Abstract
Background
Hepatocellular carcinomas (HCC) associated with inflammation that undergo radiofrequency ablation (RFA) appear to have poorer local control rates. Little is known of how mediators of inflammation influence HCC cellular thermotolerance which in part is mediated by heat shock protein 70 (HSP 70). This study determines how inflammatory mediators effect cellular thermotolerance and provides insight into how associated inflammation may impact HCC RFA local control rates.
Methods
HepG2 cell lines were cultured in control medium (CM) or CM containing conditioned medium of endotoxin-activated macrophage (CMM). Serial dilutions of CMM established microenvironments approximating low, medium and high CMM. All groups underwent a heat shock challenge (HSC) at 45° C for 10 minutes. Western blot, northern blot, densometric analysis, along with Thymidine and clonagenic assays determined how inflammation influenced multiple biologic endpoints.
Results
Cells cultured in low CMM, expressed significantly more HSP 70 RNA and protein compared to control cells after HSC. The cells also had a higher proliferative and survival rate after HSC compared to control cells. Medium CMM cultured cells had no significant difference in HSP 70 RNA and protein production or proliferation and survival rates after HSC, compared to CM cultured cells. AT high CMM the inhibitory effects of inflammatory mediators prevailed, all the measured endpoints were significantly less compared to CM cultured cells.
Conclusions
This study demonstrates that inflammation can alter the responsiveness of HCC cells to a HSC in a dose dependent manner. This study supports the clinical observation that HCC associated with chronic inflammation have worse RFA local control rates.
Keywords: Hepatocellular Carcinoma, Inflammation, Radiofrequency ablation, Heat Shock protein 70, Hepatits, Cirrhosis
Introduction
Hepatocellular carcinoma, worldwide is one of the most frequently occurring malignancies 1 with the highest incidences found in Asia and Africa. Within the United States the frequency is increasing 1. HCC usually develops in a chronic inflammatory environment (hepatitis or cirrhosis) most typically as the result of viral hepatitis or alcohol abuse. Studies have found that the associated chronic hepatitis and the by products of chronic inflammation (fibrosis and cirrhosis) negatively impact treatment outcomes 2. The most effective therapies for HCC are surgical resection or transplantation; unfortunately the majority of patients are not candidates for these therapies.
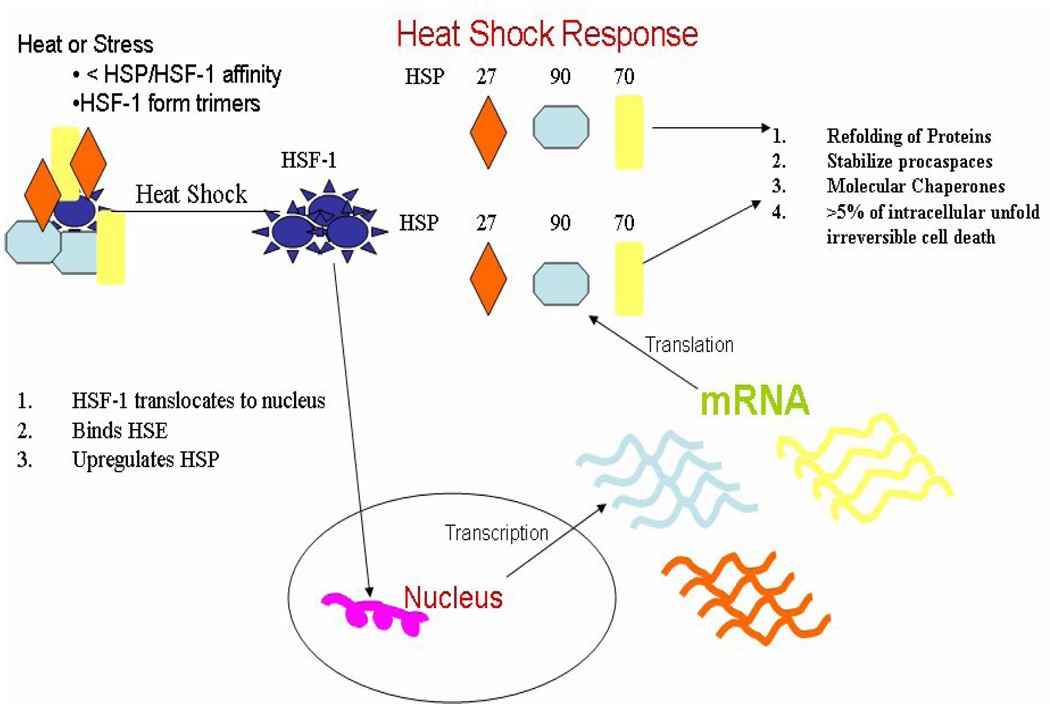
Regional therapies have been developed to treat unresectable lesions. Radiofrequency ablation (RFA) is one of these local therapies which have proven increasingly popular due to its ease of use and low associated morbidity. RFA generates thermal temperatures of greater than ≥ 50°C within the tumor as a means of destruction. Specifically, the alternating current passing through the probe tip initiates ionic agitation that induces very high temperatures and in the cells adjacent to the probe and then transmits these high temperatures via thermal conduction through the remaining surrounding tissue. Exposure to elevated temperatures is a cellular stress against which normal and malignant cells have developed processes to counteract. Normal cells have processes that provide intrinsic and heat induced thermotolerance, while malignant cells have exaggerated forms of these same processes. There are several factors that determine a cells intrinsic and heat induced thermotolerance; protein stability, ionic composition, and pH, the most critical factor is the amount of cellular heat shock proteins 70 and 90 (HSP 70 and 90) 3. The primary mediators of heat induced thermotolerance are HSP 70, 90 and heat shock factor-1 (HSF-1), HSF-1 is the nuclear transcription factor when activated up regulates the transcription of the HSP genes 3. HSF-1 is maintained in an inactive form in the cytoplasm bound by HSP 70 and 90. As denatured proteins become abundant during a heat shock challenge (HSC), HSP 70 and 90 release HSF-1 in order to bind unfolding denatured proteins 3(Fig 1). The released HSF-1 becomes a phosphorylated trimer that translocates to the nucleus, binds to the heat shock promoter element and activates transcription of heat shock protein genes. The synthesis of HSP is a highly conserved generalized stress response that can be induced by any major cellular stress including thermal initiated protein denaturing (Fig 1).
