Abstract
Context
The usual treatment for opioid-addicted youth is detoxification and counseling. Extended medication-assisted therapy may be more helpful.
Objective
To evaluate the efficacy of continuing buprenorphine-naloxone for 12 weeks vs detoxification for opioid-addicted youth.
Design, Setting, and Patients
Clinical trial at 6 community programs from July 2003 to December 2006 including 152 patients aged 15 to 21 years who were randomized to 12 weeks of buprenorphine-naloxone or a 14-day taper (detox).
Interventions
Patients in the 12-week buprenorphine-naloxone group were prescribed up to 24 mg per day for 9 weeks and then tapered to week 12; patients in the detox group were prescribed up to 14 mg per day and then tapered to day 14. All were offered weekly individual and group counseling.
Main Outcome Measure
Opioid-positive urine test result at weeks 4, 8, and 12.
Results
The number of patients younger than 18 years was too small to analyze separately, but overall, patients in the detox group had higher proportions of opioid-positive urine test results at weeks 4 and 8 but not at week 12 ( = 4.93, P = .09). At week 4, 59 detox patients had positive results (61%; 95% confidence interval [CI] = 47%-75%) vs 58 12-week buprenorphine-naloxone patients (26%; 95% CI = 14%-38%). At week 8, 53 detox patients had positive results (54%; 95% CI = 38%-70%) vs 52 12-week buprenorphine-naloxone patients (23%; 95% CI = 11%-35%). At week 12, 53 detox patients had positive results (51%; 95% CI = 35%-67%) vs 49 12-week buprenorphine-naloxone patients (43%; 95% CI = 29%-57%). By week 12, 16 of 78 detox patients (20.5%) remained in treatment vs 52 of 74 12-week buprenorphine-naloxone patients (70%; = 32.90, P < .001). During weeks 1 through 12, patients in the 12-week buprenorphine-naloxone group reported less opioid use ( = 18.45, P < .001), less injecting ( = 6.00, P = .01), and less nonstudy addiction treatment ( = 25.82, P < .001). High levels of opioid use occurred in both groups at follow-up. Four of 83 patients who tested negative for hepatitis C at baseline were positive for hepatitis C at week 12.
Conclusions
Continuing treatment with buprenorphine-naloxone improved outcome compared with short-term detoxification. Further research is necessary to assess the efficacy and safety of longer-term treatment with buprenorphine for young individuals with opioid dependence.
Recent concern has focused on opioid use among youth. For example, the proportion of 12th graders reporting past-year heroin use increased from 0.6% in 1992 to 0.9% in 2006. Similar increases occurred with pharmaceutical opioids—3.3% in 1992 to 9.5% in 20041—and recent data show that 13.4% of individuals aged 12 years or older who reported new use of heroin in the past 13 to 24 months meet criteria from the Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition) (DSM-IV) for dependence.2
The usual treatment for opioid-addicted youth is short-term detoxification and individual or group therapy in residential or outpatient settings over weeks or months. Clinicians report that relapse is high, yet many programs remain strongly committed to this approach and, except for treating withdrawal, do not use agonist medication. A few observational reports of methadone maintenance for opioid-addicted youth from the 1970s showed positive results3,4; however, only 1 controlled study of addiction-related pharmacotherapy for opioid-addicted youth has been published. It showed less use and more transitions to naltrexone at 30 days in patients receiving buprenorphine vs clonidine.5
Buprenorphine is a schedule III, μ-opioid partial agonist with a greater margin of safety than full agonists and a less intensive withdrawal.6-8 It is approved for treatment of individuals aged 16 years and older, although it was studied mainly in adults who were addicted for 5 to 10 years or longer.9-16 It has been combined with naloxone in a 4:1 ratio in an attempt to reduce abuse if crushed and injected, and a recent Finnish study found that this combination reduced its “street” value, often a surrogate for abuse liability.17
Based on the dangers associated with untreated opioid addiction, the commitment of programs treating opioid-addicted youth to nonmedication therapies, and favorable results with buprenorphine in other studies, we initiated a randomized trial of more extended treatment vs the usual short-term detoxification among opioid-dependent youth. The study was conducted at 6 sites in the National Institute on Drug Abuse (NIDA) Clinical Trials Network.
Methods
Sites
Six programs participated: Ayundantes, Española, New Mexico; Brandywine Counseling, Newark, Delaware; Duke Addictions Program, Durham, North Carolina; Mercy Recovery, Westbrook, Maine; Mountain Manor Treatment Center, Baltimore, Maryland; and the University of New Mexico Addiction and Substance Abuse Programs, Albuquerque. Four were methadone programs and 2 were adolescent programs that started using buprenorphine-naloxone for the study. Recruitment was stopped at the Newark (n = 3 patients) and Española (n = 8 patients) sites midway through the study due to slow enrollment; however, treatment and follow-up of randomized patients continued. The numbers of patients at other sites ranged from 29 to 52. The institutional review boards at the University of Pennsylvania and at each trial site approved the study.
Participants and Consent/Assent
The study was open to individuals aged 14 to 21 years who met DSM-IV criteria for opioid dependence with physiologic features18 and who sought outpatient treatment. Participants aged 18 to 21 years had to provide written consent and correctly answer 9 of 10 questions testing their understanding of the study; for participants aged 14 to 17 years, written assent and written parental consent were required and both participants and their parents had to pass the quiz. Exclusion criteria were having medical or psychiatric conditions likely to make participation difficult or unsafe; abusing alcohol or sedatives or using benzodiazepines for more than 15 days in the last 28 days; having had a sedative overdose in the past 6 months; being unable to provide a urine test result negative for benzodiazepine and methadone (in up to 3 attempts); receiving other addiction treatment; being likely to be incarcerated or to leave the area; breastfeeding or being pregnant; being unable or unwilling to use effective birth control; or receiving psychotropic medication other than a selective serotonin reuptake inhibitor. Participants defined their race and ethnicity using a demographic form standardized for the Clinical Trials Network according to National Institutes of Health policy.
Enrollment and Randomization
Patients were enrolled between July 2003 and December 2005 and randomized to 14-day outpatient detoxification (detox) or 12 weeks of treatment with buprenorphine-naloxone. Randomization occurred through an automated 24-hour service at the Veterans Affairs Cooperative Studies Program in Perry Point, Maryland, that was programmed to randomize patients separately by site. At each site, a biased-coin randomization19 protected against severe imbalance of sex, ethnicity, route of administration, and age across the treatment groups. Age was dichotomized as 14 to 18 years or 18 to 21 years, ethnicity as the majority ethnic group vs all others within the site, and route of administration as injecting or noninjecting. Balance was assessed by comparing the group sum of the binary indicators as each new patient was randomized. If both groups were balanced when a new patient was being randomized, then each group had an allocation probability of 1/2; if there was an imbalance, then the group with the higher score on the sum of indicators received an allocation probability of 1/3 and the other group a probability of 2/3. The indicator data were analyzed by K.D. and K.G.L.
