Abstract
Objective
Long chain omega-3 polyunsaturated fatty acids (n-3 PUFA) are increasingly believed to be cardioprotective. We tested the hypothesis that erythrocyte n-3 PUFA levels, as measured by the omega-3 index (O3I), are inversely related to mortality in hemodialysis patients.
Design and Study Population
Retrospective study of 93 prevalent urban U.S. hemodialysis patients with baseline n-3 PUFA blood levels.
Main Outcome Measure
Mortality rate over a median period of 755 days.
Results
The median omega-3 index was 4.69 mean weight %. During the follow up period, 19 patients died and 8 underwent renal transplantation. The probability of survival was significantly greater in patients with an O3I above the median (p=0.025). Univariate analyses found that increasing age and the Charlson Comorbidity Index (CCI) were associated with reduced survival, while a higher O3I and black race were linked with greater survival. In a multivariate model only the CCI score clearly predicted mortality (Hazard Ratio (95% CI); 1.31(1.06, 1.62)), though a protective trend was observed with an O3I above the median (2.48(0.88, 6.95)).
Conclusion
Though this modest sized study did not find a statistically significant relationship between erythrocyte n-3 PUFA levels and mortality, an inverse association was suggested. The existence of such a relationship will need to be confirmed in cohorts with greater statistical power.
INDEX WORDS: dialysis, mortality, omega-3, fish oil, eicosapentaenoic, docosahexaenoic
INTRODUCTION
The long chain omega-3 polyunsaturated fatty acids (n-3 PUFA) eicosapentaenoic (EPA, 20:5n-3) and docosahexaenoic acids (DHA, 22:6n-3) are important biological mediators obtained principally from dietary marine sources. A growing body of laboratory and clinical data suggest that n-3 PUFA have cardioprotective benefits and reduce mortality, perhaps through their anti-inflammatory, anti-arrhythmic, lipid-lowering, and/or anti-hypertensive effects (1). Based on this evidence the American Heart Association (AHA) recently recommended that individuals at high cardiovascular risk consume 1 g daily of fish oil (a rich source of EPA and DHA) (1), since supplementation rapidly and effectively raises blood n-3 PUFA levels (2).
We have previously demonstrated that hemodialysis patients consume amounts of dietary fish far below AHA guidelines and consequently have suboptimal blood n-3 PUFA content (2, 3). Inadequate n-3 PUFA intake may potentiate the already heightened cardiovascular risk state seen with kidney failure. Though an inverse link between fish consumption and mortality in dialysis patients has been reported (4), there are no data examining the relationship between blood n-3 PUFA content and death. Such data would help confirm that n-3 PUFA, and not fish, are the true mediators of cardioprotection.
We tested the hypothesis that erythrocyte n-3 PUFA levels, a reflection of long-term consumption, are inversely related to risk of death in a cohort of hemodialysis patients.
MATERIALS AND METHODS
Study Population and Design
Ninety three prevalent hemodialysis patients from Indiana University (Indianapolis, IN) affiliated dialysis units underwent blood draws between March 2005 and April 2006. A detailed description of the study cohort has been provided elsewhere (2, 3, 5). Briefly, the subjects were free of acute illness, had blood omega-3 levels measured at baseline, were followed until time of death, and adhered to the following exclusion criteria: age less than 18 years; fish oil or omega-3 supplementation within the past six months; pregnancy; current enrollment in a dietary study; compliance with a diet that prohibits fish intake; inability to give written informed consent; and malabsorption syndromes such as chronic pancreatitis or chronic diarrheal illnesses. The relevant institutional review boards approved the study protocol, and all patients gave written informed consent after reviewing a written summary of the study plan. The study adhered to the Declaration of Helsinki.
Measurements and Laboratory Tests
Blood samples were obtained immediately prior to hemodialysis treatment while in the fasting state and immediately placed on ice. Plasma and whole red blood cells (RBC) were separated by centrifugation within the hour, washed several times with 0.9% saline, and stored at −80° C. Lipids from RBC were extracted with chloroform/methanol (2:1, v/v) and fatty acid methyl esters (FAME) prepared by derivatization using boron trifluoride (BF3) in methanol (10% w/w, Supelco Inc. Bellefonte, PA, U.S.A.) as previously described (6). FAME were extracted with isooctane and analyzed by a gas chromatograph (Agilent 6890 Plus, autosampler 7683, Chemstation Rev. A.08.03; Agilent Technologies, Inc., Wilmington, DE, U.S.A.) equipped with a flame ionization detector and a DB-23 fused silica capillary column (30 m, 0.53 mm i.d., 0.5 µm film thickness; Agilent) using helium as the carrier gas. FAME were identified by comparison of their retention times with authentic standards (Nu-Chek-Prep, Inc., Elysian, MN, U.S.A.) and fatty acid values expressed as weight percentages. The summation of erythrocyte EPA and DHA content, the omega-3 index, has been linked to clinical outcomes and was used in this study as a marker of blood n-3 PUFA content (7).
