Abstract
 Dirhodium caprolactamate [Rh2(cap)4] is a highly effective catalyst for the oxidative Mannich reaction. The reaction proceeds via C-H oxidation of a tertiary amine followed by nucleophilic capture. This green transformation is conducted in protic solvent using inexpensive T-HYDRO® (70% t–BuOOH in water). Synthetically valuable γ-aminoalkyl-butenolides are obtained.
Dirhodium caprolactamate [Rh2(cap)4] is a highly effective catalyst for the oxidative Mannich reaction. The reaction proceeds via C-H oxidation of a tertiary amine followed by nucleophilic capture. This green transformation is conducted in protic solvent using inexpensive T-HYDRO® (70% t–BuOOH in water). Synthetically valuable γ-aminoalkyl-butenolides are obtained.
The Mannich reaction remains a fundamentally important carbon-carbon bond forming reaction in organic synthesis.1 Access to valuable Mannich addition products is made possible via reactive iminium ions that undergo capture with a rich assortment of nucleophiles. However, despite its well-documented scope and utility, methods for the formation of iminium ions remain limited. Strategies such as condensation or α-fragmentation often require harsh reaction conditions and/or stoichometric metal additives (Fig. 1).2 The oxidative Mannich reaction is an attractive alternative which involves the direct catalytic C-H oxidation of a 3° amine followed by nucleophilic capture (vide infra).3,4 Herein we describe a mild, selective, and efficient oxidative Mannich reaction catalyzed by dirhodium caprolactamate [Rh2(cap)4] for the rapid construction of γ-aminoalkyl-butenolides.
Figure 1.
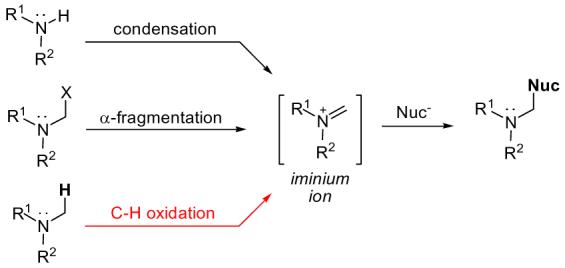
Iminium ion formation and nucleophilic capture
Rh2(cap)4 (1) has been shown to be an effective catalyst for allylic and benzylic oxidation in conjunction with tert-butyl hydroperoxide.5 We surmised that this technology could be extended to amines considering the oxidation potential of N,N-dimethylaniline 2 (relative to hydrocarbons).6 Inspired by the research of Martin,7 we chose 2-triisopropoxysilylfuran 3 to intercept the iminium ion formed in situ from catalytic C-H oxidation (Scheme 1). Siloxyfurans, which are easily prepared and bench-stable, allow the incorporation of functionally useful γ-butyrolactone moieties.8 As an architectural element, γ-butyrolactones are found in approximately 10% of all natural products.9
Scheme 1.

We initiated our investigation using conditions previously developed for benzylic oxidation5b (Table 1, entry 1) and found only trace amounts of Mannich product 4. Mindful of the stabilization afforded to iminium ions by polar solvents, we examined the reaction in methanol. No Mannich reaction was observed in the presence of base additive; however, removal of NaHCO3 from the reaction yielded 4 in 17% over 9 hours (entries 2 and 3). Heating the solution to 60 °C dramatically increased both rate and product yield (entry 4). Ethanol, a solvent of choice for green chemistry,10 was also suitable for the oxidative Mannich reaction albeit moderate yield (entry 5). Final modifications to the stoichiometry of the reaction provided optimal conditions for the formation of 4 at both 1.0 and 0.1 mol% catalyst loading (entries 6 and 7, respectively). It should be noted that all of the reactions were performed in the presence of air using inexpensive T-HYDRO® (70% t-BuOOH in water) with undistilled reagent-grade solvents.
Table 1.