Medication and Dosing
Reckitt Benckiser Pharmaceuticals Inc (Richmond, Virginia) provided medication, and the NIDA coordinated its distribution. Patients receiving buprenorphine-naloxone were instructed to not use heroin or other opioids for at least 6 hours and to be experiencing mild/moderate withdrawal prior to the first dose. The properties of buprenorphine-naloxone were explained during the consent/assent process and reviewed again prior to the first dose so patients understood they needed to hold the medication under the tongue until it dissolved and that it was likely to cause withdrawal if dissolved and injected by someone who was opioid dependent. Medication was administered on site 5 to 7 days per week (patients received take-home doses on days they were not medicated on site if a site was not open 7 days a week), and research assistants or site physicians directly observed it. The first dose was 2-mg buprenorphine with 0.5-mg naloxone. Study personnel observed the patient for 1.5 to 2 hours, and a second dose of 2 to 6 mg (expressed as buprenorphine) was administered if appropriate. On day 2, patients received the dose from day 1 unless considered overmedicated or undermedicated by a clinical assessment, were observed for 1.5 to 2 hours, and the dose adjusted by 2 to 6 mg as needed. On day 3, patients were given the dose from day 2 unless it needed adjustment, observed for 1.5 to 2 hours, and given another adjustment if needed.
Patients in the 12-week buprenorphine-naloxone group received up to a maximum amount of 24 mg per day and began a taper at week 9 that ended by week 12. Patients in the detox group received up to a maximum amount of 14-mg buprenorphine per day and ended their taper by day 14. If a patient missed 3 consecutive days of doses, medication was stopped; it was not restarted for patients in the detox group. Medication was restarted for patients in the 12-week buprenorphine-naloxone group if they returned within 7 days of the last dose. Patients who restarted were given half the amount of the last dose received and observed for 1.5 hours. If the medication was tolerated, they received a portion or the remainder of the dose. Patients who dropped out for missing medication were encouraged to continue in counseling treatment. Adverse events were assessed by weekly vital signs, assessments for sedation and withdrawal, and questions about additional medications received and adverse effects in weeks 1 through 12; similar assessments were done at months 6, 9, and 12. Electrocardiograms and liver enzyme levels were analyzed at baseline and at 4 and 12 weeks.
Drug Counseling
Patients were scheduled for 1 individual and 1 group session per week with more frequent sessions if needed. Most counselors were licensed clinical addictions specialists or had master's degrees in counseling or social work. Counseling used methods in NIDA manuals20,21 and was standardized by a 3-hour training. One to 3 counselors treated study patients at each site and were supervised using local procedures. Counseling encouraged making positive relationships and stopping drug use, taking medication as prescribed, tolerating stressful events without using drugs, keeping appointments, teaching ways to avoid drug-using situations, educating about addiction, giving positive feedback for achieving goals, referring for treatment of associated problems, and participating in age-appropriate self-help groups.
Primary and Secondary Outcomes
The primary outcome was opioid-positive urine test results at weeks 4, 8, and 12. Urine samples were tested for adulteration (color, specific gravity, temperature), although most patients were not observed during the collection because it was difficult to match female staff with female patients and vice-versa. Two tests were used: the Sure-Step (Inverness Medical Innovations, Bedford, England) that identifies amphetamine, barbiturate, benzodiazepines, cocaine, methadone, methamphetamine, morphine, hydrocodone, hydromorphone, oxycodone, phencyclidine, and tetrahydrocannabinol; and the Rapid One OXY (American Bio Medica Corp, Kinderhook, New York), which is more sensitive to oxycodone.
Secondary outcomes were dropout from the assigned condition, self-reported use, injecting, enrollment in addiction treatment outside the assigned condition, other drug use, and adverse events. Patients were considered dropouts if they missed medication for 3 consecutive days if in the detox group or 7 consecutive days if in the 12-week buprenorphine-naloxone group, did not have an individual or group session lasting 30 minutes or more for 14 consecutive days, enrolled in other addiction treatment, asked to be withdrawn, went to jail, or died. Follow-up visits at months 6, 9, and 12 included assessing self-reported use of opioids, alcohol, marijuana, and cocaine and injecting in the past month and determining whether patients were receiving other addiction treatment. Research assistants likely knew group assignments because the study was not blinded. Patients were paid $5 each for weekly assessments and $75 each for assessments at weeks 4, 8, and 12 and months 6, 9, and 12.
Statistical Methods
General estimating equation (GEE) models compared groups on longitudinal outcomes using a compound symmetry, working correlation structure and empirical standard errors that can accommodate dichotomous dependent variables.22 Explanatory variables in models examining urine test–confirmed opioid use were baseline status, site, treatment group, time (as a categorical variable), and treatment group × time interactions. Sample sizes for 2 sites (those with 3 and 8 patients) prevented assessment of group × site interactions. In analyses excluding these sites, group × site interactions were not observed; thus, the models presented include data from all sites and do not include a group × site interaction term.
A pattern-mixture model23 was used to assess the impact of missing data on urine test results. Pattern mixture models extend the basic repeated measures by including a variable that describes the main patterns of missing data as a main effect and an interaction with other variables (week and group). Significant interactions with the missing data indicator on the main variables suggest that its effects differ across levels of missing data and that missing data may not be ignorable. Following suggested guidelines,23 we used time of last data provision (a categorical variable representing week 4, 8, or 12) as the missing variable. Another approach often taken is to impute missing tests as positive. If results obtained for the original and imputed models differ substantially, missing data may not be ignorable. Both methods were used to evaluate the effects of data on the primary outcome wherein missing urine test results were counted as opioid positive.
General estimating equation models examined group differences for binary secondary outcomes (retention, self-reported drug use, injecting). Models were similar to those outlined previously except that baseline status was not included in the self-reported opioid and retention analyses due to lack of variability. When models failed to converge (ie, self-reported cocaine and marijuana use, injection use), Mantel-Haenszel analyses were performed that examined use during the whole time period and stratified on site. To assess group differences on cross-sectional outcomes, logistic regression analyses were used for binary outcomes (nonstudy treatment, received other treatment), and a generalized linear model was used for number of counseling sessions attended. These models included terms for condition and site.