Figure 1.
Schematic presentation of the major steps involved in thermal induction of heat shock protein (HSP) expression. Cytoplasmic HSPs lose affinity for HSF-1 and bind to denatured proteins. HSF-1 phosphorylated trimers translocate to the nucleus binds to HSE (HSP promoter element) leads to upregulation and transcription of the HSPs. These mRNA undergo translation and the proteins perform chaperone and protective functions to allow cells to survive.
Studies have indicated that the inflammation associated with hepatitis, cirrhosis and fibrosis, negatively impact local control rates in patients who undergo hepatic resections 4. Other Studies determined that inflammation negatively impacted RFA local control rates 5, 6, with higher incomplete ablation and local recurrence rates 5. Because HCC do develop in a chronic inflammatory environment, e.g. as a result of hepatitis B or C infection, exposure to aflatoxin B, or chronic alcohol abuse, the associated inflammation and its mediators (cytokines small molecular inflammatory mediators and reactive oxygen species) are invariably important to HCC growth and development 7–9. Previous studies identified pro-inflammatory or T-helper cells type 2 induced cytokines predominate in the chronic inflammatory environment associated with hepatitis 10, 11. Furthermore studies have quantitated the amount of cytokine expression to be slightly elevated compared to healthy un-infected control patients 11. In addition to the protective and carcinogenic aspects of chronic inflammation, the physical constraints of ablating lesions larger than 3 cm or proximity of heat sinks can allow some malignant cells to experience sub-lethal ablation temperatures. An understanding of how a chronic inflammatory environment affects the HCC intrinsic and heat induced thermotolerance is important in the development of strategies that will improve RFA local control rates. We hypothesized that the cytokines and other by products produced as a result of chronic inflammation in the HCC microenvironment would improve heat dependent thermotolerance and allow more cells to survive suboptimal RFA temperatures. This study was performed to provide a molecular basis in support of the clinical observation that inflammation and its mediators, negatively impacts RFA local control rates.
Methods
Experiments
All assays where performed in three independent experimental series and each series included triplicate cultures for all data points.
Cell Culture
The human hepatocellular carcinoma cell line (HepG2) was obtained from the American Type Culture Collection (Manassas, VA). Cells were cultured in (DMEM) with 10% fetal calf serum and termed control medium (CM) in a humidified incubator at 37°C with 5% CO2 atmosphere. Primary cultures of human pulmonary macrophages served as a source of inflammatory mediators. The macrophages were mechanically extracted from residual resected human normal lung tissue and isolated by histopaque gradient according to IRB approved tissue procurement protocol CIC 00-91 and non-therapeutic protocol CIC 00-17. The isolated cells were plated at a density of 3×105 cell/cm2, in RPMI -1640 containing 10% FCS. After 45 min. adherent cells (> 95% macrophages) were incubated in medium (1ml/ 1×106 cells) containing 1µg/ml LPS. Conditioned medium, termed CMM was collected after 16 hours, cleared of cellular debris and filtered through 0.2 µm membranes. The concentrations of cytokines were determined by multiplex immunobead flow cytometry (Luminex Inc., Autsin TX). Major inflammatory cytokines that were identified are TNF-α, IL-1β, IL-6, IL-8, IL-10 and G-CSF (concentration range 1–300ng/ml) 12. CMM was used for treatment of HepG2 cells after diluting at 1:10, 1:100, and 1:1000 in CM and these concentrations were termed high CMM, medium CMM and low CMM.
