Abstract
Background
Preliminary evidence is equivocal regarding the role of exhaled nitric oxide in clinical asthma management. This study evaluates the usefulness of eNO as an adjunct to asthma guidelines-based clinical care among inner-city adolescents and young adults.
Methods
A randomized, double-blind, parallel-group trial was conducted with 546 inner-city participants, aged 12–20 years, with persistent asthma (Clinicaltrials.gov Identifier: NCT00114413). A run-in characterization period of 3 weeks on an initial controller regimen preceded a 46-week double-blind treatment strategy. Participants were randomized to either, treatment based on NAEPP guidelines alone (Reference Group) or the guidelines plus FENO measurements (FENO Group). Primary outcome was asthma symptom days and secondary outcome was acute asthma exacerbations.
Findings
During the 46-week treatment period, the number of asthma symptom days, pulmonary function, unscheduled care visits, and hospitalizations did not differ between the treatment groups (mean asthma symptom days were 1.93 [95% CI 1.74-2.11] in the FENO group vs. 1.89 [1.71-1.74] in the control group; difference 0.04 [-0.29-0.22], p=0.7796). The FENO Group received a significantly higher inhaled corticosteroid dose (118.9 mcg/day difference, 95% CI: 48.5-189.3, P=0.0010) as compared to the Reference Group. Asthma symptoms remained low in both groups following randomization with 57% (306/534) of the participants well controlled for at least 80% of visits..
Interpretation
A coordinated asthma management program facilitated achieving good control in the majority of participants. The addition of FENO as a control indicator resulted in a higher dose of inhaled corticosteroids without a clinically important improvement in symptomatic asthma control.
Keywords: asthma, biomarker, exhaled nitric oxide, inhaled corticosteroid, inner-city asthma, long-acting ß2-agonist, medication adherence, asthma exacerbations, asthma outcomes, asthma guidelines, impairment, risk
Background
Asthma is a complex respiratory disorder characterized by variable and recurring symptoms, airflow obstruction, and underlying airway inflammation. In 2007, the NHLBI-Expert Panel 3 updated Guidelines for the Diagnosis and Management of Asthma proposed that, in order to achieve asthma control, treatment should aim at regulating the current manifestations of impairment, i.e., symptoms, need for rescue treatment, limitations of activity, and pulmonary function, as well as reducing future risk1, 2.
Asthma symptoms and exacerbations are theoretically linked to underlying airway inflammation but are not direct indicators of the inflammatory state. The application of biomarkers that are more closely associated with airway inflammation could improve asthma control by better directing treatment. FENO is a marker of airway inflammation3 and is increased during periods of uncontrolled asthma4-12 and reduced during treatment with anti-inflammatory agents13-21. Although previous trials have evaluated the use of FENO as an alternative to conventional symptom-driven therapy modification22-25, studies to date have not evaluated a clinically more relevant question, whether adding FENO to guideline-based management can improve asthma control.
The NIAID Inner-City Asthma Consortium elected to study the application of FENO measurement as an adjuvant to guideline-directed management of asthma in a population of inner-city adolescents and young adults characterized by high levels of atopy, allergen exposure, and poor asthma control26-30.
Methods
A total of 546 participants, aged 12 to 20 years, with asthma were enrolled at ten centers (see Appendix). Eligibility was limited to residents of urban census tracts in which at least 20 percent of households had incomes below the federal poverty threshold. Participants had a physician diagnosis of asthma. Individuals receiving long-term control therapy were required to have symptoms of persistent asthma or evidence of uncontrolled disease. Individuals not receiving long-term control therapy were required to have both symptoms of persistent asthma and evidence of uncontrolled disease defined by NAEPP guidelines1, 2. The protocol was approved by all institutional review boards. Written informed consent was obtained from each participant or their parent or legal guardian. Adolescents ages 12 to 17 provided assent.
Study Design
The study was a randomized, double-blind, parallel-group trial with a 3-week run-in to characterize participants, establish treatment, and evaluate adherence (Figure 1e-repository). At the initial visit, current medication regimens and adherence, asthma symptoms, pulmonary function, skin test sensitivities and control levels (Table 1e-repository) were assessed. Physicians selected one of six treatment steps (Table 2e-repository). Trained asthma counselors reinforced medication use, adherence, and environmental control. Participants were excluded after the run-in if controller adherence was <25%. Participants with a urinary cotinine >100 mg/ml were ineligible to exclude active smokers. All prescribed medications were provided without charge, and study participants were given a 24-hour telephone number for medical advice.
