II, General Valve Corporation, New Jersey, USA).
Analysis of in vitro data
Effects of NPS on postsynaptic currents (PSCs) were analyzed as follows. After baseline stabilization, PSCs were recorded upon alternating stimulation at 0.05 Hz. Amplitudes of evoked EPSCs were calculated and averaged from 15 consecutive EPSCs during baseline conditions (before application of NPS) and 15 consecutive EPSCs during near-maximal action of NPS from original current traces in a given neuron. Obtained values were averaged from different neurons, and are presented as mean amplitude (in pA) under baseline conditions and in the presence of NPS. Normalized mean amplitudes were obtained from two lines of experiments. First, EPSC amplitudes obtained during action of NPS were normalized with respect to baseline EPSCs in individual neurons, and averaging the normalized values from different neurons yielded NPS responses in % baseline. Second, in a group of control cells (without application of NPS), EPSCs were monitored over the same time course as for NPS experiments, amplitudes at time points matching maximal NPS action were normalized with respect to baseline EPSCs, and averaging the normalized values from different neurons yielded control responses in % baseline. Data for wash-out were obtained following the same procedures, but at time points after decline of NPS action. For analyses of putative monosynaptic responses, signals smaller than two times the standard deviation of baseline-noise were declared as failures and were excluded. Paired-pulse experiments consisted of two consecutive stimuli with an interval of 100 ms. Traces containing failures produced by at least one of the two stimuli were rejected from analysis. The paired-pulse ratio was calculated (Amplitude EPSC2 / Amplitude EPSC1) during baseline-stimulation and in presence of NPS. Miniature EPSCs were recorded over a time-period of 150 s (100 traces with 1.5 s duration at a sampling-rate of 5 kHz) to determine control amplitudes and frequencies of glutamate receptor mediated currents. 25 min after NPS-application recordings of mEPSCs were repeated. Data sets were imported to “MiniAnalysis” (Synaptosoft Inc., Decatur, GA, USA) and amplitudes and frequencies prior and post NPS application were identified. Mean frequencies and amplitudes of individual recordings were averaged and tested for significance. Data are presented as means with standard error of the mean (± SEM). For statistical comparison ANOVA or Student´s t-test were used, as applicable.
Cell type-specific quantitative real-time RT- PCR
After establishing whole-cell configuration with the patched neuron, in some cases after recording (5 min), the cell was lifted above the slice, the cell content was sucked into the pipette and transferred into 3 µl carrier RNA buffer (RNeasy Micro Kit, Qiagen) by breaking the tip of the pipette and expelling approx. 3 µl of solution with positive pressure. The pipette solution (6 µl) was supplemented with a recombinant ribonuclease inhibitor (0.24 U/µl; RNasin; Promega, Madison, WI, USA). Cytoplasm from single, identified cells were pooled (lpara IN: two groups of 20 and 21 pooled cells; mpara IN: 21 pooled cells; LA PN: three groups of pooled cells: 12, 17, 16, respectively), and the RNA was isolated without DNase treatment (all primers designed to be intron-spanning) using an RNA isolation kit (RNeasy Micro Kit, Qiagen). Reverse transcription (RT) protocol was used in cDNA preparation from isolated RNA (Sosulina et al., 2006) and subsequently pre-amplified as modified from Allison et al. (2006). A single-plex PCR (20 cycles) was performed (denaturation at 94°C, 25 s; annealing at 51°C, 2 min for the first 5 cycles, and 45 s for the remaining cycles; extension at.72°C, 60 s; final elongation at 72°C, 7 min) for hypoxanthine-guanine phosphoribosyltransferase (HPRT, reference gene; Chen et al., 2001) and NPSR. Relative quantitative real-time PCR was performed on pre-amplified cDNA using FAM-labeled detection assays (TaqMan®, Applied Biosystems) for the NPSR and HPRT in conjunction with the Real Master Mix (5Prime) in an ABI Prism 7000 Sequence Detection System (Applied Biosystems; 50 cycles of 15 s at 95 °C and 1min at 60 °C preceded by a 2 min decontamination step at 50°C and 10 min denaturation step at 95 °C). Primer sequences are specified in Suppl. Data. Transcript levels of the NPSR were normalized to HPRT using the equation 2−ΔC(t), where C(t) is the mean cycle threshold level and ΔC(t) = (C(t) NPSR) − (C(t) HPRT) (Livak and Schmittgen, 2001).
