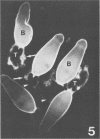
Abstract
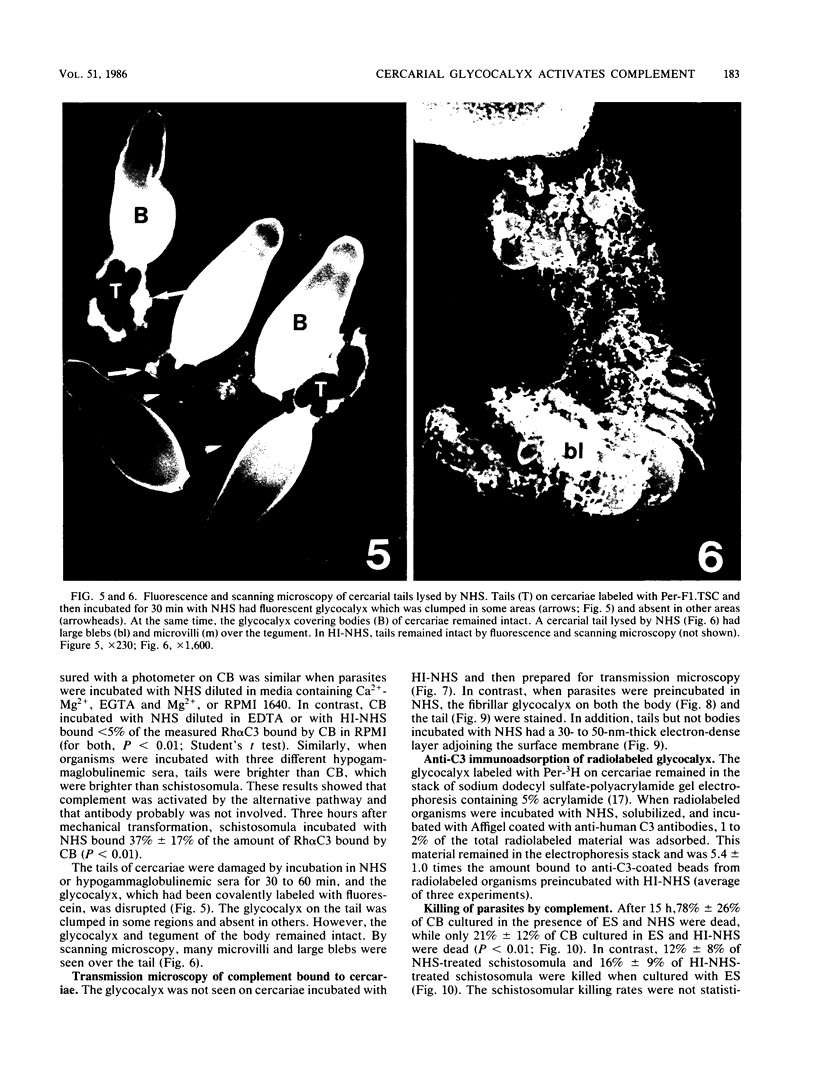
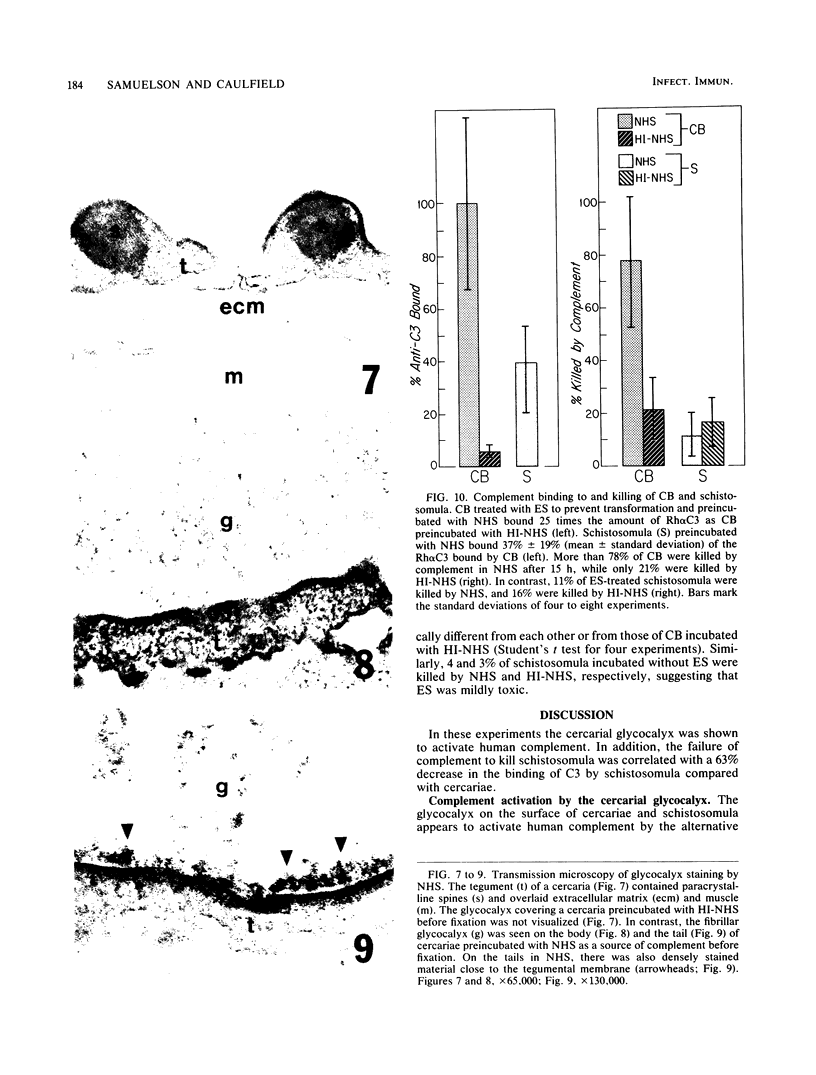
Human complement activation by cercariae and schistosomula of the human parasite Schistosoma mansoni was studied in vitro. Cercariae are composed of tails which are shed after infection of the host and bodies which transform into the larvae or schistosomula after infection. After incubation in fresh normal human serum (NHS), cercarial tails bound more anti-C3 antibodies than did cercarial bodies (CB), and the tails were rapidly lysed, while the attached CB remained intact. Complement activation by cercariae was dependent on the alternative pathway but was independent of antibody, as shown by C3 deposition by hypogammaglobulinemic human sera. By transmission microscopy, the fibrillar glycocalyx on both CB and tails was stained by NHS but not by heat-inactivated serum (HI-NHS). The glycocalyx was labeled with periodate and tritiated borohydride, and parasites were incubated in NHS and HI-NHS. After solubilization, the labeled glycocalyx on organisms incubated in NHS but not HI-NHS bound anti-C3 antibodies. Of the CB incubated with eserine sulfate to prevent transformation, 78% +/- 10% were dead after culture for 24 h in NHS. In contrast, 21% +/- 12% of the CB were dead after culture in HI-NHS. Schistosomula incubated in NHS bound 37% of the amount of anti-C3 antibodies bound by cercariae but were not killed by NHS. In conclusion, the cercarial glycocalyx activated human complement, and schistosomula were less susceptible to killing than cercariae because they had less glycocalyx and activated less complement.