All subjects completed a demographic, social, and fish consumption questionnaire. The Charlson Comorbidity Index (CCI), an index of overall disease burden that has been validated to predict mortality, was estimated for each patient (8). Serum albumin was measured by standard laboratory technique.
Statistical Analysis
Variables are expressed as mean ± SD or frequency. Study entry characteristics were compared between patients with omega-3 index above the median and below the median by use of a Student’s t-test, Wilcoxon Rank Sum test or chi-square. The primary outcome was time to death. Kaplan-Meier survival curves were compared between the two groups using log-rank test. Transplanted patients were censored at time of transplantation. The relative risk of death was estimated for individual covariates with a Cox proportional-hazards model. Sex, race, fish servings, age, serum albumin, body mass index and Charlson Comorbidity Index were considered for possible confounding factors. Variables significantly associated with outcome at the univariate Cox regression analysis (i.e. omega-3 index, Charlson Comorbidity Index, age, race) were entered in a multivariate model and adjusted omega-3 index effect was assessed based on the likelihood-ratio test. All analyses used two-sided P values with significance if ≤ 0.05. Data were analyzed with SAS 9.3.1 (SAS Institute, Inc., Cary, NC).
RESULTS
Forty seven and 46 patients had levels below and above the median omega-3 index of 4.69 mean weight %, respectively. As seen in Table 1, the latter group included more blacks and consumed more dietary fish.
Table 1.
Study Subject Characteristics
| Omega-3 Index | |||
|---|---|---|---|
| Characteristics | Below Median (n=47) |
Above Median (n=46) |
P |
| Age* | 53 ± 13 | 56 ± 12 | 0.24 |
| Sex (% male) | 72.3 | 58.7 | 0.17 |
| Race (%) | <0.01 | ||
| White | 36.2 | 10.9 | |
| Black | 63.8 | 89.1 | |
| Body Mass Index (kg/m2)* | 29 ± 8 | 31 ± 9 | 0.11 |
| Serum Albumin (g/dl)* | 3.7 ± 0.4 | 3.7 ± 0.4 | 0.82 |
| Diabetes Mellitus (%) | 19 | 13 | 0.43 |
| Peripheral Vascular Disease (%) | 34 | 24 | 0.29 |
| Myocardial Infarction (%) | 19 | 26 | 0.43 |
| Charlson Comorbidity Index* | 4.9 ± 2.0 | 4.4 ± 2.0 | 0.22 |
| Weekly Fish Servings (%) | <0.01 | ||
| <1 | 59.1 | 23.9 | |
| ≥1 | 40.9 | 76.1 | |
mean ± SD
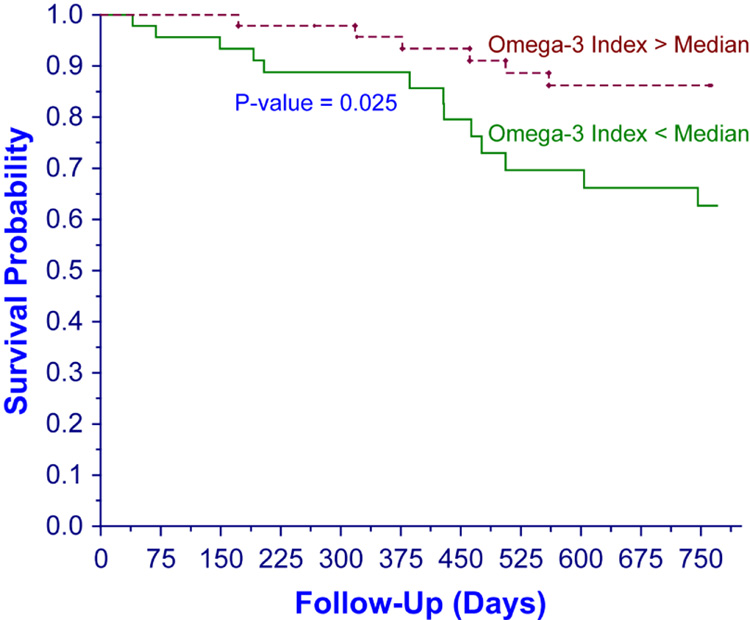
Over a median follow up period of 755 days (range: 40–770), 19 patients died and 8 underwent renal transplantation. Erythrocyte omega-3 index levels (median (range)) in persons who died and survived were 4.9 (1.3–8.1) and 4.3 (1.6–6.8) mean weight %, respectively. As shown in the Figure, the unadjusted probability of survival was significantly greater in patients with omega-3 index above the median (p = 0.025).