Heat Shock Challenge
After 24 hour pre-treatment of the cells in CM or the three concentrations of CMM at 37°C, the cells were subjected to a HSC by placing cells in a 45°C water bath for 10 minutes. Experimental controls consisted of the same pretreatment but by placing the cells in a 37°C water bath for 10 minutes and were labeled non-HSC cells. All cells were then returned to the incubator, and then harvested at 4, 8 and 24 hours, to determine the cells viability, cytokine regulated expression of acute phase proteins and level of HSP 70 production.
Western Blot Analysis
Replicate aliquots of whole cell lysates, containing 1.0 or 2.0µg of protein were electrophoresed on 7.5% polyacrylamide gels. The proteins were transferred to protean membranes (Schleicher & Schuell, Keene, NH). Immediately after transfer, the proteins on the membranes were stained with Ponceau red to verify loading of equal amounts of protein per samples. In each experimental series, two replicate blots were cut horizontally at the ~ 60 kDa size position; the upper sections were used to probe for HSP 70 and the lower sections for total ERK. Replicate separations and immunoblot analyses instead of re-probing of membranes were applied, due to difficulties in complete removal of the antibodies from the first round of reaction. The membranes were reacted with antibodies to HSP 70(Santa Cruz Santa Cruz, CA) and total form of ERK1/2, (Santa Cruz Santa Cruz, CA). The membranes were incubated with the appropriate peroxidase-conjugated secondary antibodies (ICN Biomedical, Aurora, OH) and the antibody binding was visualized by enhanced chemiluminescence reaction (Amersham Biosciences Piscataway, NJ). In each experimental series, immunoblots were exposed to X-ray films for various lengths of time (1 sec to 3.0 min) to obtain images that are in the linear range of signal detection.
Densometric Analysis
The chemiluminescence images of immunoblots were scanned with a high-resolution desktop scanner. The digital images were quantified with Image Quant Software 5.0 (Amersham Biosciences Chalfont Buckinghamshire Eng.) The net pixel value for each protein band that was within the linear range of detection was normalized to the co-analyzed standard and used to calculate the relative difference to the untreated control cells in each experimental series. The pixel values determined for the untreated controls were defined as being equal to 1.0. In each experimental set, the pixel values of the basal or intrinsic level and those induced by cytokine or HSC treatments were then expressed relative to the basal level.
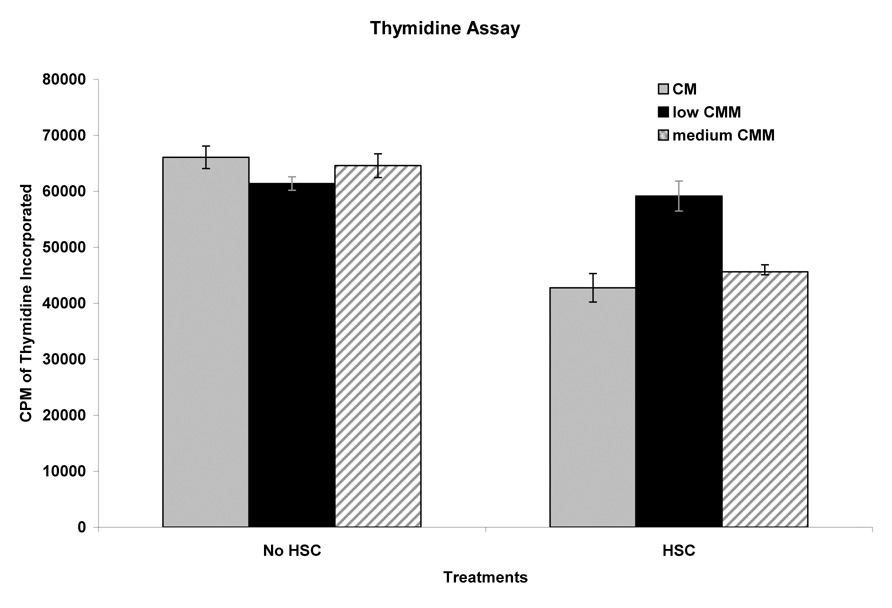
Thymidine Assay
HepG2 cells were seeded into 24 well culture plates (5×104 cells per well). These cells were cultured in their respective culture medium (CM, low CMM, medium CMM, and high CMM) for 23 hours prior to undergoing a HSC or non-HSC. One hour later, 1µCi of [3H] thymidine (Amersham Biosciences) was added to each culture and incubation continued for additional 16 hours. Cells were released by trypsin, transferred onto paper filter by the cell harvester (Tomtec, Hamden CT) and the amount of incorporated tritium was calculated by a scintillation counter (Trilux microbeta, Turku Finland). The mean of the net values of the duplicate wells were expressed relative to the incorporation determined for the control cultures in each of the series, which were defined as 100%.