The study was designed to have 80% power to detect a difference of 18% between the groups at each of the 3 time points at a significance level of 5% and, assuming a 30% loss to attrition, a within-subject repeated-measures correlation of 0.5. With an additional adjustment to allow for nesting effects due to multiple sites, this yielded a required sample size of 120 per group. The study randomized only 78 patients to detox and 74 patients to receive 12 weeks of buprenorphine-naloxone, rather than the 120 originally planned. With the same assumptions as used for the original design, this would yield a power of only 58% for the original target effect. In the study, the attrition, within-person correlation, and site effects were comparable with the design assumptions. However, the effect sizes at weeks 4 and 8 were larger than expected (35% and 31%, rather than the planned 18%) while the effect at 12 weeks was smaller (8% rather than 18%). Thus, although power was lower for the designed effect, the observed effects were larger. Statistical analyses were performed with SAS version 9.1 (SAS Institute, Cary, North Carolina).
Results
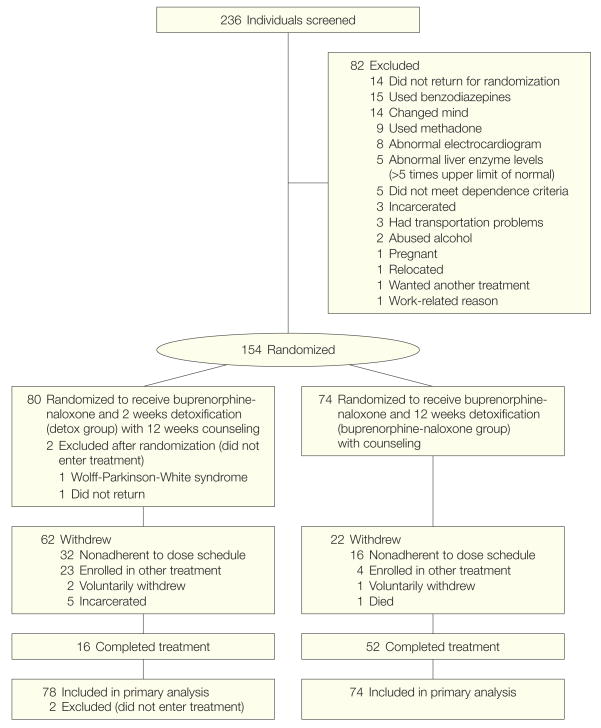
Of 236 patients screened, 154 were randomized and 152 entered treatment (Figure 1). The most common reasons for exclusion were use of benzodiazepines and failure to return. There were no significant group differences in sex, race, years of drug use, injecting in the past 30 days, age, hepatitis C status, work status, educational level, or marital status (Table 1). Although the study was open to individuals aged 14 to 21 years, only one 15-year-old and no 14-year-olds enrolled. Maximum doses for detox patients were as follows: 24 (31%) received 2 to 8 mg and 53 (68%) received 9 to 14 mg. For patients receiving 12 weeks of buprenorphine-naloxone, 20 (27%) received 2 to 8 mg, 43 (59%) received 9 to 16 mg, and 10 (14%) received 17 to 24 mg.
Figure 1.
Participation in Trial of Buprenorphine-Naloxone for Treatment of Opioid-Addicted Youth
Table 1.
Participant Characteristicsa
| No. (%) | ||
|---|---|---|
| Characteristic | Detoxification Group
(n = 78) |
12-Week Buprenorphine-Naloxone Group
(n = 74) |
| Male sex | 48 (61.5) | 42 (56.8) |
|
| ||
| Age, mean (SD), y | 19.2 (1.6) | 19.14 (1.4) |
|
| ||
| < 18 y | 14 (18) | 12 (16) |
|
| ||
| Race/ethnicity | ||
| White | 56 (71.8) | 56 (75.7) |
|
| ||
| African American | 2 (2.6) | 1 (1.4) |
|
| ||
| Hispanic | 20 (25.6) | 18 (24.3) |
|
| ||
| Filipino | 1 (1.3) | 0 |
|
| ||
| Main problem heroin | 41 (53) | 42 (57) |
|
| ||
| Main problem other opiate/analgesics | 25 (32) | 27 (36) |
|
| ||
| Main problem polydrug | 11 (14) | 5 (7) |
|
| ||
| Heroin use, median, yb | 1 (1/2) | 1 (0/3) |
|
| ||
| Opiate use, median, yb | 1 (0/2) | 1 (0/3) |
|
| ||
| Cocaine use, median, yb | 0 (0/1) | 0 (0/1) |
|
| ||
| Marijuana use, median, yb | 4 (2/6) | 3 (1/6) |
|
| ||
| Injecting (past 30 d) | 36 (48) | 35 (47) |
|
| ||
| Positive for hepatitis C | 16 (20.5) | 12 (16.2) |
|
| ||
| Education, mean (SD), y | 11.3 (1.5) | 11.0 (1.7) |
|
| ||
| In school (past 6 mo) | 17 (21.8) | 21 (28.4) |
|
| ||
| Working (past 6 mo) | 56 (71.8) | 53 (71.6) |
No between-group differences were observed for the following variables used in the stratified randomization: sex (P = .68), race white/nonwhite (P = .65), injecting/not injecting (P = .93), and age under 18 y/18-21 y (P = .78).
Because of the skewness of the data, values presented reflect medians; first and third quartiles are presented in parentheses.
Primary Outcome During Treatment: Opioid-Positive Urine Test Results
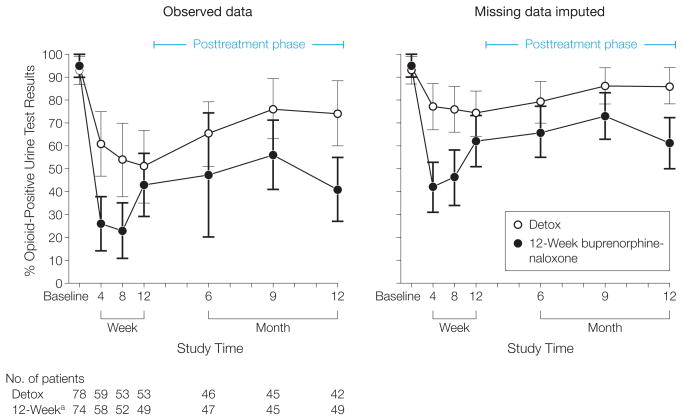
Patients were contacted at all assessment points regardless of whether they remained in treatment. The number of detox patients and 12-week buprenorphine-naloxone patients providing urine at weeks 4, 8, and 12 is in Figure 2; 41 detox and 49 12-week buprenorphine-naloxone patients provided all samples through week 12.
Figure 2.