After run-in, subjects were assigned by centralized block randomization with a block size of ten to receive either a Reference Group (guideline-based care) or FENO Group (exhaled nitric oxide (eNO) added to guideline-based care). The randomization sequence was generated from a random number table and was stratified by site. Investigators and patients were blinded to treatment assignment.
At each visit conducted every 6 to 8 weeks, FENO, lung function, asthma symptoms, rescue medication use, adherence, healthcare utilization, and missed school days were evaluated (Figure 1e-repository). Adherence was based on Diskus® built-in dose counter and structured questionnaire.
Treatment Determination
Symptoms, rescue medication use, pulmonary function, and adherence were used to determine control level (4 levels; Level 1 = well controlled). FENO was measured (flow rate 50 ml/s) with a rapid-response chemiluminescent analyzer (NIOX™ System, Aerocrine, Sweden) following American Thoracic Society guidelines31. FENO was measured for each participant at every visit, but only influenced treatment of the FENO Group. Control level and FENO data were entered into a computer program which generated two treatment options for the blinded physician, one for the Reference Group and another for the FENO Group. The treatment options were derived from protocol-defined treatment steps (Table 2e-repository). Medication was adjusted based on control and adherence (Table 3e-repository). Medication was only reduced after two consecutive visits with good control (Control level 1). When adherence was ≥50%, and FENO was elevated, the FENO Group was eligible to receive an additional one step increase in treatment compared to what would be given to the Reference Group. For safety reasons, FENO was not allowed to increase treatment on the third consecutive visit without elevated symptoms. Also low FENO alone was not allowed to reduce therapy without a corresponding reduction in symptoms. An unblinded coordinator dispensed the appropriate treatment plan based on the participant’s group assignment.
Primary Outcome Measures
The primary outcome was the mean of maximum symptom days per two-week recall at each visit during the 46-week treatment period. Maximum symptom days, as used in previous inner-city asthma studies32, 33 were defined as the largest value among the following variables reported over the prior 2 weeks: (1) number of days with wheezing, chest tightness or cough; (2) number of nights of sleep disturbance; (3) number of days when activities were affected. This measure allows asthma symptoms to be correctly gauged whether the study participant expresses their asthma as reduction in play, sleep disturbance, or wheeze is reported. The mean of maximum symptom days for all visits was then calculated. The study was powered with a 90% confidence level of detecting at least a 0.70 difference in maximum symptom days per two weeks.
Statistical Methods
The average maximum symptom days per person per 2 weeks in the control group was assumed to be 4.2 days (SD: 2.4) for power calculations33. To detect a clinically meaningful group difference of 0.70 days per person per two weeks with 90% power (α=0.05 two sided), 165 subjects per group were required. Anticipating that 30-35% of subjects would not complete the study, we augmented the sample by 34% and targeted a total enrollment of 500 participants (250 per group); the final enrollment was 546. The difference in post-randomization asthma-related outcomes between groups was analyzed with a linear mixed model with fixed effects for treatment group and visit, with adjustment for levels at randomization and study site.
Utilization outcomes include hospitalizations, unscheduled ED or clinic visits, prednisone courses for asthma and asthma exacerbations. Asthma exacerbations are a composite outcome that includes hospitalizations, unscheduled visits, and prednisone use. These were rare events, so instead of analyzing the data longitudinally, we summed the events over the course of the study and analyzed using a logistic regression of any versus none.
Analyses were performed according to intention to treat with alpha level 0.05. Post-hoc sub-analyses were conducted to identify characteristics associated with favorable response to FENO -based management. Sub-analyses were conducted for heterogeneity of treatment effects across a fixed set of 9 characteristics using a statistical test for interaction.34 All statistical analyses, including the block randomization procedure outlined earlier, were performed using SAS software (version 9.1.3, SAS Institute).
Role of the Funding Source
This trial was funded through a contract with the Division of Allergy, Immunology, and Transplantation, NIAID/NIH. DAIT staff participated in protocol development, study oversight, regulatory reporting, and monitoring study conduct. NIH staff, principal investigator, and all co-investigators did not have access to outcome data until the trial was closed. Thereafter, the principal investigator, all co-investigators, and NIH staff had access to all study data. S. Szefler had final responsibility for the decision to submit for publication.
Findings
Study Population
Between September 2004 and December 2005, 780 subjects were screened and 546 were randomized, mean age 14.4 years (Interquartile range (IQR): 13–16 years) (Figure 1). Symptoms at randomization were high (Table 1): over three-quarters of participants exceeded Control Level 1 and 57% (313/546) of participants were Control Levels 3 and 4, consistent with moderate to severe asthma. Forced expiratory volume in 1 second (FEV1) and the ratio of FEV1 over forced vital capacity (FEV1/FVC) were modestly reduced with 22.5% (119/529) of participants having an FEV1 % <80% predicted 35. Most participants had at least one positive skin test (87.9%, 467/531) with the median number of positive tests being 5 (IQR:2-7) of 14 total tests placed. FENO levels at randomization were generally elevated with 63.6% (347/546) of participants having FENO ≥20 ppb.