Northern Blot Analysis
HepG2 cells were pre-treated with CM, low CMM, medium CMM and high CMM for 24 hours then subjected to a HSC or non-HSC. After additional incubation for 4 hours total cellular RNA were extracted with Trizol (Invitrogen). Northern blot analysis of RNA was carried out as described previously 13. Briefly, duplicate aliquots RNA were separated on formaldehyde-containing agarose gels and transferred to nitrocellulose membrane (Schleicher & Schuell, Keene, NH). The RNA was hybridized to P32labelled random-primed cDNA to HSP 70 or α1-acid glycoprotein (AGP). The pattern was visualized by autoradiography and quantified by phosphor-imaging (Molecular Dynamics)
Clonagenic Assay
HepG2 cells were pre-treated with CM, low CMM, medium CMM, and high CMM for 24 hours. After HSC the cells were released by trypsin and aliquots of 500 cells/10ml were plated onto 10cm plates. The cells were cultured for seven days in their respective medium, changing medium after day 3. The adherent cell were fixed and stained. Clones of 25 cells or greater were counted and represented the cells surviving the HSC. The non-HSC cells served as the experimental controls.
Results
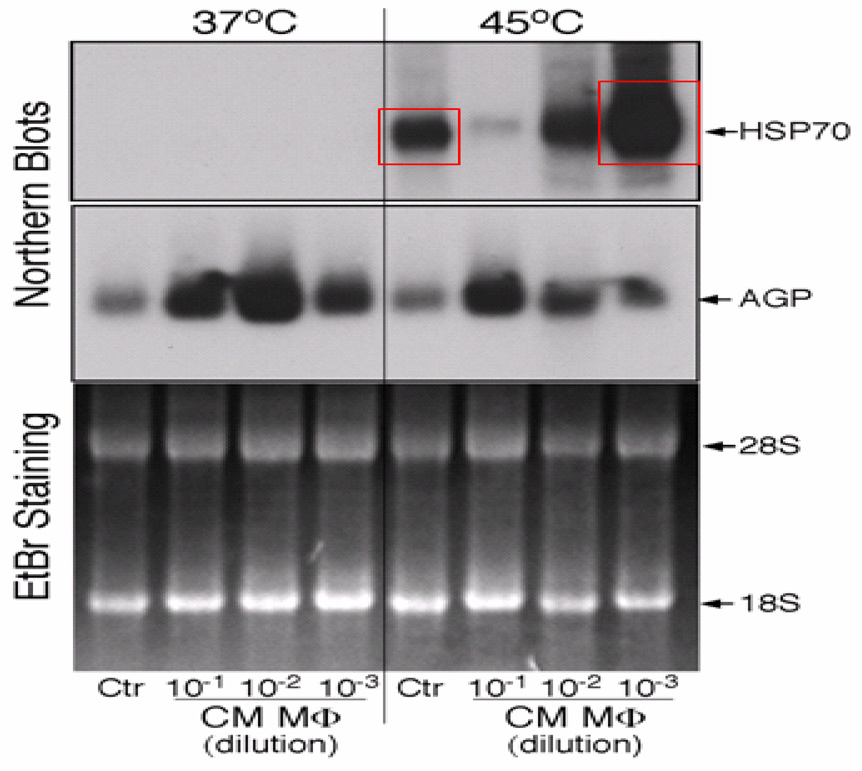
Induction of HSP70 mRNA and protein was used as a measure for the responsiveness of HCC to HSC. An optimal induction of HSP70 expression was achieved by 10 min incubation at 45°C (Fig. 2). Four hours following HSC, HSP70 mRNA was detectable by northern blotting and was strongly increased over the 37°C control (Fig. 2, lane 5 versus lane 1). The cytokine-specific response of certain acute phase proteins has been applied as an in vivo and in vitro marker for inflammation 13. Treatment of HepG2 cells for 24 h with CMM resulted in dose-dependent induction of AGP mRNA (Fig. 2, lanes 2–4). Maximal induction was achieved with 1/100 diluted CMM. Using CMM at the 1/10 dilution, the high concentrations of cytokines become inhibitory. Northern blot analysis also indicated that CMM was not effective in inducing HSP70 mRNA. However, CMM pretreatment modified the effect of HSC. (Fig. 2, lanes 5–8). Low CMM enhanced the HSC induced expression of HSP 70 mRNA by 5.5-fold (Fig. 2, lane 8). This stimulatory effect was unusually sensitive to the CMM dose. In HepG2 cells treated with medium CMM, HSP70 mRNA induction by HSC was approximately equal to that of the control (Fig. 2, lane 7), whereas high CMM inhibited the HSC effects by 7-fold (Fig. 2, lane 6). Because of the prominent inhibitory activity of high CMM, this treatment condition was not used for further characterization of HepG2 cells response.