Percentage of Opioid-Positive Urine Test Results at Baseline and Weeks 4, 8, and 12 and Follow-up Months 6, 9, and 12
Detox indicates detoxification group. Error bars indicate 95% confidence intervals.
a12-Week buprenorphine-naloxone group.
At week 4, 59 detox patients had positive results (61%; 95% confidence interval [CI] = 47%-75%) vs 58 12-week buprenorphine-naloxone patients (26%; 95% CI = 14%-38%). At week 8, 53 detox patients had positive results (54%; 95% CI = 38%-70%) vs 52 12-week buprenorphine-naloxone patients (23%; 95% CI = 11%-35%). At week 12, 53 detox patients had positive results (51%; 95% CI = 35%-67%) vs 49 12-week buprenorphine-naloxone patients (43%; 95% CI = 29%-57%).
A GEE model that ignored missing data showed a marginal group × time interaction ( = 4.93, P = .09). While not attaining the usual 5% significance, it likely reflected a lack of power for interaction effects rather than constant treatment effects at each time point. Therefore we retained the term in our model, thus allowing different effects at each time point. Results were that detox patients were more likely to provide opiate-positive urine at week 4 (odds ratio [OR] = 7.05; 95% CI = 2.87-17.29; = 18.21, P < .001) and week 8 (OR = 5.07; 95% CI = 2.02-12.79; = 12.79, P = .001) but not week 12 (OR = 1.84, 95% CI = 0.75-4.49; = 1.78, P = .18).
While inclusion of the group × time interaction gave a summary of the data, removing the interaction and accepting equal buprenorphine effects at each time point yielded a significant main effect for buprenorphine ( = 18.32, P < .001) across 12 weeks. Similar results were obtained when missing urine test results were imputed positive (Figure 2), in which case the group × time interaction was slightly more significant ( = 5.74, P = .06). Removal of the interaction yielded a main effect for buprenorphine across 12 weeks ( = 19.07, P < .001). Results of the pattern mixture model predicting opioid-positive urine test results revealed no interaction of dropout time with group or week (dropout time × group: = 0.03, P = .86; dropout time × week: = 0.14, P = .71; dropout time × group × week: = 0.06, P = .81). Because there were no interactions pertaining to dropout time, results suggested that missing data were not invalidating the group effect.
Secondary Outcomes During Treatment
Patients in the detox group were less likely to remain in the assigned treatment than those in the 12-week buprenorphine-naloxone group (OR = 0.13, 95% CI = 0.07-0.26, = 32.90, P < .001) (Table 2). Among 78 detox patients, 16 (20.5%) completed; among 74 in the 12-week buprenorphine-naloxone group, 52 (70%) completed. The most common reason for noncompletion was missing 2 weeks of counseling. Detox patients were more likely to report opioid use (OR = 4.30, 95% CI = 2.25-8.22; = 18.45, P < .001), marijuana use (OR = 6.15, 95% CI = 2.10-18.01; = 12.23, P = .001), and injection (OR = 3.54, 95% CI = 1.27-9.87; = 6.00, P = .01). In addition, detox patients were more likely to report enrollment in other addiction treatment (OR = 13.09, 95% CI = 3.73-45.89; = 25.82, P < .001) and cocaine use (OR = 16.39, 95% CI = 3.07-87.47; = 14.47, P = .001), although the CIs suggest that the estimates are somewhat unstable due to small cell counts. Groups did not differ in rates of self-reported alcohol use, (P = .42). Patients in the 12-week buprenorphine-naloxone group attended more counseling sessions (mean No. of sessions = 11.77, 95% CI = 9.73-13.81) than patients in the detox group (mean No. of sessions = 5.06, 95% CI = 3.62-6.50; F1, 145 = 33.70, P < .001).
Table 2.
Secondary Outcomes
| % (95% CI) | |||||
|---|---|---|---|---|---|
| Outcome | Time | Detoxification Group | 12-Week Buprenorphine-Naloxone Group | OR (95% CI) GEE Score Test of Group Effect | |
| Weeks 4, 8, and 12 | |||||
| Retention in trial | 4 wk | 45 (34-56) | 84 (75-93) |
|
0.13 (0.07-0.26) = 32.90, P < .001 |
|
| |||||
| 8 wk | 27 (17-37) | 74 (64-84) | |||
|
| |||||
| 12 wk | 21 (12-30) | 70 (59-81) | |||
|
| |||||
| Nonstudy treatment | 1-12 wk | 35 (24-46) | 4 (0-9) |
|
13.09 (3.73-45.89) = 25.82, P < .001 |
|
| |||||
| Any opioid use past week (self-report) | 4 wk | 63 (49-77) | 26 (14-38) |
|
4.30 (2.25-8.22) = 18.45, P < .001 |
|
| |||||
| 8 wk | 52 (37-67) | 19 (8-30) | |||
|
| |||||
| 12 wk | 55 (40-70) | 38 (24-52) | |||
|
| |||||
| Any alcohol use past week (self-report) | 4 wk | 46 (31-61) | 28 (16-40) |
|
1.35 (0.66-2.77) = 0.64, P = .42 |
|
| |||||
| 8 wk | 36 (21-51) | 29 (16-42) | |||
|
| |||||
| 12 wk | 24 (11-37) | 22 (10-34) | |||
|
| |||||
| Any marijuana use past week (self-report)a | 4 wk | 24 (11-37) | 9 (1-17) |
|
6.15 (2.10-18.01) = 12.23, P = .001 |
|
| |||||
| 8 wk | 19 (7-31) | 6 (0-13) | |||
|
| |||||
| 12 wk | 26 (12-40) | 16 (6-26) | |||
|
| |||||
| Any cocaine use past week (self-report)a | 4 wk | 15 (4-26) | 2 (0-6) |
|
16.39 (3.07-87.47) = 14.47, P = .001 |
|
| |||||
| 8 wk | 14 (3-25) | 2 (0-6) | |||
|
| |||||
| 12 wk | 12 (2-22) | 2 (0-6) | |||
|
| |||||
| Counseling sessions attended, mean, No.b | 1-12 wk | 5.06 (3.62-6.50) | 11.77 (9.73-13.81) | F1, 145 = 33.70, P < .001 | |
|
| |||||
| Injecting past 30 d (self-report)a | 4 wk | 37 (23-51) | 21 (10-32) |
|
3.54 (1.27-9.87) = 6.00, P = .01 |
|
| |||||
| 8 wk | 26 (12-40) | 13 (4-22) | |||
|
| |||||
| 12 wk | 33 (18-48) | 16 (6-26) | |||
|
| |||||
| Months 6, 9, and 12 | |||||
| Any opioid use past month (self-report)c | 6 mo | 63 (49-77) | 72 (59-85) |
|
1.34 (0.70-2.57) = 0.80, P = .37 |
|
| |||||
| 9 mo | 70 (56-84) | 53 (38-68) | |||
|
| |||||
| 12 mo | 72 (58-86) | 53 (39-67) | |||
|
| |||||
| Any alcohol use past month (self-report) | 6 mo | 46 (31-61) | 37 (23-51) |
|
1.30 (0.67-2.53) = 0.60, P = .44 |
|
| |||||
| 9 mo | 43 (28-58) | 36 (22-50) | |||
|
| |||||
| 12 mo | 47 (32-62) | 43 (29-57) | |||
|
| |||||
| Any marijuana use past month (self-report)a | 6 mo | 30 (16-44) | 24 (11-37) |
|
1.33 (0.55-3.18) = 0.39, P = .53 |
|
| |||||
| 9 mo | 27 (14-40) | 22 (10-34) | |||
|
| |||||