Full text
PDF
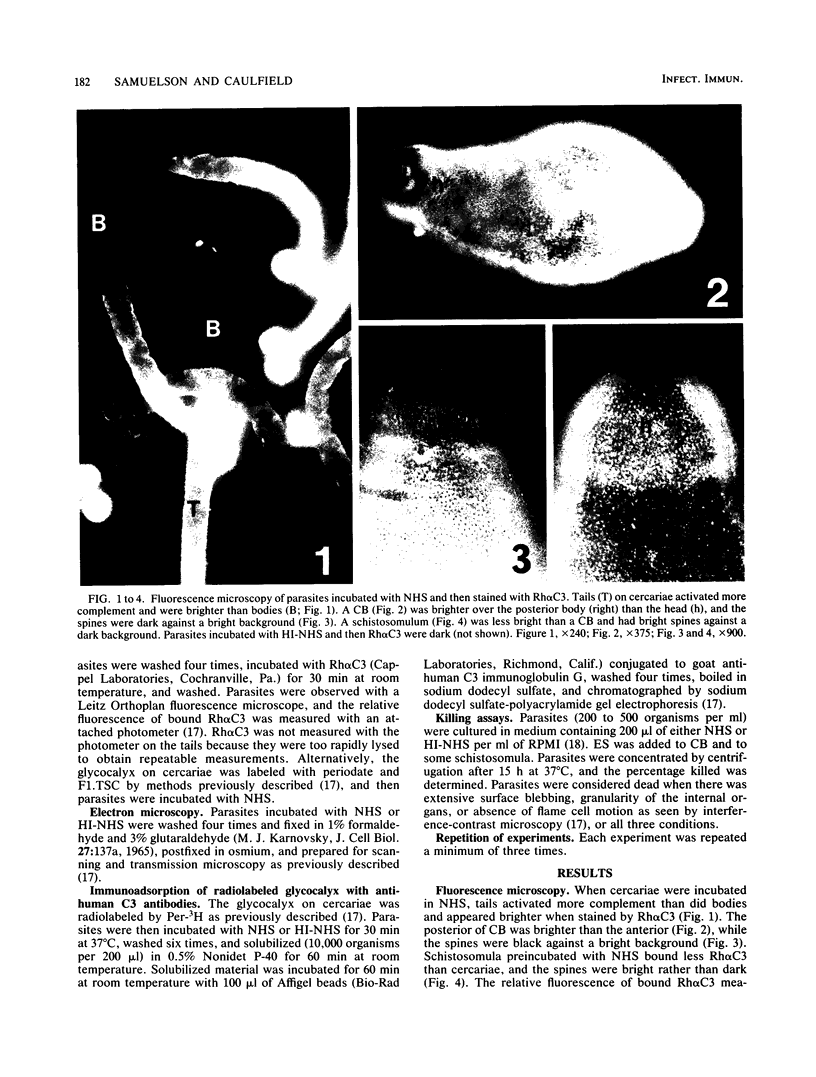


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dean D. A. Decreased binding of cytotoxic antibody by developing Schistosoma mansoni. Evidence for a surface change independent of host antigen adsorption and membrane turnover. J Parasitol. 1977 Jun;63(3):418–426. [PubMed] [Google Scholar]
- Dean D. A., Mangold B. L., Georgi J. R., Jacobson R. H. Comparison of Schistosoma mansoni migration patterns in normal and irradiated cercaria-immunized mice by means of autoradiographic analysis. Evidence that worm elimination occurs after the skin phase in immunized mice. Am J Trop Med Hyg. 1984 Jan;33(1):89–96. doi: 10.4269/ajtmh.1984.33.89. [DOI] [PubMed] [Google Scholar]
- Dessein A., Samuelson J. C., Butterworth A. E., Hogan M., Sherry B. A., Vadas M. A., David J. R. Immune evasion by Schistosoma mansoni: loss of susceptibility to antibody or complement-dependent eosinophil attack by schistosomula cultured in medium free of macromolecules. Parasitology. 1981 Jun;82(Pt 3):357–374. doi: 10.1017/s0031182000066890. [DOI] [PubMed] [Google Scholar]
- Dias Da Silva W., Kasatchkine M. D. Schistosoma mansoni: activation of the alternative pathway of human complement by schistosomula. Exp Parasitol. 1980 Oct;50(2):278–286. doi: 10.1016/0014-4894(80)90029-6. [DOI] [PubMed] [Google Scholar]
- Grossman N., Leive L. Complement activation via the alternative pathway by purified Salmonella lipopolysaccharide is affected by its structure but not its O-antigen length. J Immunol. 1984 Jan;132(1):376–385. [PubMed] [Google Scholar]
- Hockley D. J., McLaren D. J. Schistosoma mansoni: changes in the outer membrane of the tegument during development from cercaria to adult worm. Int J Parasitol. 1973 Jan;3(1):13–25. doi: 10.1016/0020-7519(73)90004-0. [DOI] [PubMed] [Google Scholar]
- Joiner K. A., Goldman R., Schmetz M., Berger M., Hammer C. H., Frank M. M., Leive L. A quantitative analysis of C3 binding to O-antigen capsule, lipopolysaccharide, and outer membrane protein of E. coli 0111B4. J Immunol. 1984 Jan;132(1):369–375. [PubMed] [Google Scholar]
- Kemp W. M., Damian R. T., Greene N. D. Schistosoma mansoni: immunohistochemical localization of the CHR reaction in glycocalyx of cercaria. Exp Parasitol. 1973 Feb;33(1):27–33. doi: 10.1016/0014-4894(73)90006-4. [DOI] [PubMed] [Google Scholar]
- Kemp W. M. Ultrastructure of the Cercarienhüllen Reaktion of Schistosoma mansoni. J Parasitol. 1970 Aug;56(4):713–723. [PubMed] [Google Scholar]
- Levi-Schaffer F., Schryer M. D., Somlarsky M. The resistance of schistosomula of Schistosoma mansoni to antibodies and complement in vitro does not correlate with the binding of antibodies to the surface of the parasite. J Immunol. 1982 Dec;129(6):2744–2751. [PubMed] [Google Scholar]
