Abstract
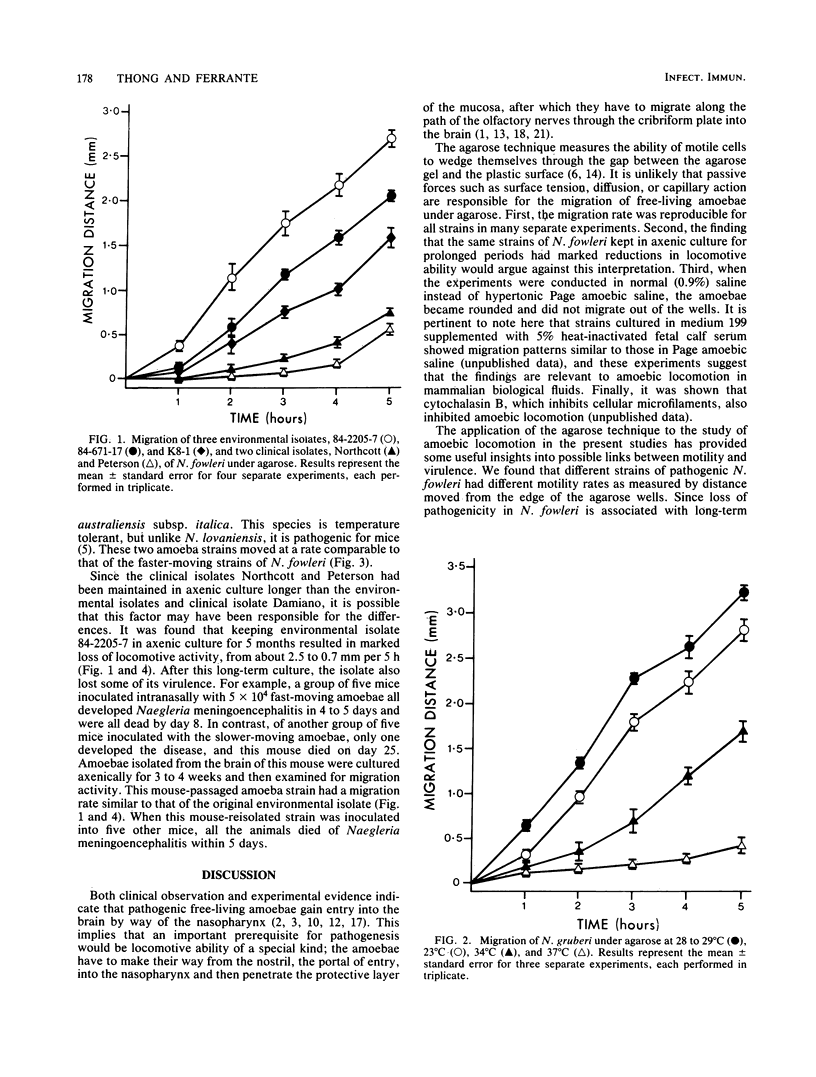
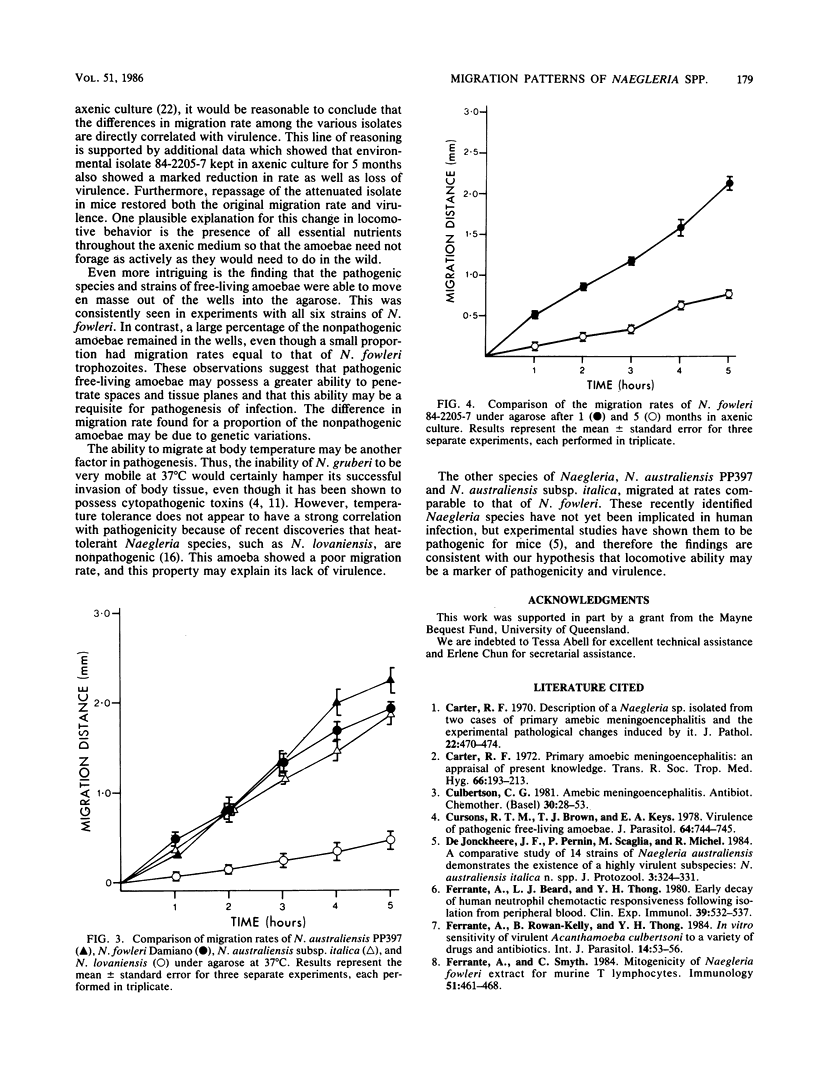
Four species of Naegleria were tested for their ability to migrate under agarose. Pathogenic N. fowleri strains exhibited rapid locomotion at 37 degrees C. Environmental isolates of N. fowleri moved faster than clinical isolates which had been kept in axenic culture for longer periods, and this result was confirmed by using the 84-2205-7 strain kept in axenic culture for 1 or 5 months. Nonpathogenic N. gruberi strains migrated actively at 28 degrees C but not at 37 degrees C; moreover, even at 28 degrees C, active amoebae constituted only a small proportion of the whole. The temperature-tolerant, nonpathogenic species N. lovaniensis moved more slowly than N. fowleri at 37 degrees C. In contrast, N. australiensis, which is temperature tolerant as well as pathogenic for mice, migrated at a rate comparable to that of N. fowleri. There appears to be a direct correlation between the locomotive ability of free-living amoebae and their pathogenic potential.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carter R. F. Primary amoebic meningo-encephalitis. An appraisal of present knowledge. Trans R Soc Trop Med Hyg. 1972;66(2):193–213. doi: 10.1016/0035-9203(72)90147-2. [DOI] [PubMed] [Google Scholar]
- Carter R. F. Sensitivity to amphotericin B of a Naegleria sp. isolated from a case of primary amoebic meningoencephalitis. J Clin Pathol. 1969 Jul;22(4):470–474. doi: 10.1136/jcp.22.4.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culbertson C. G. Amebic meningoencephalitis. Antibiot Chemother (1971) 1981;30:28–53. doi: 10.1159/000398093. [DOI] [PubMed] [Google Scholar]
- Cursons R. T., Brown T. J., Keys E. A. Virulence of pathogenic free-living amebae. J Parasitol. 1978 Aug;64(4):744–745. [PubMed] [Google Scholar]