Supplementary Material
Acknowledgements
Thanks are due to Dr. Yuchio Yanagawa, Gunma University, Japan, for kindly providing litters for breeding of GAD67-EGFP mice. We would like to thank E. Boening, A. Markovic, E. Nass, S. Ruppel for expert technical assistance, and Dr. P. Coulon for fluorescence-microscopy and for critical reading of the manuscript. This work was supported by the Deutsche Forschungsgemeinschaft (DFG Pa 336/15-1 to HCP; SFB-TRR 58 TP A2, A3 to HCP and TS), the Max-Planck-Research Award (to HCP), the fund "Innovative Medical Research" of the University of Münster Medical School (SO220608 to LS, LE210613 to JL) and the National Institute of Mental Health (NIMH, grant MH-71313 to RKR). SS is supported by the International Human Frontier Science Program Organization.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allison DW, Ohran AJ, Stobbs SH, Mameli M, Valenzuela CF, Sudweeks SN, Ray AP, Henriksen SJ, Steffensen SC. Connexin-36 gap junctions mediate electrical coupling between ventral tegmental area GABA neurons. Synapse. 2006;60:20–31. doi: 10.1002/syn.20272. [DOI] [PubMed] [Google Scholar]
- Bouton ME, Westbrook RF, Corcoran KA, Maren S. Contextual and temporal modulation of extinction: behavioral and biological mechanisms. Biol Psychiatry. 2006;60:352–360. doi: 10.1016/j.biopsych.2005.12.015. [DOI] [PubMed] [Google Scholar]
- Brasier DJ, Feldman DE. Synapse-specific expression of functional presynaptic NMDA receptors in rat somatosensory cortex. J Neurosci. 2008;28:2199–2211. doi: 10.1523/JNEUROSCI.3915-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debanne D, Guérineau NC, Gähwiler BH, Thompson SM. Paired-pulse facilitation and depression at unitary synapses in rat hippocampus: quantal fluctuation affects subsequent release. J Physiol. 1996;491:163–176. doi: 10.1113/jphysiol.1996.sp021204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geracitano R, Kaufmann WA, Szabo G, Ferraguti F, Capogna M. Synaptic heterogeneity between mouse paracapsular intercalated neurons of the amygdala. J Physiol. 2007;585:117–134. doi: 10.1113/jphysiol.2007.142570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herry C, Trifilieff P, Micheau J, Lüthi A, Mons N. Extinction of auditory fear conditioning requires MAPK/ERK activation in the basolateral amygdala. Eur. J. Neurosci. 2006;24:261–269. doi: 10.1111/j.1460-9568.2006.04893.x. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Brandenburg N, Lane M, Roy-Byrne P, Stang PD, Stein DJ, Wittchen HU. Rethinking the duration requirement for generalized anxiety disorder: evidence from the National Comorbidity Survey Replication. Psychol Med. 2005;35:1073–1082. doi: 10.1017/s0033291705004538. [DOI] [PubMed] [Google Scholar]
- Kim J, Lee S, Park K, Hong I, Song B, Son G, Park H, Kim WR, Park E, Choe HK, Kim H, Lee C, Sun W, Kim K, Shin KS, Choi S. Amygdala depotentiation and fear extinction. Proc Natl Acad Sci U S A. 2007;104:20955–20960. doi: 10.1073/pnas.0710548105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laxmi TR, Stork O, Pape HC. Generalisation of conditioned fear and its behavioural expression in mice. Behav Brain Res. 2003;145:89–98. doi: 10.1016/s0166-4328(03)00101-3. Erratum in: Behav Brain Res. 2994, 141, 337. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Likhtik E, Pelletier JG, Paz R, Paré D. Prefrontal control of the amygdala. J Neurosci. 