Abstract
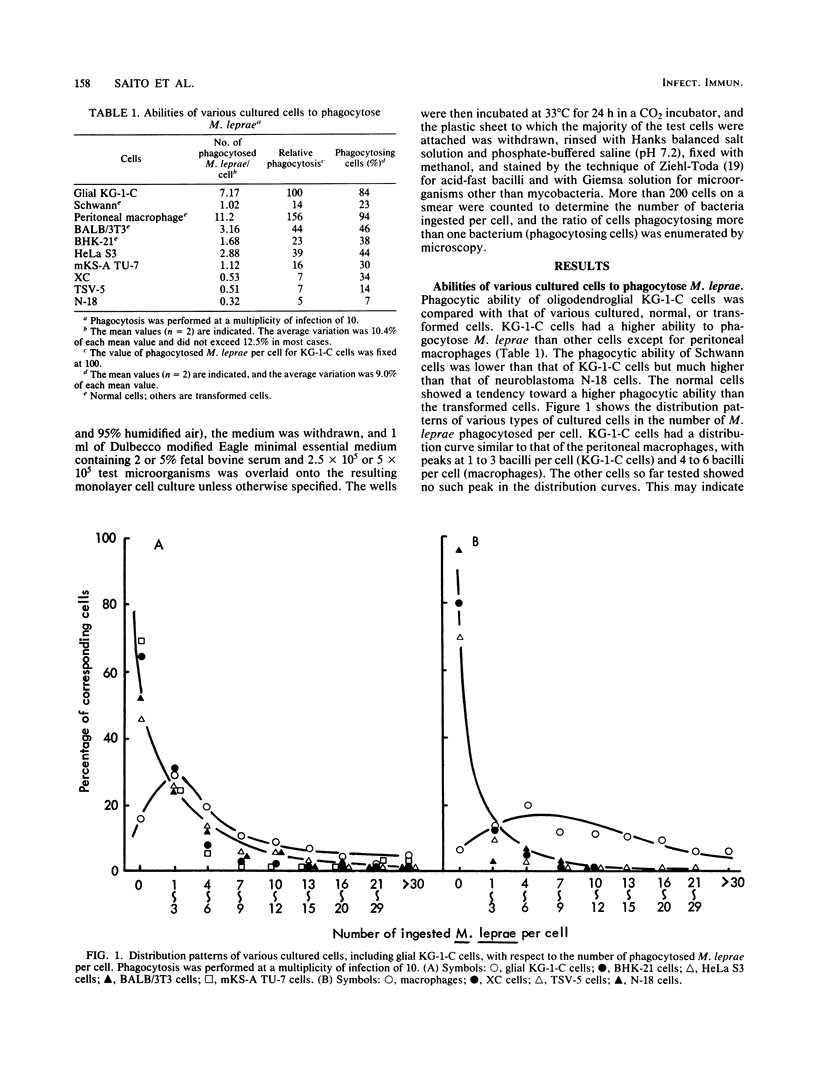
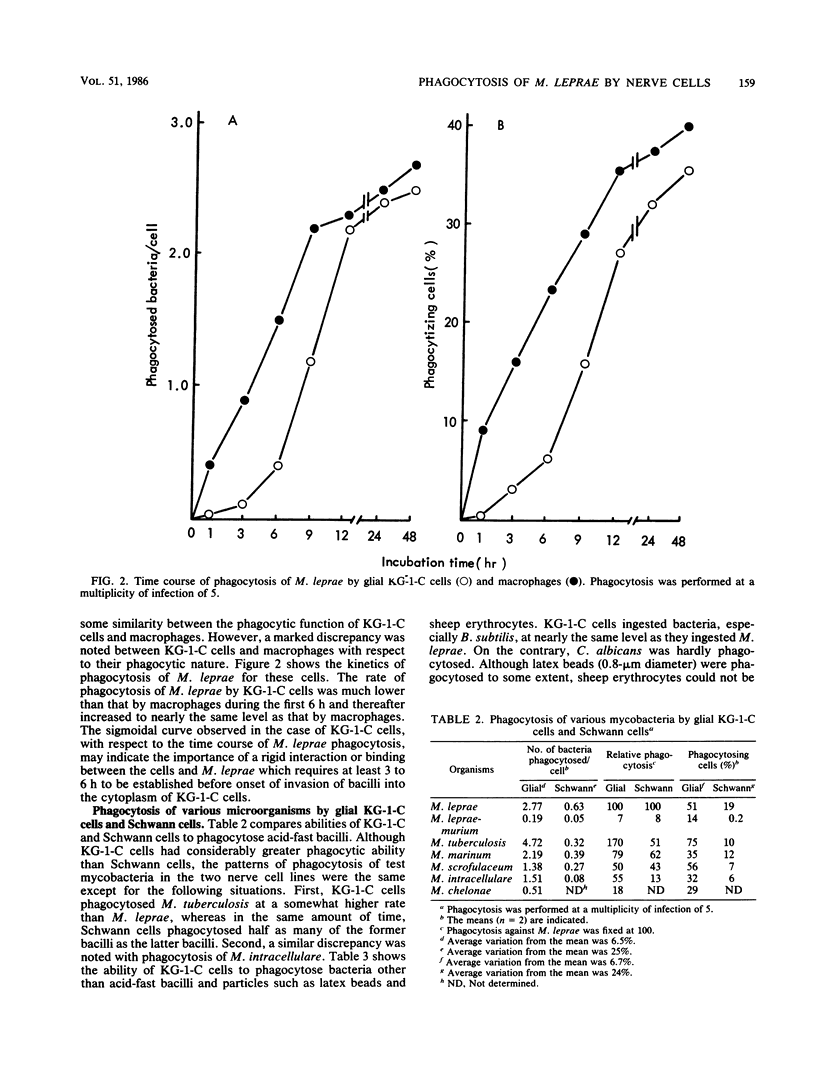
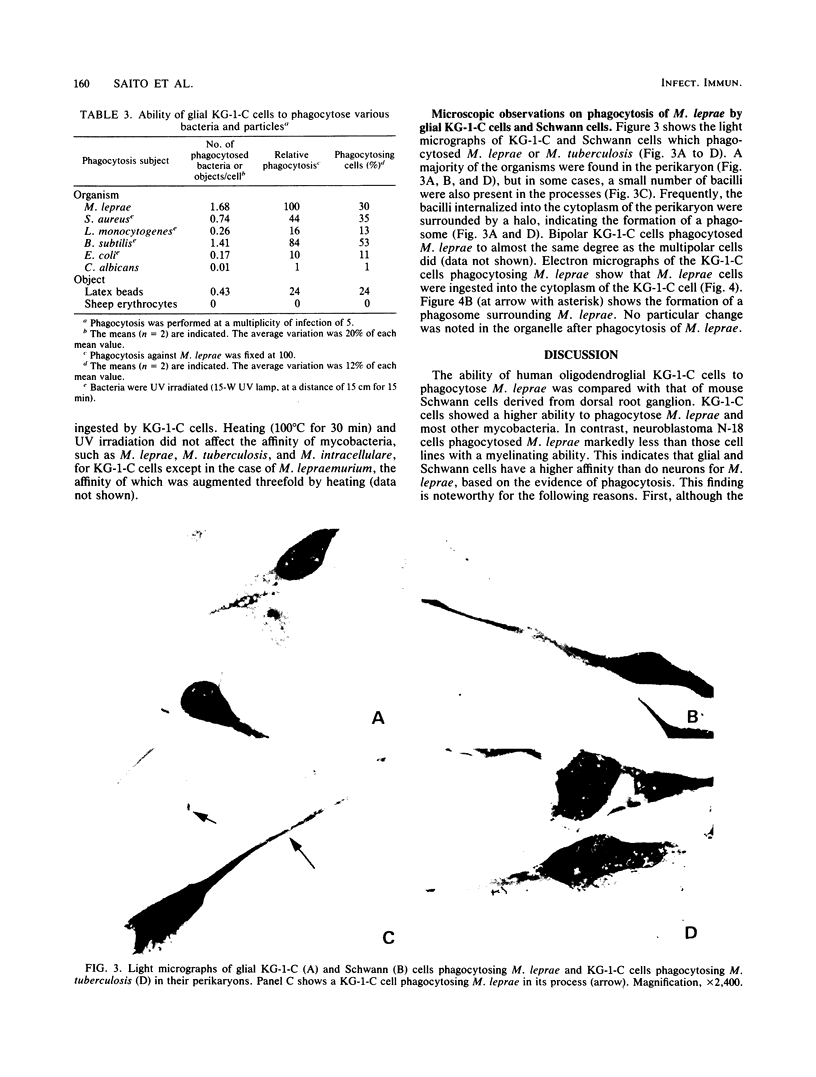
Human oligodendroglial KG-1-C cells derived from human cerebral mixed glioma and mouse Schwann cells derived from dorsal root ganglion were studied with respect to their abilities to phagocytose various mycobacteria, especially Mycobacterium leprae, and other microorganisms. KG-1-C cells phagocytosed M. leprae at a markedly higher rate than BALB/3T3, BHK 21, HeLa S3, mKS-A TU-7, XC, TSV-5, N-18, and Schwann cells but at a lower rate than peritoneal macrophages. Schwann cells also exhibited substantial phagocytic ability against M. leprae, and their phagocytic rate against M. leprae was much higher than that of N-18 cells, derived from neurons. KG-1-C and Schwann cells phagocytosed mycobacteria other than M. leprae, and their phagocytic patterns with various mycobacteria were similar, thereby suggesting that their abilities to phagocytose mycobacteria were based on the same cellular mechanism. The time course of phagocytosis of M. leprae by KG-1-C cells markedly differed from that by macrophages, indicating differences in the cellular mechanisms of M. leprae phagocytosis. KG-1-C cells also ingested microorganisms other than acid-fast bacilli, such as Staphylococcus aureus, Listeria monocytogenes, Bacillus subtilis, and Escherichia coli but not Candida albicans. They also phagocytosed latex beads (0.8-micron diameter) but not sheep erythrocytes. Microscopically, most mycobacterial cells were ingested in the perikaryon of KG-1-C cells and Schwann cells.