Development of the Oxidative Mannich Reaction
 | |||
|---|---|---|---|
| entry | conditionsa | key | yield (4)b |
| 1 | 1 (1.0 mol%), CH2Cl2, NaHCO3 (50 mol%), rt | -- | |
| 2 | 1 (1.0 mol%), MeOH, NaHCO3 (50 mol%), rt | -- | |
| 3 | 1 (1.0 mol%), MeOH, rt | 17 | |
| 4 | 1 (1.0 mol%), MeOH, 60 °C | 86 | |
| 5 | 1 (1.0 mol%), EtOH, 60 °C | 51 | |
| 6 | 1 (1.0 mol%), 2 (2.0 equiv), 3 (1.0 equiv), MeOH, 60 °C | 96 (95c) | |
| 7 | 1 (0.1 mol%), 2 (2.0 equiv), 3 (1.0 equiv), MeOH, 60 °C | 90 (78c) | |
Reactions were performed using 2 (1.0 equiv), 3 (1.5 equiv), T-HYDRO® (1.2 equiv), and solvent (0.27 M/[substrate]) unless otherwise noted.
Yield was determined by 1H NMR using an internal standard.
Isolated yield of the analytically pure compound after chromatography (SiO2).
The oxidative Mannich reaction catalyzed by 1 was easily extended to a diverse collection of amine substrates (Table 2). Noteworthy examples include an amine containing a proximal olefin (entry 4) as well as an aromatic aldehyde (entry 5). Unsymmetrical amines (entries 6 and 7) were α-CH3 selective (regioisomeric α-methylene addition-products were not observed). Substituted 2-siloxyfurans (entries 10 and 11), including a useful 5-allyl-2-siloxyfuran11 (entry 12), were also viable participants in the oxidative Mannich reaction.
Table 2.
The Oxidative Mannich Reaction Catalyzed by Rh2(cap)4a
| entry | amine | 2-siloxyfuran | product | yieldb |
|---|---|---|---|---|
| 1 | 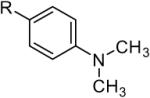 |
3 |  |
76 (79)c 89 (84) 78 (66) |
| 2 | 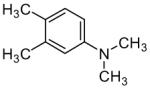 |
3 |  |
78 |
| 3 |  |
3 |  |
57d |
| 4 |  |
3 |  |
60 |
| 5 |  |
3 |  |
50 |
| 6 |  |
3 |  |
64 |
| 7 |  |
3 |  |
53 |
| 8 |  |
3 |  |
79 |
| 9 |  |
3 | 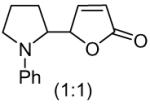 |
89 |
| 10 | 2 |  |
 |
86 |
| 11 | 2 |  |
72 | |
| 12 | 2 |  |
 |
75 |
Reaction conditions: amine (2.0 equiv), 2-siloxyfuran (1.0 equiv), Rh2(cap)4 (1.0 mol%), T-HYDRO® (1.2 equiv), MeOH (0.27 M/[2-siloxyfuran]), 60 °C, 3-5 hr.
Isolated yield after chromatography.
Yield in parentheses were for reactions performed using 0.1 mol% Rh2(cap)4 over 16 hrs.
Reaction time 1 hr.
Mechanistically, we have determined that the reaction proceeds through an iminium ion generated from α-CH3 amine oxidation followed by nucleophilic capture. In the absence of exogenous siloxyfuran, the oxidation of 2 in MeOH yielded α-methoxyamine 5 (62% yield, 30 minutes, rt) without evidence of mixed-peroxide 6 (Scheme 2, eq 1).12 However, replacing methanol as the solvent with non-nucleophilic CH2Cl2 gave 613 in 60% yield under the same conditions (eq 2).14 We isolated 6 and observed an equilibrium in MeOH between 5 and 6 that largely favored the mixed-peroxide (Keq ≈ 3 × 10−2). From close examination of the oxidation of 2 in MeOH (eq 1), the production of 5 to the exclusion of 6 in the reaction (at low conversion) clearly indicated that 5 was produced as the kinetic product from a catalytically generated iminium ion rather than an equilibrium product from 6 . Therefore, we have ruled out the intermediacy of 6 in the dirhodium-catalyzed oxidative Mannich reaction. The intermediacy of 5 and its overall contribution towards carbon-carbon bond formation is under investigation.15
Scheme 2.

In conclusion, we have developed an oxidative Mannich reaction catalyzed by Rh2(cap)4 that allows for synthesis of valuable γ-aminoalkyl-butenolides from readily available amines. Efforts are currently underway to extend this technology to other nucleophiles as well as assessing the role of Rh2(cap)4 in the catalytic generation of iminium ions.
