Abstract
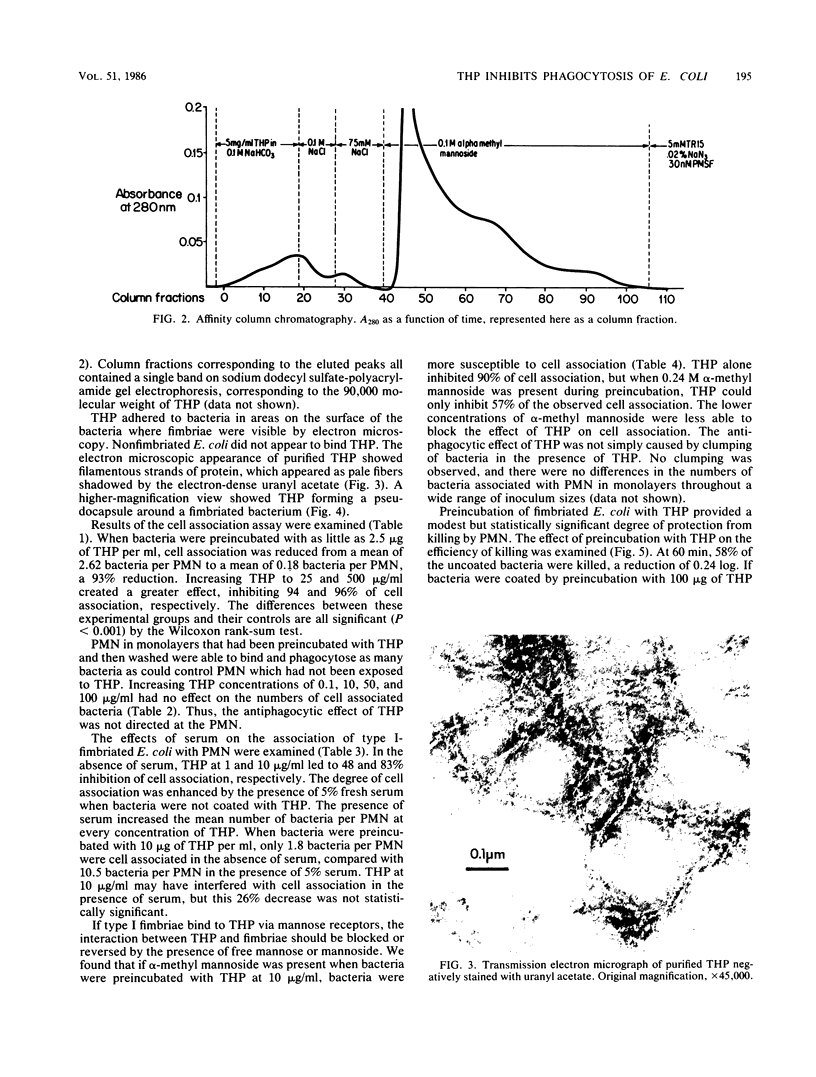
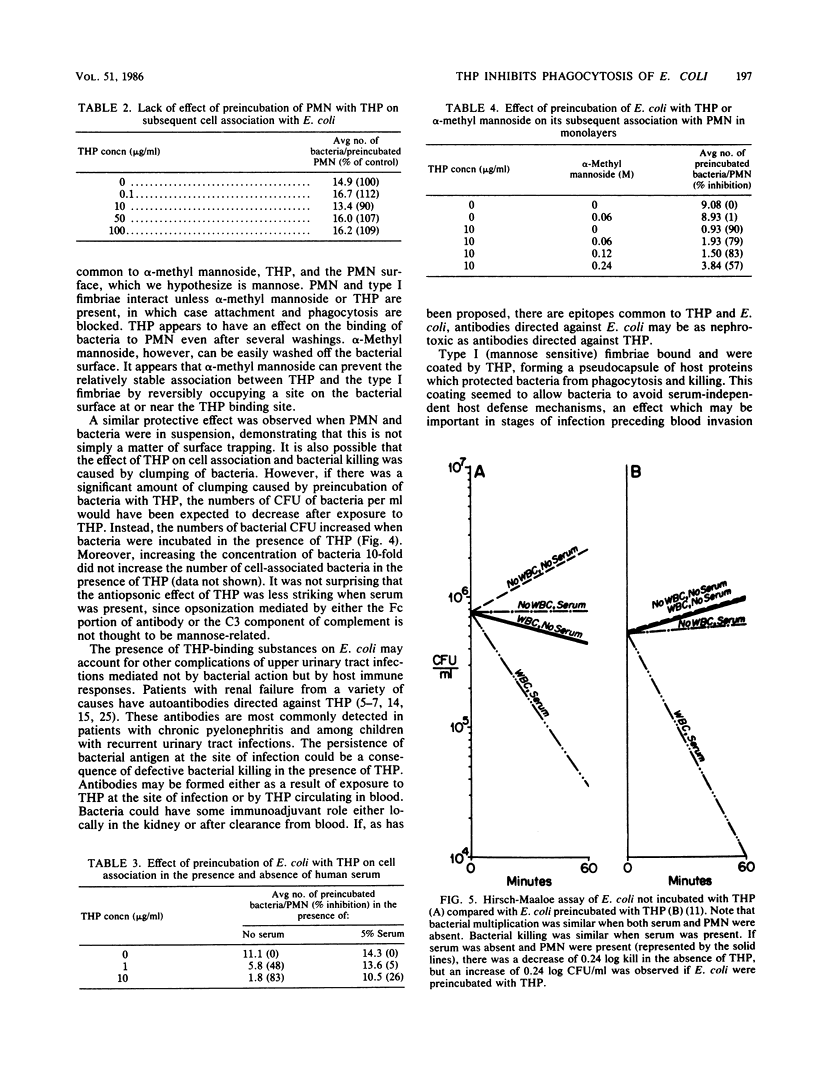
Human polymorphonuclear leukocytes (PMN) ingest type I (mannose sensitive) fimbriated Escherichia coli even in the absence of antibody, complement, or other serum opsonins. Our studies suggest that the Tamm-Horsfall urinary glycoprotein (THP) interferes with serum-independent ingestion. Electron micrographs showed that dissolved THP adhered to type I fimbriae and formed a pseudocapsule around bacteria bearing type I fimbriae. Phase-variant bacteria grown on blood agar neither expressed fimbriae nor bound THP. Affinity column chromatography demonstrated mannose-sensitive binding between purified type I fimbriae and purified THP. The ability of human PMN to bind and ingest type I-fimbriated E. coli was diminished if the bacteria had been coated by exposure to THP at physiologic concentrations. At 1 h, PMN were associated with an average of 2.62 uncoated bacteria, but with only 0.18 coated bacteria (P less than 0.001). alpha-Methyl mannoside blocked the observed effect of THP on binding and phagocytosis in a dose-dependent fashion: increased mannoside led to increased blocking. PMN preincubated with THP were able to bind and phagocytose normally. There did not appear to be any significant clumping of bacteria in suspension to account for these effects. Bactericidal assays with leukocytes in suspension demonstrated protection of THP-coated bacteria. At 1 h, PMN killed 42% of noncoated E. coli (a decrease of 0.24 log), but the number of THP-coated bacteria increased by 75% (an increase of 0.24 log). These observations may partially explain the virulence of E. coli in the bladder and kidney, where serum activity is low and THP is abundant.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beachey E. H. Bacterial adherence: adhesin-receptor interactions mediating the attachment of bacteria to mucosal surface. J Infect Dis. 1981 Mar;143(3):325–345. doi: 10.1093/infdis/143.3.325. [DOI] [PubMed] [Google Scholar]
- Blumenstock E., Jann K. Adhesion of piliated Escherichia coli strains to phagocytes: differences between bacteria with mannose-sensitive pili and those with mannose-resistant pili. Infect Immun. 1982 Jan;35(1):264–269. doi: 10.1128/iai.35.1.264-269.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eden C. S., Eriksson B., Hanson L. A. Adhesion of Escherichia coli to human uroepithelial cells in vitro. Infect Immun. 1977 Dec;18(3):767–774. doi: 10.1128/iai.18.3.767-774.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edén C. S., Freter R., Hagberg L., Hull R., Hull S., Leffler H., Schoolnik G. Inhibition of experimental ascending urinary tract infection by an epithelial cell-surface receptor analogue. Nature. 1982 Aug 5;298(5874):560–562. doi: 10.1038/298560a0. [DOI] [PubMed] [Google Scholar]