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bunge R. P., Wood P. Studies on the transplantation of spinal cord tissue in the rat. I. The development of a culture system for hemisections of embryonic spinal cord. Brain Res. 1973 Jul 27;57(2):261–276. doi: 10.1016/0006-8993(73)90135-2. [DOI] [PubMed] [Google Scholar]
- Dastur D. K., Pandya S. S., Antia N. H. Nerves in the arm in leprosy. 2. Pathology, pathogenesis and clinical correlations. Int J Lepr Other Mycobact Dis. 1970 Jan-Mar;38(1):30–48. [PubMed] [Google Scholar]
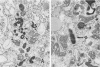
- Job C. K. Mycobacterium leprae in nerve lesions in lepromatous leprosy. An electron microscopic study. Arch Pathol. 1970 Mar;89(3):195–207. [PubMed] [Google Scholar]
- Miyake E. Establishment of a human oligodendroglial cell line. Acta Neuropathol. 1979 Apr 12;46(1-2):51–55. doi: 10.1007/BF00684804. [DOI] [PubMed] [Google Scholar]
- Miyake E., Ohta M., Matsushima T. A human glial cell line synthesizing nervous tissue-specific (S100) protein. Gan. 1978 Oct;69(5):737–738. [PubMed] [Google Scholar]
- Mudge A. W. Schwann cells induce morphological transformation of sensory neurones in vitro. Nature. 1984 May 24;309(5966):367–369. doi: 10.1038/309367a0. [DOI] [PubMed] [Google Scholar]
- Mukoyama M., Sasaki N. [Peripheral nerve involvement in leprosy: neuropathological study on autopsy cases (author's transl)]. No To Shinkei. 1979 Apr;31(4):403–408. [PubMed] [Google Scholar]
- NISHIURA M., HARADA N., IMAEDA T. Electron microscopy of ultra-thin sections of lepromatous peripheral nerves. Int J Lepr. 1957 Oct-Dec;25(4 Pt 1):323–328. [PubMed] [Google Scholar]