| 12 mo | 23 (10-36) | 22 (10-34) | |||
|
| |||||
| Any cocaine use past month (self-report)a | 6 mo | 20 (8-32) | 11 (2-20) |
|
3.84 (1.47-10.02) = 7.45, P = .006 |
|
| |||||
| 9 mo | 30 (16-44) | 7 (0-15) | |||
|
| |||||
| 12 mo | 30 (16-44) | 18 (7-29) | |||
|
| |||||
| Injecting past 30 d (self-report) | 6 mo | 26 (13-39) | 37 (23-51) |
|
1.60 (0.71-3.60) = 1.23, P = .23 |
|
| |||||
| 9 mo | 35 (21-49) | 20 (8-32) | |||
|
| |||||
| 12 mo | 28 (14-42) | 18 (7-29) | |||
|
| |||||
| In addiction treatment | 6 mo | 37 (23-51) | 45 (30-60) |
|
0.61 (0.35-1.09) = 2.67, P = .10 |
|
| |||||
| 9 mo | 31 (17-45) | 44 (29-59) | |||
|
| |||||
| 12 mo | 40 (25-55) | 53 (39-67) | |||
Abbreviations: CI, confidence interval; GEE, general estimating equation; OR, odds ratio.
Mantel-Haenszel analysis stratifying by site for binary outcome of use across 12 weeks. General estimating equation models failed to converge.
F test from analysis-of-covariance model.
Significant group × time interaction, = 6.99, P = .03; groups differed significantly at month 12.
Posttreatment Outcomes: Months 6, 9, and 12
Opioid-positive urine test results at months 6, 9, and 12 are shown in Figure 2. Patients in the detox group provided higher proportions of positive urine test results than patients in the 12-week buprenorphine-naloxone group when missing values were not imputed (OR = 2.65, 95% CI = 1.28-5.50, = 6.64, P = .01), although high rates were seen in both groups (12-week buprenorphine-naloxone group: 41%-56%; mean rate = 48%; detox: 65%-76%; mean rate = 72%). Similar results were observed when missing values were imputed as positive (OR = 2.85, 95% CI = 1.52-5.33, = 9.67, P = .002), although rates were necessarily higher (12-week buprenorphine-naloxone: 61%-73%; mean = 71%; detox: 79%-86%, mean rate = 83%). There was a trend ( = 2.67, P = .10) for fewer detox patients to be in other addiction treatment (OR = 0.61, 95% CI = 0.35-1.09). Although detox patients displayed significantly more self-reported cocaine use than 12-week buprenorphine-naloxone patients (OR = 3.84, 95% CI = 1.47-10.02; = 7.45, P = .006), the 2 groups did not differ in rates of self-reported use of alcohol (OR = 1.30, 95% CI = 0.67-2.53; = 0.60, P = .44) or marijuana (OR = 1.33, 95% CI = 0.55-3.18, = 0.39, P = .53) and injecting (OR = 1.60, 95% CI = 0.71-3.60, = 1.23, P = .23).
Adverse Events
The sample size was not sufficiently large to draw conclusions about safety; however, no serious adverse events attributable to buprenorphine-naloxone were reported and no patients were removed for adverse events. Headaches were the most common events, reported by 16% to 21% of patients in both groups. Other problems were reported by less than 10% of patients and were typical of problems seen in primary care or problems with opioids (eg, nausea, insomnia, stomachache, vomiting, anxiety). One death occurred in a 19-year-old patient in the 12-week buprenorphine-naloxone group who dropped out after 3 doses and was not located until her obituary appeared in a newspaper 3 months later. The medical examiner report cited methadone overdose as the cause. Four of 83 patients who tested negative for hepatitis C at baseline were positive at week 12, 2 in each group.
Comment
Opioid-positive urine test results, retention in the trial, self-reported opioid use, injecting behavior, enrollment in nonstudy treatment, and use of cocaine and marijuana strongly favored patients in the 12-week buprenorphine-naloxone group during weeks 1 through 12. They had much less use of opioids, cocaine, and marijuana; much better treatment retention; and much less injecting and need for additional treatment while on medication. The exception of these results favoring the 12-week buprenorphine-naloxone group was their urine test results at week 12 when the dose taper ended. A similar loss of differences was seen in self-reported opioid use and injecting at 6, 9, and 12 months. Taken together, these data show that stopping buprenorphine-naloxone had comparably negative effects in both groups, with effects occurring earlier and with somewhat greater severity in patients in the detox group. Although patients were young and reported regular opioid use for 1.5 years on average, their findings resembled those after detoxification of opioid-dependent adults with much longer periods of addiction. Interestingly, 12-week buprenorphine-naloxone patients had lower proportions of opioid-positive urine test results at follow-up, although differences with detox patients were much less than in weeks 1 through 12, possibly because 12-week buprenorphine-naloxone patients tended to be more engaged in longer-term treatment.
The 18% prevalence of hepatitis C and conversion of 4 of 83 patients from negative to positive by week 12 is alarming, but it is a known consequence of injection use because hepatitis C is easily acquired by sharing equipment.24 This finding, and data showing that methadone or buprenorphine maintenance reduces risk of infection with human immunodeficiency virus (HIV) and overdose death,25-31 points to benefits that can be associated with the prompt use of buprenorphine-naloxone—for extended periods—as part of standard outpatient treatment. The data do not provide much information on how long buprenorphine-naloxone should be continued, but considering the potential for rapid re-addiction following medication cessation, overdose death, infection with HIV, and addiction-related psychosocial impairments, they show that detoxification, whether performed over 2 weeks or 3 months, was largely ineffective for young patients with short periods of addiction when done under similar outpatient conditions. Stated differently, these data suggest that once DSM-IV criteria for opioid dependence with physiologic features are met, the course of addiction appears similar regardless of its length and that clinicians should be in no hurry to stop an effective medication simply because the patient is young and has been addicted for a short time.