- Edén C. S., Hansson H. A. Escherichia coli pili as possible mediators of attachment to human urinary tract epithelial cells. Infect Immun. 1978 Jul;21(1):229–237. doi: 10.1128/iai.21.1.229-237.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eshdat Y., Silverblatt F. J., Sharon N. Dissociation and reassembly of Escherichia coli type 1 pili. J Bacteriol. 1981 Oct;148(1):308–314. doi: 10.1128/jb.148.1.308-314.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasth A., Ahlstedt S., Hanson L. A., Jann B., Jann K., Kaijser B. Cross-reactions between the Tamm-Horsfall glycoprotein and Escherichia coli. Int Arch Allergy Appl Immunol. 1980;63(3):303–311. doi: 10.1159/000232640. [DOI] [PubMed] [Google Scholar]
- Fasth A., Bengtsson U., Kaijser B., Wieslander J. Antibodies to Tamm-Horsfall protein associated with renal damage and urinary tract infections in adults. Kidney Int. 1981 Oct;20(4):500–504. doi: 10.1038/ki.1981.167. [DOI] [PubMed] [Google Scholar]
- Fasth A., Bjure J., Hellström M., Jacobsson B., Jodal U. Autoantibodies to Tamm-Horsfall glycoprotein in children with renal damage associated with urinary tract infections. Acta Paediatr Scand. 1980 Nov;69(6):709–715. doi: 10.1111/j.1651-2227.1980.tb07138.x. [DOI] [PubMed] [Google Scholar]
- Fasth A., Hanson L. A., Jodal U., Peterson H. Autoantibodies to Tamm-Horsfall protein associated with urinary tract infections in girls. J Pediatr. 1979 Jul;95(1):54–60. doi: 10.1016/s0022-3476(79)80081-5. [DOI] [PubMed] [Google Scholar]
- Finne J., Leinonen M., Mäkelä P. H. Antigenic similarities between brain components and bacteria causing meningitis. Implications for vaccine development and pathogenesis. Lancet. 1983 Aug 13;2(8346):355–357. doi: 10.1016/s0140-6736(83)90340-9. [DOI] [PubMed] [Google Scholar]
- HIRSCH J. G., STRAUSS B. STUDIES ON HEAT-LABILE OPSONIN IN RABBIT SERUM. J Immunol. 1964 Jan;92:145–154. [PubMed] [Google Scholar]
- Hagberg L., Hull R., Hull S., Falkow S., Freter R., Svanborg Edén C. Contribution of adhesion to bacterial persistence in the mouse urinary tract. Infect Immun. 1983 Apr;40(1):265–272. doi: 10.1128/iai.40.1.265-272.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyer J. R., Seiler M. W. Pathophysiology of Tamm-Horsfall protein. Kidney Int. 1979 Sep;16(3):279–289. doi: 10.1038/ki.1979.130. [DOI] [PubMed] [Google Scholar]