Figure 2.
HSC regulation of HSP 70 mRNA. HepG2 cell were pre-treated with low CMM for 24h. After HSC the cells were continued in culture for 4h. Total RNA was extracted and aliquots of 10µg were analyzed by Northern blot for mRNA encoding HSP70 and α-glycoprotein (AGP). Ethidium bromide (EtBr) staining of the separated RNA was used as loading controls.
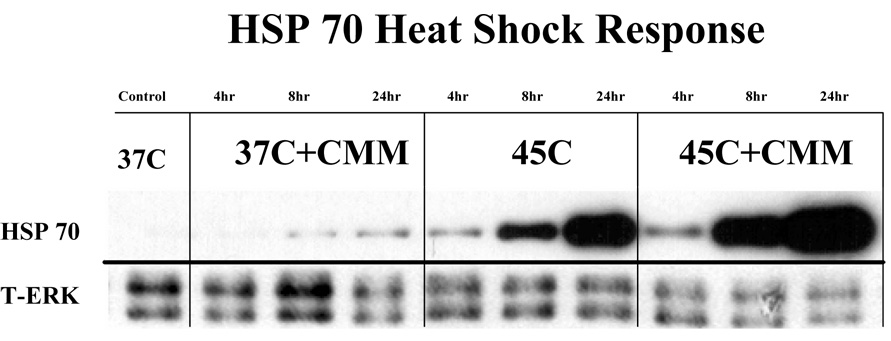
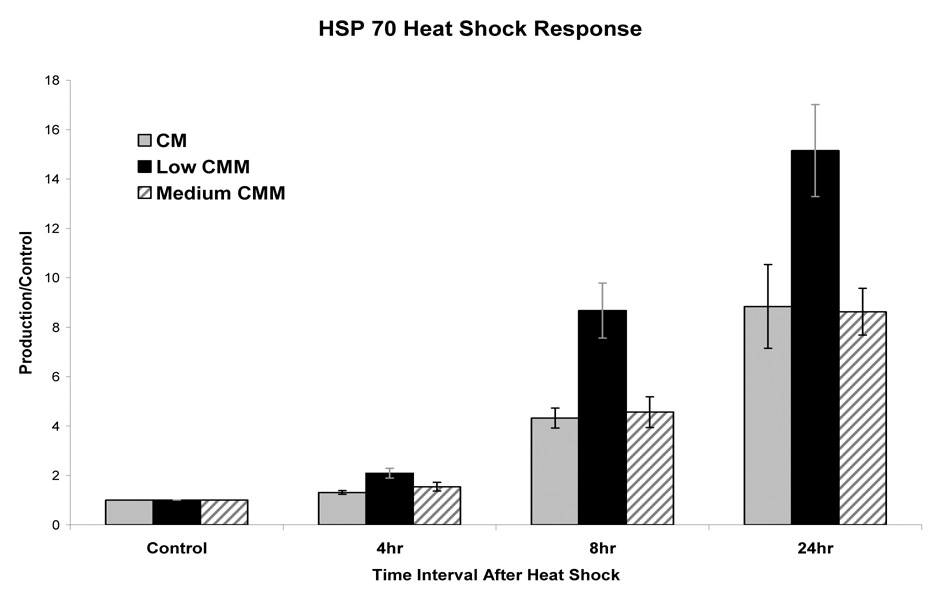
The HSC-mediated induction of HSP70 mRNA correlated with a significantly increased amount of HSP70 protein. The immunodetectable level of HSP70 reached maximal level by 24 h (Fig 3) and quantification of the changes indicated a significant increase (Fig. 4). There was no detectable change in the amount HSP70 in cells cultured in CM or low CMM at 4hours without a HSC. However the cells cultured in low CMM but not exposed to HSC had detectable level of HSP70 at 8 and 24 hours post-HSC. However this level was not consistently detected and did not yield a statistically significant response (Fig. 4). The cells cultured in low CM had a 4.4-fold and 7.0-fold increases in the amount of HSP 70 at 8 and 24 h after HSC when compared to cells cultured in CM (Fig. 3&Fig. 4). Consistent with the mRNA results, cells cultured in medium CMM were comparable to control cells (Fig. 4) and cells in high CMM produced less HSP70 after HSC compared to CM cultured cells (data not shown). The reduced expression of HSP70 protein in medium and high CMM treated cells was tentatively attributed to the inhibitory effect of high concentration of inflammatory mediators.
Figure 3.
HSC induced expression of HSP70. HepG2 cells were pre-treated with low CMM for 24h. After HSC, the cells were cultured for the indicated periods of time. Aliquots of cell lysates (2µg) were analyzed by western blotting for HSP70 protein. Total ERK 1/2 levels served as loading controls.
Figure 4.