Figure.
Survival by Omega-3 Index
Univariate analyses found that an increasing age [HR (95% CI); 1.05 (1.01, 1.09)] and CCI score [1.47 (1.2, 1.8)] were associated with reduced survival, while a higher omega-3 index (above vs. below median, [0.35 (0.13, 0.92)]), and black race [vs. white; 0.25 (0.10, 0.62)] were linked to greater survival. When examined as a continous variable, an increasing omega-3 index was associated with improved survival [0.64 (0.44, 0.94)]. A multivariate Cox model (Table 2) found that only the CCI score clearly predicted mortality, though a protective trend was observed in persons with an omega-3 index above the median.
Table 2.
Hazard of Death in a Multivariate Model
| Variable | Hazard Ratio* (95% CI) |
P value |
|---|---|---|
| Omega-3 Index (<median) | 2.48 (0.88, 6.95) | 0.085 |
| Charlson Comorbidity Index Score | 1.31 (1.06, 1.62) | 0.011 |
| Age | 1.03 (0.99, 1.07) | 0.118 |
| Black (versus white) | 0.50 (0.18, 1.37) | 0.174 |
Hazard ratio was per one unit increase for continuous variables
DISCUSSION
Our study is the first to directly examine the relationship in hemodialysis patients between blood n-3 PUFA content and mortality. Though the findings were not statistically significant, they did suggest an inverse association, especially considering the cohort’s modest size and limited statistical power. Identifying a link between blood n-3 PUFA levels and risk of clinical events in the kidney failure population is a worthwhile goal simply because, as we have recently demonstrated (2), levels are easily modifiable by oral supplementation.
N-3 PUFA play important and increasingly recognized roles in a number of biological processes that influence cardiovascular health, including eicosanoid production, regulation of the inflammatory and immune systems, lipid metabolism and blood pressure, and cell membrane function and stability (9–12). There is robust observational evidence (13–15) and mounting data from clinical supplementation trials (16–18) that n-3 PUFA reduce the risk of cardiovascular events, sudden cardiac death, and overall mortality. The evidence is persuasive enough to have convinced the AHA and other international health organizations to establish n-3 PUFA intake guidelines for at-risk individuals (1, 19).
The cardiovascular effects of n-3 PUFAs have not been extensively explored in the dialysis population, though the available evidence does suggest a benefit. Kutner et al observed that fish consumption was associated with reduced mortality in a retrospective analysis of 216 incident dialysis patients (4). N-3 PUFA were not measured in this study. A more recent, randomized, secondary prevention study in Danish hemodialysis patients found that fish oil supplementation 1.7 g/day reduced the risk of myocardial infarctions but not the primary composite outcomes of total deaths and cardiovascular events (20). Though well designed overall, the study was underpowered to detect the primary outcome.
In conclusion, though our modest sized study did not find a clear, statistically significant link between n-3 PUFA blood levels and mortality, it did suggest an inverse relationship. The existence of such a relationship will need to be confirmed in cohorts with greater statistical power.
ACKNOWLEDGMENTS
We greatly appreciate the excellent clinical, laboratory, and funding support provided by Qian Li, M.D., Akber Saifullah, M.D., the National Kidney Foundation of Indiana, Inc. (NKFI), the National Institutes of Health (K23 RR019615-01A1), and the General Clinical Research Center at Indiana University School of Medicine (M01 RR00750).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Kris-Etherton PM, Harris WS, Appel LJ. Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation. 2002;106:2747–2757. doi: 10.1161/01.cir.0000038493.65177.94. [DOI] [PubMed] [Google Scholar]
- 2.Saifullah A, Watkins B, Saha C, Li Y, Moe S, Friedman A. Oral fish oil supplementation raises blood omega-3 levels and lowers C-reactive protein in haemodialysis patients—a pilot study. Nephrol Dial Transplant. 2007;22:3561–3567. doi: 10.1093/ndt/gfm422. [DOI] [PubMed] [Google Scholar]
- 3.Friedman AN, Moe SM, Perkins SM, Li Y, Watkins BA. Fish consumption and omega-3 fatty acid status and determinants in long-term hemodialysis. American Journal of Kidney Diseases. 2006;47:1064–1071. doi: 10.1053/j.ajkd.2006.03.033. [DOI] [PubMed] [Google Scholar]