- Machado A. J., Gazzinelli G., Pellegrino J., Dias de Silva W. Schistosoma mansoni: the role of the complement C3-activating system in the cercaricidal action of normal serum. Exp Parasitol. 1975 Aug;38(1):20–29. doi: 10.1016/0014-4894(75)90034-x. [DOI] [PubMed] [Google Scholar]
- Ramalho-Pinto F. J., Gazzinelli G., Howells R. E., Mota-Santos T. A., Figueiredo E. A., Pellegrino J. Schistosoma mansoni: defined system for stepwise transformation of cercaria to schistosomule in vitro. Exp Parasitol. 1974 Dec;36(3):360–372. doi: 10.1016/0014-4894(74)90076-9. [DOI] [PubMed] [Google Scholar]
- Ratnoff W. D., Fearon D. T., Austen K. F. The role of antibody in the activation of the alternative complement pathway. Springer Semin Immunopathol. 1983;6(4):361–371. doi: 10.1007/BF02116280. [DOI] [PubMed] [Google Scholar]
- Ruppel A., Rother U., Diesfeld H. J. Schistosoma mansoni: development of primary infections in mice genetically deficient or intact in the fifth component of complement. Parasitology. 1982 Oct;85(Pt 2):315–323. doi: 10.1017/s0031182000055293. [DOI] [PubMed] [Google Scholar]
- Samuelson J. C., Caulfield J. P. The cercarial glycocalyx of Schistosoma mansoni. J Cell Biol. 1985 May;100(5):1423–1434. doi: 10.1083/jcb.100.5.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuelson J. C., Sher A., Caulfield J. P. Newly transformed schistosomula spontaneously lose surface antigens and C3 acceptor sites during culture. J Immunol. 1980 Apr;124(4):2055–2057. [PubMed] [Google Scholar]
- Santoro F., Lachmann P. J., Capron A., Capron M. Activation of complement by Schistosoma mansoni schistosomula: killing of parasites by the alternative pathway and requirement of IgG for classical pathway activation. J Immunol. 1979 Oct;123(4):1551–1557. [PubMed] [Google Scholar]
- Santoro F., Vandemeulebroucke B., Liebart M. C., Capron A. Schistosoma mansoni: role in vivo of complement in primary infection of mice. Exp Parasitol. 1982 Aug;54(1):40–46. doi: 10.1016/0014-4894(82)90108-4. [DOI] [PubMed] [Google Scholar]
- Smithers S. R., Gammage K. Recovery of Schistosoma mansoni from the skin, lungs and hepatic portal system of naive mice and mice previously exposed to S. mansoni: evidence for two phases of parasite attrition in immune mice. Parasitology. 1980 Apr;80(2):289–300. doi: 10.1017/s0031182000000755. [DOI] [PubMed] [Google Scholar]
- Tavares C. A., Gazzinelli G., Mota-Santos T. A., Dias Da Silva W. Schistosoma mansoni: complement-mediated cytotoxic activity in vitro and effect of decomplementation on acquired immunity in mice. Exp Parasitol. 1978 Dec;46(2):145–151. doi: 10.1016/0014-4894(78)90126-1. [DOI] [PubMed] [Google Scholar]
- Winkelstein J. A., Tomasz A. Activation of the alternative complement pathway by pneumococcal cell wall teichoic acid. J Immunol. 1978 Jan;120(1):174–178. [PubMed] [Google Scholar]