Densometric analysis of heat induced HSP 70 protein expression. Western blots from 3 independent experimental series were quantified and normalized to non-HSC controls (defined as 1). Error bars represent the calculated standard error of the mean.
HepG2 cells marginally responded to low CMM with a 7% lower incorporation of thymidine when compared to cells cultured in CM and a non-HSC (Fig. 5). However, when HepG2 cells cultured in CM were subjected to HSC, DNA synthesis was decreased by 35%. In cells pretreated with low CMM, the HSC-mediated reduction of DNA synthesis was not evident in cells in low CMM (Fig. 5).
Figure 5.
Effect of HSC on DNA synthesis of HepG2 cells. HepG2 cells were pretreated with low medium or medium CMM. After HSC, the cells were maintained for 40h and [3H] thymidine incorporation was determined, data points determined from 3 independent experiments and error bars represent calculated standard error of the mean.
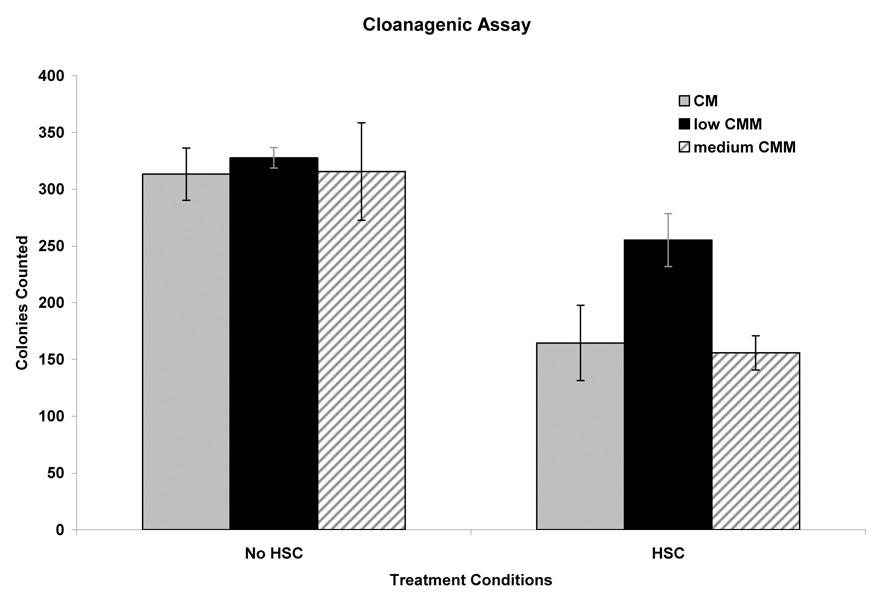
Clonogenic assay mirrored the findings of the thymidine incorporation. The recovery of countable clones for HepG2 cells cultured in CM and those cultured in low or medium CMM at 37° C were essentially identical. The values indicated a ~ 65% clonogenicity of the HepG2 cell culture. Only in high CMM could we detect a minor reduction in clone formation when compared to those cells cultured in CM at 37°C (data not shown) Recovery of clones after HSC was reduced by ~50% (~30% clonogenicity of the HSC-treated culture) However, when the cells are pretreated with low CMM, the recovery of proliferating clones was reduced only by 12% (51% clonogenicity) (Fig. 5). From these results we concluded that low CMM improved the HepG2 cells’ ability to survive a HSC. However cells cultured in high CMM had an almost immeasurable amount of countable clone formation when compared to those cells cultured in CM at 37°C.
Discussion
The results of this study provides evidence that low CMM, which most likely resembles the physiologic level of cytokines and other inflammatory mediators identified in a chronic inflammatory environment, may impact cellular processes of HCC. Since hepatocarcinomas are associated with a certain level of chronic inflammation, the effects of inflammatory mediators on the HSC was mimicked by pretreatment of HepG2 cells with the conditioned medium of endotoxin-activated lung macrophages (CMM). CMM is considered to represent a physiologically relevant combination of macrophage-derived inflammatory cytokines and other mediators that is expected to act in paracrine fashion at the tissue sites of production as well as at systemic level when these enter the circulation. Because the majority of HCC develop in a chronic inflammatory environment an understanding of this interplay between mediators of inflammation and HCC is critical to understanding of hepatocarcinogenesis and the development of novel HCC treatment modalities7. The results reveal that HepG2 cells cultured in low CMM are more thermotolerant than cells cultured in CM or other concentrations of CMM. Furthermore the relevant concentration of inflammatory mediators in low CMM produce an exaggerated thermally induced HSP 70 response. The cells in low CMM have a 5.5 fold higher HSP 70 mRNA which translated into a 4 fold and 7 fold increase in HSP 70 at 8 and 24 hours after HSC (Fig 2&3). Also cells in low CMM showed improved survival and maintenance of proliferation rates after HSC compared to cells in CM (Fig 4&5).