- De Jonckheere J. F., Pernin P., Scaglia M., Michel R. A comparative study of 14 strains of Naegleria australiensis demonstrates the existence of a highly virulent subspecies: N. australiensis italica n. spp. J Protozool. 1984 May;31(2):324–331. doi: 10.1111/j.1550-7408.1984.tb02971.x. [DOI] [PubMed] [Google Scholar]
- Ferrante A., Beard L. J., Thong Y. H. Early decay of human neutrophil chemotactic responsiveness following isolation from peripheral blood. Clin Exp Immunol. 1980 Feb;39(2):532–537. [PMC free article] [PubMed] [Google Scholar]
- Ferrante A., Rowan-Kelly B., Thong Y. H. In vitro sensitivity of virulent Acanthamoeba culbertsoni to a variety of drugs and antibiotics. Int J Parasitol. 1984 Feb;14(1):53–56. doi: 10.1016/0020-7519(84)90011-0. [DOI] [PubMed] [Google Scholar]
- Ferrante A., Smyth C. Mitogenicity of Naegleria fowleri extract for murine T lymphocytes. Immunology. 1984 Mar;51(3):461–468. [PMC free article] [PubMed] [Google Scholar]
- John D. T. Primary amebic meningoencephalitis and the biology of Naegleria fowleri. Annu Rev Microbiol. 1982;36:101–123. doi: 10.1146/annurev.mi.36.100182.000533. [DOI] [PubMed] [Google Scholar]
- Marciano-Cabral F. M., Patterson M., John D. T., Bradley S. G. Cytopathogenicity of Naegleria fowleri and Naegleria gruberi for established mammalian cell cultures. J Parasitol. 1982 Dec;68(6):1110–1116. [PubMed] [Google Scholar]
- Martinez J., Duma R. J., Nelson E. C., Moretta F. L. Experimental naegleria meningoencephalitis in mice. Penetration of the olfactory mucosal epithelium by Naegleria and pathologic changes produced: a light and electron microscope study. Lab Invest. 1973 Aug;29(2):121–133. [PubMed] [Google Scholar]
- Nelson R. D., Quie P. G., Simmons R. L. Chemotaxis under agarose: a new and simple method for measuring chemotaxis and spontaneous migration of human polymorphonuclear leukocytes and monocytes. J Immunol. 1975 Dec;115(6):1650–1656. [PubMed] [Google Scholar]