Limitations
The small proportion of patients younger than 18 years was not sufficient to meaningfully analyze their outcomes. A similar limitation was the almost total absence of young African American individuals, yet this finding was consistent with other data showing that they are much less affected by opioid addiction than young white individuals.32,33 We could not detect the surreptitious use of buprenorphine since it was not part of the urine testing; however, its use would probably magnify group differences because more detox than 12-week buprenorphine-naloxone patients used unprescribed opioids. The lack of blinding of evaluators was another limitation, but the assessments were objective (urine tests, dropout) or self-reported and unlikely to influence results. The frequent observed dosing ensured that patients took the medication as prescribed, but results might not be as good under less highly supervised conditions where more take-home doses are prescribed.
The low follow-up rate was another limitation; however, missing data did not appear to negate the main findings because analyses remained consistent even with conservative imputation of missing data. Although the findings were internally consistent and consonant with prestudy hypotheses, the follow-up problem made it difficult to estimate the number of patients who achieved recovery, defined as a “voluntarily maintained lifestyle characterized by sobriety, personal health, and citizenship.”34
We had no way to compare these results with intensive outpatient therapy, residential treatment, therapeutic community, or naltrexone. It was impossible to design a random assignment study including the first 3 options because they are in limited supply, and the programs we contacted did not feel comfortable using an agonist medication with this population except for short-term detoxification. Naltrexone may be more useful than it has been with opioid-addicted adults, especially if parents supervise adherence35,36 or an extended-release formulation is used; however, this formulation is not approved for opioid dependence.
We detected no adverse effects attributable to buprenorphine-naloxone; however, the number of patients was too small to adequately capture them and the study did not assess adverse effects beyond 12 months. Although undetected adverse effects are a constant risk, it is difficult to imagine an unfavorable risk/benefit ratio, at least in the short-term, considering the risks associated with the level of opioid use that was detected in the absence of medication. Similarly, we did not learn of any diversion, but the risk of this adverse event is greater in settings where more take-home doses are permitted.
Clinical Implications
Because much opioid addiction treatment has shifted from inpatient to outpatient where buprenorphine-naloxone can be administered, having it available in primary care, family practice, and adolescent programs has the potential to expand the treatment options currently available to opioid-addicted youth and significantly improve outcomes. Other effective medications, or longer and more intensive psychosocial treatments, may have similarly positive results. Studies are needed to explore these possibilities and to assess the efficacy and safety of longer-term treatment with buprenorphine for young individuals with opioid dependence.
Acknowledgments
Funding/Support: This study was supported by the following grants from the National Institute on Drug Abuse: U10-DA013711 to Duke University (Dr Patkar); U10-DA015831 to Harvard University (Dr Weiss); U10-DA13034 to Johns Hopkins University (Dr Stitzer); K12-DA000357 (Dr Subramaniam); U10-DA1533 to the University of New Mexico (Dr Miller); and U10-DA13043 and KO5-DA17009 (Dr Woody). Reckitt Benckiser provided medication for the study.
Footnotes
Trial Registration clinicaltrials.gov Identifier: NCT00078130
Author Contributions: Dr Woody had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Woody, Poole, Forman, McNicholas, Blaine, Lynch, Fudala.
Acquisition of data: Woody, Poole, Subramaniam, Bogenschutz, Abbott, Patkar, Publicker, McCain, Potter, Forman, Vetter, Lynch.
Analysis and interpretation of data: Woody, Poole, Dugosh, Bogenschutz, Patkar, McCain, Vetter, McNicholas, Lynch, Fudala.
Drafting of the manuscript: Woody, Poole, Subramaniam, Dugosh, McCain, Lynch.
Critical revision of the manuscript for important intellectual content: Woody, Poole, Subramaniam, Bogenschutz, Abbott, Patkar, Publicker, Potter, Forman, Vetter, McNicholas, Blaine, Fudala.
Statistical analysis: Dugosh, Lynch, Poole.
Obtained funding: Woody, Forman.
Administrative, technical, or material support: Woody, Poole, Subramaniam, Bogenschutz, Abbott, Patkar, Publicker, McCain, Potter, Forman, Vetter, Blaine, Lynch, Fudala.
Study supervision: Woody, Poole, Abbott, Patkar, McCain, Forman, Vetter, McNicholas, Lynch.
Financial Disclosures: Dr Woody reported being a member of the RADARS postmarketing study external advisory group whose job is to assess abuse of prescription medications. Denver Health administers RADARS and Abbott, Cephalon, Endo, Pricara/Ortho-NcNeil, Purdue Pharma, and Shire subscribe to its data. Dr Woody reported that Ortho-McNeil and Purdue Pharma funded similar work by him prior to his joining RADARS. Dr Woody reported that Schering-Plough, the European distributor for buprenorphine-naloxone, funded his travel costs to meetings in Sweden and Finland in June 2008 to present data from this study. Dr Bogenschutz reported receiving research funding from Forest and Lilly and having a confidentiality agreement with Lilly. Dr Forman reported being a faculty member at the University of Pennsylvania and co–principal investigator with Dr Woody on the Delaware Valley Node of the NIDA Clinical Trials Network until December 2005 when he joined Alkermes. Dr Patkar reported being a consultant to Bristol-Meyers Squibb, GlaxoSmithKline, and Reckitt Benckiser and being on the speakers' bureau for and receiving honoraria from Bristol-Meyers Squibb, Forest, GlaxoSmithKline, Janssen, Jazz Pharmaceuticals Lundbeck, McNeil Consumer & Specialty Inc, Organon, and Pfizer. Dr Publicker reported having been a speaker for Cephalon, Forest, and Reckitt Benckiser. Dr McNicholas reported having conducted training programs to certify physicians in the use of buprenorphine. Her expenses have been paid by unrestricted grants to universities that were often provided by Reckitt Benckiser. Dr Fudala reported having been employed by the University of Pennsylvania and Philadelphia VA Medical Center from 1991 until he joined Reckitt Benckiser in June 2005 and reported having been a consultant to Johnson & Johnson and Purdue Pharma.
Role of Sponsor: The protocol review committee of the Clinical Trials Network (CTN) of the National Institute on Drug Abuse provided guidance and final approval for the study design. The director and deputy director of the Center for the Clinical Trials Network (CCTN), the data and safety monitoring board of the CCTN, the operations coordinating committee of the CTN, and quarterly site visits from a subcontractor to the CCTN monitored the conduct, data collection, and data management. The publications committee of the CTN gave final approval of the analysis and interpretation of the data and approved the manuscript. Reckitt Benckiser provided medication for the study.