2005;25:7429–7437. doi: 10.1523/JNEUROSCI.2314-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu KT, Walker DL, Davis M. Mitogen-activated protein kinase cascade in the basolateral nucleus of amygdala is involved in extinction of fear-potentiated startle. J Neurosci. 2001;21:RC162. doi: 10.1523/JNEUROSCI.21-16-j0005.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz B. The endocannabinoid system and extinction learning. Mol Neurobiol. 2007;36:92–101. doi: 10.1007/s12035-007-8004-x. [DOI] [PubMed] [Google Scholar]
- Maren S, Quirk GJ. Neuronal signalling of fear memory. Nat Rev Neurosci. 2004;5:844–852. doi: 10.1038/nrn1535. [DOI] [PubMed] [Google Scholar]
- Marowsky A, Fritschy JM, Vogt KE. Functional mapping of GABA A receptor subtypes in the amygdala. Eur J Neurosci. 2004;20:1281–1289. doi: 10.1111/j.1460-9568.2004.03574.x. [DOI] [PubMed] [Google Scholar]
- Marowsky A, Yanagawa Y, Obata K, Vogt KE. A specialized subclass of interneurons mediates dopaminergic facilitation of amygdala function. Neuron. 2005;48:1025–1037. doi: 10.1016/j.neuron.2005.10.029. [DOI] [PubMed] [Google Scholar]
- Marsicano G, Wotjak CT, Azad SC, Bisogno T, Rammes G, Cascio MG, Hermann H, Tang J, Hofmann C, Zieglgänsberger W, Di Marzo V, Lutz B. The endogenous cannabinoid system controls extinction of aversive memories. Nature. 2002;418:530–534. doi: 10.1038/nature00839. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, et al. Cortico-cortical and cortico-amygdaloid projections of the rat occipital cortex: a Phaseolus vulgaris leucoagglutinin study. Neuroscience. 1996;71:55–75. doi: 10.1016/0306-4522(95)00416-5. [DOI] [PubMed] [Google Scholar]
- Myers KM, Ressler KJ, Davis M. Different mechanisms of fear extinction dependent on length of time since fear acquisition. Learn Mem. 2006;13:216–223. doi: 10.1101/lm.119806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers KM, Davis M. Mechanisms of fear extinction. Mol Psychiatry. 2007;12:120–150. doi: 10.1038/sj.mp.4001939. [DOI] [PubMed] [Google Scholar]
- Okamura N, Habay SA, Zeng J, Chamberlin AR, Reinscheid RK. Synthesis and pharmacological in vitro and in vivo profile of SHA 68 (3-Oxo-1,1-diphenyl-tetrahydro-oxazolo[3,4-a]pyrazine-7-carboxylic acid 4-fluoro-benzylamide), a selective antagonist of the Neuropeptide S receptor. J Pharmacol Exp Ther. 2008 doi: 10.1124/jpet.107.135103. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paré D, Smith Y. The intercalated cell masses project to the central and medial nuclei of the amygdala in cats. Neuroscience. 1993;57:1077–1090. doi: 10.1016/0306-4522(93)90050-p. [DOI] [PubMed] [Google Scholar]
- Paré D, Royer S, Smith Y, Lang EJ. Contextual inhibitory gating of impulse traffic in the intra-amygdaloid network. Ann N Y Acad Sci. 2003;985:78–91. doi: 10.1111/j.1749-6632.2003.tb07073.x. [DOI] [PubMed] [Google Scholar]
- Paré D, Quirk GJ, Ledoux JE. New vistas on amygdala networks in conditioned fear. J Neurophysiol. 2004;92:1–9. doi: 10.1152/jn.00153.2004. [DOI] [PubMed] [Google Scholar]
- Phelps EA, LeDoux JE. Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron. 2005;48:175–187. doi: 10.1016/j.neuron.2005.09.025. [DOI] [PubMed] [Google Scholar]