- Källenius G., Möllby R., Svenson S. B., Helin I., Hultberg H., Cedergren B., Winberg J. Occurrence of P-fimbriated Escherichia coli in urinary tract infections. Lancet. 1981 Dec 19;2(8260-61):1369–1372. doi: 10.1016/s0140-6736(81)92797-5. [DOI] [PubMed] [Google Scholar]
- Mayrer A. R., Kashgarian M., Ruddle N. H., Marier R., Hodson C. J., Richards F. F., Andriole V. T. Tubulointerstitial nephritis and immunologic responses to Tamm-Horsfall protein in rabbits challenged with homologous urine or Tamm-Horsfall protein. J Immunol. 1982 Jun;128(6):2634–2642. [PubMed] [Google Scholar]
- Mayrer A. R., Miniter P., Andriole V. T. Immunopathogenesis of chronic pyelonephritis. Am J Med. 1983 Jul 28;75(1B):59–70. doi: 10.1016/0002-9343(83)90074-8. [DOI] [PubMed] [Google Scholar]
- Ofek I., Beachey E. H., Bisno A. L. Resistance of Neisseria gonorrhoeae to phagocytosis: relationship to colonial morphology and surface pili. J Infect Dis. 1974 Mar;129(3):310–316. doi: 10.1093/infdis/129.3.310. [DOI] [PubMed] [Google Scholar]
- Orskov I., Ferencz A., Orskov F. Tamm-Horsfall protein or uromucoid is the normal urinary slime that traps type 1 fimbriated Escherichia coli. Lancet. 1980 Apr 19;1(8173):887–887. doi: 10.1016/s0140-6736(80)91396-3. [DOI] [PubMed] [Google Scholar]
- Silverblatt F. J., Dreyer J. S., Schauer S. Effect of pili on susceptibility of Escherichia coli to phagocytosis. Infect Immun. 1979 Apr;24(1):218–223. doi: 10.1128/iai.24.1.218-223.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smithers S. R., Terry R. J., Hockley D. J. Host antigens in schistosomiasis. Proc R Soc Lond B Biol Sci. 1969 Feb 25;171(1025):483–494. doi: 10.1098/rspb.1969.0007. [DOI] [PubMed] [Google Scholar]
- TAMM I., HORSFALL F. L., Jr A mucoprotein derived from human urine which reacts with influenza, mumps, and Newcastle disease viruses. J Exp Med. 1952 Jan;95(1):71–97. doi: 10.1084/jem.95.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitnack E., Beachey E. H. Antiopsonic activity of fibrinogen bound to M protein on the surface of group A streptococci. J Clin Invest. 1982 Apr;69(4):1042–1045. doi: 10.1172/JCI110508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Work J., Andriole V. T. Tamm-Horsfall protein antibody in patients with end-stage kidney disease. Yale J Biol Med. 1980 Mar-Apr;53(2):133–148. [PMC free article] [PubMed] [Google Scholar]