Additional Contributions: Charles P. O'Brien, MD, PhD, provided administrative support; Doreen Cardillo provided technical support for research staff; Cynthia Clark, CRNP, designed case report forms; Chris Petro, MA, developed the Web-based system that was used for data collection (all at University of Pennsylvania). Howard Moss, MD, provided suggestions for outcome measures (Philadelphia VA Medical Center; now with the National Institute on Alcohol and Alcohol Abuse, Rockville, Maryland). A. Thomas McLellan, PhD, provided critical review of the manuscript (Treatment Research Institute and University of Pennsylvania). The following provided additional medical support at their sites: Hilary Connery, MD, and Edward McCarthy, MD (Mercy Recovery); Marc Fishman, MD (Mountain Manor); and Robert Kushner, MD (University of New Mexico Addiction and Substance Abuse Programs). The following Principal Investigators provided administrative support for study teams at their sites: Leonard Handlesman, MD (Duke University; now deceased); William A. Miller, PhD (University of New Mexico); Maxine Stitzer, PhD (Johns Hopkins); and Roger Weiss, MD (Harvard University).
The following individuals collected data at participating sites: Hilary S. Connery, MD, PhD; Jennifer Sharpe Potter, PhD, MPH; and Scott Provost, MM, MSW, McLean Hospital Alcohol and Drug Abuse Treatment Program; Michael Bogenschutz, MD; Wendy Johnson, Robert Kushner, MD; Craig L. Pacheco, MS; Patrick Abbott, MD; Meredith Pampell, Marilyn W. Diener, RN; Dinah Lopez, LPN; Socorro Lopez-Mazon, RN; Anna Maria Padilla Morgan, MA; and Gloria A. White, RN, University of New Mexico Center on Alcoholism, Substance Abuse and Addictions; Leonard Pena, Matthew Lujan, Jafet Gonzalez, MD; Danny Jaramillo, Gabriella Ortiz, RN; and Edna Gonzales, RN, Ayundantes; Botonya Harris, Brandy Alsop, Janice Sneed, MHS, CADC; Joe Glick, MD; Kimberly Fisher, LPN; Maria C. Mancuso, MD; Michele S. Hofstetter, LPN; Pamela D. Stearn, MS; Stuart Narrod, MD; Thelma K. Malone, RN, CD, MHS; and Tracey Dale, LPN, NCCDN, Brandywine Treatment Center; Ashwin Patkar, MD; Emily Brickman, Florine Melvin, Karen McCain, MSN, FNP; Kevin D. Watkins, Leonard Handlesman, MD; Melissa Williams, Neena Ajwani, Peggy Arias, RN; Renita Woodall, and Roxanne Ellington, LCAS, Duke Psychiatry–Duke Addictions Programs; Thomas E. Allen, LCSW, LADC; Sarah Braun, Christine C. Evans, RNC, CARN; Christopher Coose, MS, LADC; Edward A. McCarthy, MD; Elizabeth K. Clay, RN; Lisa DiPietro, RN; Mark Publicker, MD; Sally Van Snepson, PA-C, LAc, MS; and Burma Wilkins, Mercy Addiction Treatment Clinic; Adrienne N. Dixon, MS, PA-C; Amber M. Harris, BA; Angie Wu, RN; Carligher Long, Cindy Voss, Elsie Lopez, LPN; Geetha Subramaniam, MD; Marc Fishman, MD; Paul Harrell III, and Shannon Garrett, MSW, Mountain Manor Treatment Clinic. None of those listed received funding for this work other than full or partial salary from the National Institute on Drug Abuse for their work on the study.
References
- 1.Monitoring the future: national survey results on drug use, 1975-2007: Table 5.2, Long-term trends in annual percentage of use of various drugs in grade 12. [October 2, 2008];:202. http://www.monitoringthefuture.org/pubs/monographs/vol1_2007.pdf.
- 2.US Department of Health and Human Services, Substance Abuse and Mental Health Services Administration; [October 10, 2008]. Highlights of recent reports on substance abuse and mental health. Office of Applied Studies http://www.oas.samhsa.gov/highlights.htm. [Google Scholar]
- 3.Nightingale SL, Wurmser L, Platt PC, et al. Adolescents on methadone: preliminary observations. Presented at: Third Annual Conference on Methadone Treatment; November 14-16, 1970; New York, New York. [Google Scholar]
- 4.Millman RB, Nyswander ME. Slow detoxification of adolescent heroin addicts in New York City. Presented at: Third Annual Conference on Methadone Treatment; November 14-16, 1970; New York, New York. [Google Scholar]
- 5.Marsch LA, Bickel WK, Badger GJ, et al. Comparison of pharmacological treatments for opioid-dependent adolescents. Arch Gen Psychiatry. 2005;62(10):1157–1164. doi: 10.1001/archpsyc.62.10.1157. [DOI] [PubMed] [Google Scholar]
- 6.Jasinski DR, Pevnick JS, Griffith JD. Human pharmacology and abuse potential of the analgesic buprenorphine: a potential agent for treating narcotic addiction. Arch Gen Psychiatry. 1978;35(4):501–516. doi: 10.1001/archpsyc.1978.01770280111012. [DOI] [PubMed] [Google Scholar]
- 7.Lewis JW. Buprenorphine. Drug Alcohol Depend. 1985;14(3-4):363–372. doi: 10.1016/0376-8716(85)90067-5. [DOI] [PubMed] [Google Scholar]
- 8.Walsh SL, Preston KL, Stitzer ML, Cone EJ, Bigelow GE. Clinical pharmacology of buprenorphine: ceiling effects at high doses. Clin Pharmacol Ther. 1994;55(5):569–580. doi: 10.1038/clpt.1994.71. [DOI] [PubMed] [Google Scholar]
- 9.Johnson RE, Jaffe JH, Fudala PJ. A controlled trial of buprenorphine treatment for opioid dependence. JAMA. 1992;267(20):2750–2755. [PubMed] [Google Scholar]
- 10.Johnson RE, Chutauepe MA, Strain EC, Walsh SL, Stitzer ML, Bigelow GE. A comparison of levomethadyl acetate, buprenorphine, and methadone for opioid dependence. N Engl J Med. 2000;343(18):1290–1297. doi: 10.1056/NEJM200011023431802. [DOI] [PubMed] [Google Scholar]
- 11.Schottenfeld RS, Pakes JR, Oliveto A, Ziedonis D, Kosten TR. Buprenorphine vs methadone maintenance treatment for concurrent opioid dependence and cocaine abuse. Arch Gen Psychiatry. 1997;54(8):713–720. doi: 10.1001/archpsyc.1997.01830200041006. [DOI] [PubMed] [Google Scholar]