- Reinscheid RK, Xu YL, Okamura N, Zeng J, Chung S, Pai R, Wang Z, Civelli O. Pharmacological characterization of human and murine neuropeptide s receptor variants. J Pharmacol Exp Ther. 2005;315:1338–1345. doi: 10.1124/jpet.105.093427. [DOI] [PubMed] [Google Scholar]
- Reinscheid RK. Phylogenetic appearance of neuropeptide S precursor proteins in tetrapods. Peptides. 2007;28:830–837. doi: 10.1016/j.peptides.2007.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repa JC, Muller J, Apergis J, Desrochers TM, Zhou Y, LeDoux JE. Two different lateral amygdala cell populations contribute to the initiation and storage of memory. Nat Neurosci. 2001;4:724–731. doi: 10.1038/89512. [DOI] [PubMed] [Google Scholar]
- Royer S, Martina M, Paré D. An inhibitory interface gates impulse traffic between the input and output stations of the amygdala. J Neurosci. 1999;19:10575–10583. doi: 10.1523/JNEUROSCI.19-23-10575.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royer S, Paré D. Bidirectional synaptic plasticity in intercalated amygdala neurons and the extinction of conditioned fear responses. Neuroscience. 2002;115:455–462. doi: 10.1016/s0306-4522(02)00455-4. [DOI] [PubMed] [Google Scholar]
- Rudolph U, Crestani F, Möhler H. GABA(A) receptor subtypes: dissecting their pharmacological functions. Trends Pharmacol Sci. 2001;22:188–194. doi: 10.1016/s0165-6147(00)01646-1. [DOI] [PubMed] [Google Scholar]
- Sosulina L, Meis S, Seifert G, Steinhäuser C, Pape HC. Classification of projection neurons and interneurons in the rat lateral amygdala based upon cluster analysis. Mol Cell Neurosci. 2006;33:57–67. doi: 10.1016/j.mcn.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Szinyei C, Heinbockel T, Montagne J, Pape HC. Putative cortical and thalamic inputs elicit convergent excitation in a population of GABAergic interneurons of the lateral amygdala. J Neurosci. 2000;20:8909–8915. doi: 10.1523/JNEUROSCI.20-23-08909.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szinyei C, Stork O, Pape HC. Contribution of NR2B subunits to synaptic transmission in amygdaloid interneurons. J Neurosci. 2003;23:2549–2556. doi: 10.1523/JNEUROSCI.23-07-02549.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamamaki N, Yanagawa Y, Tomioka R, Miyazak iJ, Obata K, Kaneko T. Green fluorescent protein expression and colocalization with calretinin, parvalbumin, and somatostatin in the GAD67-GFP knock-in mouse. J Comp Neurol. 2003;467:60–79. doi: 10.1002/cne.10905. [DOI] [PubMed] [Google Scholar]
- Xu YL, Reinscheid RK, Huitron-Resendiz S, Clark SD, Wang Z, Lin SH, Brucher FA, Zeng J, Ly NK, Henriksen SJ, de Lecea L, Civelli O. Neuropeptide S: a neuropeptide promoting arousal and anxiolytic-like effects. Neuron. 2004;43:487–497. doi: 10.1016/j.neuron.2004.08.005. [DOI] [PubMed] [Google Scholar]
- Xu YL, Gall CM, Jackson VR, Civelli O, Reinscheid RK. Distribution of neuropeptide S receptor mRNA and neurochemical characteristics of neuropeptide S-expressing neurons in the rat brain. J Comp Neurol. 2007;500:84–102. doi: 10.1002/cne.21159. [DOI] [PubMed] [Google Scholar]
- Yang YL, Lu KT. Facilitation of conditioned fear extinction by d-cycloserine is mediated by mitogen-activated protein kinase and phosphatidylinositol 3-kinase cascades and requires de novo protein synthesis in basolateral nucleus of amygdala. Neuroscience. 2005;134:247–260. doi: 10.1016/j.neuroscience.2005.04.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.