Supplementary Material
Acknowledgement
We are grateful to the NSF and the NIH (GM-46503) for their generous support. We also thank the ACS Division of Organic Chemistry for support to A.J.C. (Emmanuil Troyansky Fellowship).
Footnotes
Supporting Information Available: Experimental procedures and product characterization. This material is available free of charge via the Internet at http://pubs.acs.org.
REFERENCES
- 1.For reviews of the Mannich reaction see: Kleinman ED. In: Comprehensive Organic Synthesis. Trost BM, editor. Vol. 2. Pergamon; Oxford: 1991. p. 893. Arend M, Westermann B, Risch N. Angew. Chem., Int. Ed. 1998;37:1045. doi: 10.1002/(SICI)1521-3773(19980504)37:8<1044::AID-ANIE1044>3.0.CO;2-E. Royer J, Bonin M, Micouin L. Chem. Rev. 2004;104:2311. doi: 10.1021/cr020083x.
- 2.Speckamp WN, Moolenaar MJ. Tetrahedron. 2000;56:3817. [Google Scholar]
- 3.For metal-catalyzed oxidation of amines see: Murahashi S-I. Angew. Chem., Int. Ed. 1995;34:2443. Murahashi S-I, Imada Y. In: Transition Metals for Organic Synthesis. Beller M, Bolm C, editors. Vol. 2. Wiley-VCH; Weinheim: 2004. p. 497. Murahashi S-I, Komiya N. In: Modern Oxidation Methods. Bäckvall J-E, editor. Wiley-VCH; Weinheim: 2004. p. 165.
- 4.For oxidative Mannich reactions see: Li Z, Li C-J. J. Am. Chem. Soc. 2005;127:3672. doi: 10.1021/ja050058j. Li ZP, Li CJ. Eur. J. Org. Chem. 2005:3173. and references therein. Murahashi S, Komiya N, Terai H, Nakae T. J. Am. Chem. Soc. 2003;125:15312. doi: 10.1021/ja0390303. Murahashi S-I, Komiya N, Terai H. Angew. Chem., Int. Ed. 2005;44:6931. doi: 10.1002/anie.200501496.
- 5.For dirhodium-catalyzed oxidation see: Catino AJ, Forslund RE, Doyle MP. J. Am. Chem. Soc. 2004;126:13622. doi: 10.1021/ja045330o. Catino AJ, Nichols JM, Choi H, Gottipamula S, Doyle MP. Org. Lett. 2005;7:5167. doi: 10.1021/ol0520020.
- 6.Sumalekshmy S, Gopidas KR. Chem. Phys. Lett. 2005;413:294. [Google Scholar]
- 7.For leading reviews see: Martin SF. Acc. Chem. Res. 2002;35:895. doi: 10.1021/ar950230w. Bur SK, Martin SF. Tetrahedron. 2001;57:3221.
- 8.For a review of 2-siloxyfurans and related analogs see: Rassu G, Zanardi F, Battistini L, Casiraghi G. Chem. Soc. Rev. 2000;29:109.
- 9.Seitz M, Reiser O. Curr. Opin. Cell. Biol. 2005;9:285. doi: 10.1016/j.cbpa.2005.03.005. and references therein.
- 10.For EtOH as a green solvent see: Taber GP, Pfisterer DM, Colberg JC. Org. Process Res. Dev. 2004;8:385.
- 11.For 5-allyl-2-triisopropylsiloxyfuran in natural product synthesis see: Liras S, Davoren JE, Bordner J. Org. Lett. 2001;3:703. doi: 10.1021/ol0070482.
- 12.For the α-methoxylation of tertiary amines see: Murahashi S, Naota T, Miyaguchi N, Nakato T. Tetrahedron Lett. 1992;33:6991.
- 13.For the α-peroxidation of tertiary amines see: Murahashi S, Naota T, Yonemura K. J. Am. Chem. Soc. 1988;110:8256.
- 14.The use of CH3NO2 as a solvent gave nitromethane-captured product (not shown) and mixed-peroxide 6. For CH3NO2 capture see ref. 4a.
- 15.Nucleophilic capture of iminium ions with methanol is competitive with 2-siloxyfuran in the reaction. α-Methoxyamines are observed as side products.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