- NISHIURA M. The electron microscopic basis of the pathology of leprosy. Int J Lepr. 1960 Oct-Dec;28:357–400. [PubMed] [Google Scholar]
- Rees R. J. Enhanced susceptibility of thymectomized and irradiated mice to infection with Mycobacterium leprae. Nature. 1966 Aug 6;211(5049):657–658. doi: 10.1038/211657a0. [DOI] [PubMed] [Google Scholar]
- Rees R. J., Weddell G., Palmer E., Jamison D. G. Experimental studies on nerve fibers in leprosy. II. The reaction of human Schwann cells toward particles and leprosy bacilli. Int J Lepr. 1965 Apr-Jun;33(2):160–178. [PubMed] [Google Scholar]
- Saito H., Tomioka H. Enhanced hydrogen peroxide release from macrophages stimulated with streptococcal preparation OK-432. Infect Immun. 1979 Nov;26(2):779–782. doi: 10.1128/iai.26.2.779-782.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shetty V. P., Mehta L. N., Antia N. H., Irani P. F. Teased fibre study of early nerve lesions in leprosy and in contacts, with electrophysiological correlates. J Neurol Neurosurg Psychiatry. 1977 Jul;40(7):708–711. doi: 10.1136/jnnp.40.7.708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swift T. R. Peripheral nerve involvement in leprosy: quantitative histologic aspects. Acta Neuropathol. 1974;29(1):1–8. doi: 10.1007/BF00684386. [DOI] [PubMed] [Google Scholar]
- Yoshizumi M. O., Asbury A. K. Intra-axonal bacilli in lepromatous leprosy. A light and electron microscopic study. Acta Neuropathol. 1974 Feb 7;27(1):1–10. doi: 10.1007/BF00687235. [DOI] [PubMed] [Google Scholar]