The kinetics of induction and the role of HSP 70 in thermotolerance have been described by many authors 3, 14–17. Our data confirm in HepG2 cells, a correlation between augmented activation of the HSP 70 response and thermotolerance. While a decrease of proliferation after HSC of 35% in our study (Fig. 4), is in line with what has been described for other cells systems 18, the finding that low CMM is capable of abrogating this HSC effect is novel and important implication for the therapeutic application of heat. Song et al described the competition between HSP 70 and RAF-1 for binding to the co-chaperone protein BAG-1 determines cell proliferation after a heat shock challenge. Since binding of HSP 70 counters the effect of RAF-1/BAG-1 and the engagement of the mitogen activated protein kinase/ ERK 1/2 pathway is reduced with a concomitant reduction of DNA synthesis. The sustained proliferation of HepG2 cells after HSC when cultured in low CMM cytokine suggests that inflammatory mediators boost the activation of the ERK pathways and promote survival. However, the data also demonstrate that CMM at higher concentrations is effective in reducing proliferation even in cells that had not been subjected to HSC. Analysis of various cell lines had indicated an elevated ERK activity as a function of transformation and this activation was further enhanced by inflammatory cytokines and by CMM 19. What remains to be determined is whether the magnified HSP70 expression in CMM pre-treated cells will also be more effective in quenching the action of BAG-1. The concentration of the pro-inflammatory cytokines in low, medium and high CMM is known but complete analysis of all the components expressed as a result of macrophage activation is not known and is recognized as a limitation of this study. Because of this limitation it prohibits a definitive answer to what may be the exact cause of the robust heat induced HSP 70 production but provides information to warrant further analysis. The effects inflammatory mediators on HSC and thermotolerance were determined in the tissue culture model of HepG2 cells. The information derived from this system to HCC in will demand appropriate translational studies.
When the results of this study, which identified increased thermotolerance in HepG2 cells cultured in the presence of inflammation, is correlated with the clinical observation of poor RFA local control rates of HCC associated with chronic liver inflammation is compelling. Many Studies have made this clinical observation whether hepatitis, cirrhosis or fibrosis are used as the surrogate for chronic inflammation 2, 5. Pompili et al, looked at cirrhotic patients with HCC who underwent RFA as bridge to transplantation, their explanted liver were analyzed and identified a 56% partial ablation rate 20.
Furthermore their study identified complete necrosis of only 62% of the HCC that were ≤ 3.0cm which is less than the 1 year local control rate for HCC of similar size in other series. Harrison et al described similar findings of high local failure rates after RFA associated with hepatitis 5.
This study provides evidence of how low levels of inflammation allow cells to produce a robust thermally induced HSP 70 response, more thermo-tolerant cells that survive thermal challenges at a higher rate. Furthermore this study does provide translational based evidence of how inflammation may negatively impact HCC RFA local control rates. Additional study is warranted to determine which inflammatory factors or combination of factors are responsible for the observation and if these observations would present in an animal model
Figure 6.
Clonogenicity of HepG2 cells after HSC. The cells cultured in CM, low CMM and medium CMM at 37°C averaged 313, 327 and 325 colonies formed with the difference between the three cohorts within the standard error of the mean. After HSC CM, low CMM and medium CMM cultured cells averaged 164, 257 and 156 colonies formed. Low CMM improved the cells ability to survive a HSC.
Acknowledgements
Research supported by NIH grant CA85580 and the tissue procurement facility at Roswell Park Cancer Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jemal A, Tiwari RC, Murray T, et al. Cancer statistics, 2004. Ca: a Cancer Journal for Clinicians. 2004;54(1):8–29. doi: 10.3322/canjclin.54.1.8. [DOI] [PubMed] [Google Scholar]
- 2.Cha C, Fong Y, Jarnagin WR, et al. Predictors and patterns of recurrence after resection of hepatocellular carcinoma. Journal of the American College of Surgeons. 2003;197(5):753–758. doi: 10.1016/j.jamcollsurg.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 3.Lepock JR. Cellular effects of hyperthermia: relevance to the minum dose for thermal damage. International Journal of Hyperthermia. 2003;19(3):252–266. doi: 10.1080/0265673031000065042. [DOI] [PubMed] [Google Scholar]