- Page F. C. Taxonomic criteria for limax amoebae, with descriptions of 3 new species of Hartmannella and 3 of Vahlkampfia. J Protozool. 1967 Aug;14(3):499–521. doi: 10.1111/j.1550-7408.1967.tb02036.x. [DOI] [PubMed] [Google Scholar]
- Stevens A. R., De Jonckheere J., Willaert E. Naegleria lovaniensis new species: isolation and identification of six thermophilic strains of a new species found in association with Naegleria fowleri. Int J Parasitol. 1980 Feb;10(1):51–64. doi: 10.1016/0020-7519(80)90064-8. [DOI] [PubMed] [Google Scholar]
- Thong Y. H., Carter R. F., Ferrante A., Rowan-Kelly B. Site of expression of immunity to Naegleria fowleri in immunized mice. Parasite Immunol. 1983 Jan;5(1):67–76. doi: 10.1111/j.1365-3024.1983.tb00724.x. [DOI] [PubMed] [Google Scholar]
- Thong Y. H., Ferrante A., Rowan-Kelly B., O'Keefe D. Immunization with live amoebae, amoebic lysate and culture supernatant in experimental Naegleria meningoencephalitis. Trans R Soc Trop Med Hyg. 1980;74(5):570–576. doi: 10.1016/0035-9203(80)90141-8. [DOI] [PubMed] [Google Scholar]
- Thong Y. H., Ferrante A., Shepherd C. Phagocytic behaviour towards baker's yeast distinguishes pathogenic from non-pathogenic Naegleria. Trans R Soc Trop Med Hyg. 1978;72(2):207–209. doi: 10.1016/0035-9203(78)90067-6. [DOI] [PubMed] [Google Scholar]
- Thong Y. H. Primary amoebic meningoencephalitis: fifteen years later. Med J Aust. 1980 Apr 19;1(8):352–354. doi: 10.5694/j.1326-5377.1980.tb134919.x. [DOI] [PubMed] [Google Scholar]
- Visvesvara G. S., Callaway C. S. Light and electron microsopic observations on the pathogenesis of Naegleria fowleri in mouse brain and tissue culture. J Protozool. 1974 May;21(2):239–250. doi: 10.1111/j.1550-7408.1974.tb03648.x. [DOI] [PubMed] [Google Scholar]
- Wong M. M., Karr S. L., Jr, Chow C. K. Changes in the virulence of Naegleria fowleri maintained in vitro. J Parasitol. 1977 Oct;63(5):872–878. [PubMed] [Google Scholar]