- 12.Strain EC, Stitzer ML, Liebson IA, Bigelow GE. Buprenorphine versus methadone in the treatment of opioid dependence: self-reports, urinalysis, and addiction severity index. J Clin Psychopharmacol. 1996;16(1):58–67. doi: 10.1097/00004714-199602000-00010. [DOI] [PubMed] [Google Scholar]
- 13.Ling W, Charuvastra C, Collins JF, et al. Buprenorphine maintenance treatment of opioid dependence: a multicenter randomized clinical trial. Addiction. 1998;93(4):475–486. doi: 10.1046/j.1360-0443.1998.9344753.x. [DOI] [PubMed] [Google Scholar]
- 14.Fudala PJ, Yu E, Macfadden W, Boardman C, Chiang CN. Effects of buprenorphine and naloxone in morphine-stabilized opioid addicts. Drug Alcohol Depend. 1998;50(1):1–8. doi: 10.1016/s0376-8716(98)00008-8. [DOI] [PubMed] [Google Scholar]
- 15.Fiellin DA, Pantalon MV, Chawarski MC, et al. Counseling plus buprenorphine-naloxone maintenance therapy for opioid dependence. N Engl J Med. 2006;355(4):365–374. doi: 10.1056/NEJMoa055255. [DOI] [PubMed] [Google Scholar]
- 16.Magura S, Lee SJ, Salsitz EA, et al. Outcomes of buprenorphine maintenance in office-based practice. J Addict Dis. 2007;26(2):13–23. doi: 10.1300/J069v26n02_03. [DOI] [PubMed] [Google Scholar]
- 17.Alho H, Sinclair D, Vuori E, Holopainen A. Abuse liability of buprenorphine-naloxone tablets in untreated IV drug users. Drug Alcohol Depend. 2007;88(1):75–78. doi: 10.1016/j.drugalcdep.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 18.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 19.Efron B. Forcing a sequential experiment to be balanced. Biometrika. 1971;58:403–417. [Google Scholar]
- 20.Mercer DE, Woody GE. An individual drug counseling approach to treat cocaine addiction: the collaborative cocaine treatment study model. [September 29, 2008];Therapy Manuals for Drug Abuse: 3; NIH Publication No 99-4380. http://www.nida.nih.gov/TXManuals/IDCA/IDCA1.html.
- 21.Daley DC, Mercer DE. Counseling for cocaine addiction: the collaborative cocaine treatment study model. [September 29, 2008];Therapy Manuals for Drug Abuse: 3; NIH Publication No 99-4380. http://www.nida.nih.gov/TXManuals/IDCA/IDCA1.html.
- 22.Diggle PJ, Liang KY, Zeger SL. Analysis of Longitudinal Data. Oxford, England: Oxford Statistical Science Series; 1996. p. 13. [Google Scholar]
- 23.Hedeker D, Gibbons RD. Applications of random-effects pattern-mixture models for missing data in longitudinal studies. Psychol Methods. 1997;2:64–78. [Google Scholar]
- 24.Hahn JA, Page-Shafer K, Lum PJ, et al. Hepatitis C virus serocon version among young injection drug users: relationships and risks. J Infect Dis. 2002;186(11):1558–1564. doi: 10.1086/345554. [DOI] [PubMed] [Google Scholar]
- 25.Metzger DS, Navaline H, Woody GE. Drug abuse treatment as AIDS prevention. Public Health Rep. 1998;113(suppl 1):97–106. [PMC free article] [PubMed] [Google Scholar]
- 26.Auriacombe M, Franques P, Tignol J. Deaths attributable to methadone vs buprenorphine in France. JAMA. 2001;285(1):45. doi: 10.1001/jama.285.1.45. [DOI] [PubMed] [Google Scholar]
- 27.Lepere B, Gourarier L, Sanchez M, et al. Reduction in the number of lethal heroin overdoses in France since 1994: focus on substitution treatments [in French] Ann Med Interne (Paris) 2001;152(suppl 3):IS5–IS12. [PubMed] [Google Scholar]
- 28.Soyka M, Penning R, Wittchen U. Fatal poisoning in methadone and buprenorphine treated patients: are there differences? Pharmacopsychiatry. 2006;39(3):85–87. doi: 10.1055/s-2006-941482. [DOI] [PubMed] [Google Scholar]
- 29.Clausen T, Anchersen K, Waal H. Mortality prior to, during and after opioid maintenance treatment (OMT): a national prospective cross-registry study. Drug Alcohol Depend. 2008;94(1-3):151–157. doi: 10.1016/j.drugalcdep.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 30.Woody GE, Kane V, Lewis K, Thompson R. Premature deaths following discharge from methadone maintenance: a replication. J Addict Med. 2007;1(4):180–185. doi: 10.1097/ADM.0b013e318155980e. [DOI] [PubMed] [Google Scholar]
- 31.Kakko J, Svanborg KD, Kreek MJ, Heilig M. 1-year retention and social function after buprenorphine-assisted relapse prevention treatment for heroin dependence in Sweden: a randomized, placebo-controlled trial. Lancet. 2003;361(9358):662–668. doi: 10.1016/S0140-6736(03)12600-1. [DOI] [PubMed] [Google Scholar]
- 32.Rosenblum A, Parrino M, Schnoll SH, et al. Prescription opioid abuse among enrollees into methadone maintenance treatment. Drug Alcohol Depend. 2007;90(1):64–71. doi: 10.1016/j.drugalcdep.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 33.Broz D, Ouellet LJ. Racial and ethnic changes in heroin injection in the United States: implications for the HIV/AIDS epidemic. Drug Alcohol Depend. 2008;94(1-3):221–233. doi: 10.1016/j.drugalcdep.2007.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Betty Ford Institute Consensus Panel. What is recovery? a working definition from the Betty Ford Institute. J Subst Abuse Treat. 2007;33(3):221–228. doi: 10.1016/j.jsat.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 35.Krupitsky EM, Zvartau EE, Masalov DV, et al. Naltrexone and fluoxetine for heroin dependence treatment in St. Petersburg, Russia. J Subst Abuse Treat. 2006;31(4):319–328. doi: 10.1016/j.jsat.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 36.Fals-Stewart W, O'Farrell TJ. Behavioral family counseling and naltrexone for male opioid-dependent patients. J Consult Clin Psychol. 2003;71(3):432–442. doi: 10.1037/0022-006x.71.3.432. [DOI] [PubMed] [Google Scholar]