- 4.Bilimoria MM, Lauwers GY, Doherty DA, et al. Underlying liver disease, not tumor factors, predicts long-term survival after resection of hepatocellular carcinoma. Archives of Surgery. 2001;136(5):528–535. doi: 10.1001/archsurg.136.5.528. [DOI] [PubMed] [Google Scholar]
- 5.Harrison LE, Koneru B, Baramipour P, et al. Locoregional recurrences are frequent after radiofrequency ablation for hepatocellular carcinoma. Journal of the American College of Surgeons. 2003;197(5):759–764. doi: 10.1016/S1072-7515(03)00750-6. [see comment]. [DOI] [PubMed] [Google Scholar]
- 6.Scaife CL, Curley SA. Complication, local recurrence, and survival rates after radiofrequency ablation for hepatic malignancies. Surgical Oncology Clinics of North America. 2003;12(1):243–255. doi: 10.1016/s1055-3207(02)00088-1. [DOI] [PubMed] [Google Scholar]
- 7.Drucker CPW, Teufelhofer O, Grusch M, Ellinger A, Schulte-Hermann R, Grasl-Kraupp B. Non-parenchymal liver cells support the growth advantage in the first stages of hepatocarcinogenesis. Carcinogenesis. 2005 doi: 10.1093/carcin/bgi202. EPUB. [DOI] [PubMed] [Google Scholar]
- 8.Hershko DD, Robb BW, Luo GJ, et al. Interleukin-6 induces thermotolerance in cultured Caco-2 cells independent of the heat shock response. Cytokine. 2003;21(1):1–9. doi: 10.1016/s1043-4666(02)00488-x. [DOI] [PubMed] [Google Scholar]
- 9.Nishikawa MNS, Shiomi S, Tamori A, Koh N, Takeda T, Kubo S, Hirohashi K, Kinoshita H, Sato E, Inoue M. Somatic mutation of mitochondrial DNA in cancerous and noncancerous liver tissue in individuals with hepatocellular carcinoma. Cancer Research. 2001;61(5):1843–1845. [PubMed] [Google Scholar]
- 10.Fan XGLW, Li CZ, Wang ZC, Luo LX, Tan DM, Hu GL, Zhang Z. Circulating Th1 and Th2 cytokines in patients with hepatitis C virus infection. Mediators of Inflammation. 1998;7(4):295–297. doi: 10.1080/09629359890992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shapiro SGV, Elias N, Zuckerman E, Salman N, Lahat N. mRNA cytokine profile in peripheral blood cells from chronic hepatitis C virus (HCV)-infected patients: effects of interferon-alpha (IFN-alpha) treatment. Clinical and Experimental Immunology. 1998;114(1):55–60. doi: 10.1046/j.1365-2249.1998.00693.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Loewen GMTE, Blanchard F, Tan D, Yu J, Raza S, Matsui S, Baumann H. Transformation of human bronchial epithelial cells alters responsiveness to inflammatory cytokines. BMC Cancer. 2005;5(145) doi: 10.1186/1471-2407-5-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang YKE, Berger FG, Lim SK, Baumann H. Haptoglobin, an inflammation-inducible plasma protein. Redox Report. 2001;6(6):379–385. doi: 10.1179/135100001101536580. [DOI] [PubMed] [Google Scholar]
- 14.Beere HM, Wolf BB, Cain K, et al. Heat-shock protein 70 inhibits apoptosis by preventing recruitment of procaspase-9 to the Apaf-1 apoptosome. Nature Cell Biology. 2000;2(8):469–475. doi: 10.1038/35019501. [DOI] [PubMed] [Google Scholar]
- 15.Hosoi N, Itoh H, Koyama K, Tashima Y. Overexpression of the heat shock protein 70 confers protection against oxidative injury in HEPG2 cells. Transplantation Proceedings. 2002;34(7):2647–2649. doi: 10.1016/s0041-1345(02)03460-7. [DOI] [PubMed] [Google Scholar]
- 16.Sciandra JS, John Heat shock proteins and protection of proliferation and translation in mammalian cells. Cancer Research. 1984;44:5188–5194. [PubMed] [Google Scholar]
- 17.Yang WLND, Makizumi R, Gallos G, Ye X, Sharma RR, Ravikumar TS. Heat shock protein 70 is induced in mouse human colon tumor xenografts after sublethal radiofrequency ablation. Annals Surgical Oncology. 2004;11(4):399–406. doi: 10.1245/ASO.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 18.Song J, Takeda M, Morimoto RI. Bag1-Hsp70 mediates a physiological stress signalling pathway that regulates Raf-1/ERK and cell growth. Nature Cell Biology. 2001;3(3):276–282. doi: 10.1038/35060068. [DOI] [PubMed] [Google Scholar]
- 19.Douglas WG, Tracy E, Tan D, et al. Development of head and neck squamous cell carcinoma is associated with altered cytokine responsiveness. Molecular Cancer Research. 2004;2(10):585–593. [PubMed] [Google Scholar]
- 20.Pompili MMV, Rondinara G, Fassati LR, Piscaglia F, Agnes S, Covino M, Ravaioli M, Fagiuoli S, Gasbarrini G, Rapaccini GL. Percutaneous ablation procedures in cirrhotic patients with hepatocellular carcinoma submitted to liver transplantation: Assessment of efficacy at explant analysis and of safety for tumor recurrence. Liver Transplantation. 2005;11(9):1117–1126. doi: 10.1002/lt.20469. [DOI] [PubMed] [Google Scholar]