- 4.Kutner NG, Clow PW, Zhang R, Aviles X. Association of fish intake and survival in a cohort of incident dialysis patients. Am J Kidney Dis. 2002;39:1018–1024. doi: 10.1053/ajkd.2002.32775. [DOI] [PubMed] [Google Scholar]
- 5.Friedman A, Siddiqui R, Watkins B. Acute rise of omega-3 polyunsaturated fatty acids during hemodialysis treatment. J Ren Nutr. 2007 doi: 10.1053/j.jrn.2007.11.002. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Watkins BA, Li Y, Allen KG, Hoffmann WE, Seifert MF. Dietary ratio of (n-6)/(n-3) polyunsaturated fatty acids alters the fatty acid composition of bone compartments and biomarkers of bone formation in rats. J Nutr. 2000;130:2274–2284. doi: 10.1093/jn/130.9.2274. [DOI] [PubMed] [Google Scholar]
- 7.Harris W, Schacky Cv. The Omega-3 Index: a new risk factor for death from coronary heart disease? Prev Med. 2004;39:212–220. doi: 10.1016/j.ypmed.2004.02.030. [DOI] [PubMed] [Google Scholar]
- 8.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. Journal of Chronic Diseases. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 9.Dwyer JH, Allayee H, Dwyer KM, et al. Arachidonate 5-lipoxygenase promoter genotype, dietary arachidonic acid, and atherosclerosis. N Engl J Med. 2004;350:29–37. doi: 10.1056/NEJMoa025079. [DOI] [PubMed] [Google Scholar]
- 10.Leaf A, Kang JX, Xiao YF, Billman GE. Clinical prevention of sudden cardiac death by n-3 polyunsaturated fatty acids and mechanism of prevention of arrhythmias by n-3 fish oils. Circulation. 2003;107:2646–2652. doi: 10.1161/01.CIR.0000069566.78305.33. [DOI] [PubMed] [Google Scholar]
- 11.Appel LJ, Miller ER, 3rd, Seidler AJ, Whelton PK. Does supplementation of diet with 'fish oil' reduce blood pressure? A meta-analysis of controlled clinical trials. Arch Intern Med. 1993;153:1429–1438. [PubMed] [Google Scholar]
- 12.Phillipson BE, Rothrock DW, Connor WE, Harris WS, Illingworth DR. Reduction of plasma lipids, lipoproteins, and apoproteins by dietary fish oils in patients with hypertriglyceridemia. N Engl J Med. 1985;312:1210–1216. doi: 10.1056/NEJM198505093121902. [DOI] [PubMed] [Google Scholar]
- 13.Siscovick DS, Raghunathan TE, King I, et al. Dietary intake and cell membrane levels of long-chain n-3 polyunsaturated fatty acids and the risk of primary cardiac arrest. JAMA. 1995;274:1363–1367. doi: 10.1001/jama.1995.03530170043030. [DOI] [PubMed] [Google Scholar]
- 14.Albert CM, Campos H, Stampfer MJ, et al. Blood levels of long-chain n-3 fatty acids and the risk of sudden death. N Engl J Med. 2002;346:1113–1118. doi: 10.1056/NEJMoa012918. [DOI] [PubMed] [Google Scholar]
- 15.Wang C, Harris WS, Chung M, et al. n-3 Fatty acids from fish or fish-oil supplements, but not alpha-linolenic acid, benefit cardiovascular disease outcomes in primary- and secondary-prevention studies: a systematic review. American Journal of Clinical Nutrition. 2006;84:5–17. doi: 10.1093/ajcn/84.1.5. [DOI] [PubMed] [Google Scholar]
- 16.Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial. Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto miocardico. Lancet. 1999;354:447–455. [PubMed] [Google Scholar]
- 17.Mozaffarian D, Rimm EB. Fish intake, contaminants, and human health: evaluating the risks and the benefits. JAMA. 2006;296:1885–1899. doi: 10.1001/jama.296.15.1885. [DOI] [PubMed] [Google Scholar]
- 18.Yokoyama M, Origasa H, Matsuzaki M, et al. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomised open-label, blinded endpoint analysis. Lancet. 2007;369:1090–1098. doi: 10.1016/S0140-6736(07)60527-3. [DOI] [PubMed] [Google Scholar]
- 19.Gebauer SK, Psota TL, Harris WS, Kris-Etherton PM. n-3 fatty acid dietary recommendations and food sources to achieve essentiality and cardiovascular benefits. American Journal of Clinical Nutrition. 2006;83:1526S–1535S. doi: 10.1093/ajcn/83.6.1526S. [DOI] [PubMed] [Google Scholar]
- 20.Svensson M, Schmidt E, Jorgensen K, Christensen J. N-3 fatty acids as secondary prevention against cardiovascular events in patients who undergo chronic hemodialysis: A randomized, placebo-controlled intervention trial. Clin J Am Soc Nephrol. 2006;1:780–786. doi: 10.2215/CJN.00630206. [DOI] [PubMed] [Google Scholar]