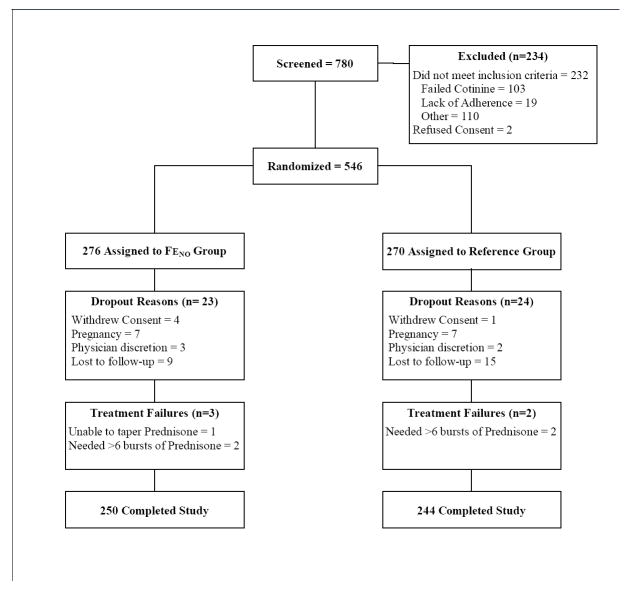
Figure 1. Consort Diagram.
Consort Diagram showing the flow of participants from enrollment to completion of study.
Table 1.
Asthma Status of 546 Randomized ACE Participants at Enrollment
| Asthma-related symptoms at enrollment (no. of days / last 2 wks) | |
| Maximum symptom days | 5.6 ± 4.6 |
| Days of wheeze, chest tightness or cough | 4.5 ± 4.1 |
| Days of interference with activities | 3.3 ± 4.1 |
| Nights of sleep disruption | 2.7 ± 3.7 |
| School days missed | 0.7 ± 1.4 |
| Asthma Control Test™ | |
| ACT™ score in the last month | 18.2 ± 4.2 |
| Lung function and exhaled nitric oxide level at enrollment | |
| FEV1 (% of predicted value) | 92.1 ± 16.6 |
| FEV1/FVC | 77.8 ± 9.4 |
| FENO (ppb) | 31.7 (14.1 – 65.4) |
| Asthma-related health care use in the year prior to enrollment (%) | |
| ≥ 1 Hospitalizations | 14.7 (80/546) |
| ≥ 1 Unscheduled visits | 68.7 (375/546) |
| ≥ 1 Prednisone courses | 52.0 (284/546) |
| ≥ 1 Exacerbations | 78.9 (431/546) |
Plus-minus values are means ± SD. Interquartile range is provided in parentheses with medians. Counts are provided in parentheses with percentages. Of the 546 study participants, more than 491 (90%) responded for all characteristics except for school days missed (398 responses).
Except for employment, there were no significant differences between groups in demographic characteristics (Table 2; Table 4e-repository). Over 90% (90.5%, 494/546) of randomized participants completed the one-year study with comparably low drop-out and treatment failure rates between groups (Figure 1).
Table 2.
Characteristics of the 546 ACE Participants at Randomization
| FENO Group
(n=276) |
Reference Group
(n=270) |
|
|---|---|---|
| Demographics | ||
| Age at recruitment (yr) | 14.4 ± 2.1 | 14.4 ± 2.1 |
| Male (%) | 52.9 (146/276) | 52.6 (142/270) |
| Race / ethnic group (%) | ||
| Black | 66.3 (183/276) | 60.7 (164/270) |
| Hispanic | 22.5 (62/276) | 23.3 (63/270) |
| Other or mixed | 11.2 (31/276) | 15.9 (43/270) |
| Caretaker completed high school (%) | 78.4 (182/232) | 74.6 (176/236) |
| ≥ 1 household member employed (%) | 85.9 (237/276) | 78.9 (213/270) |
| Household income <$15,000 (%) | 48.2 (121/251) | 56.2 (141/251) |
| Asthma characteristics | ||
| Duration of asthma (yr) | 10.7 ± 4.3 | 10.5 ± 4.3 |
| Asthma Control Test™ | ||
| ACT™ score in the last month | 21.1 ± 3.6 | 21.3 ± 3.2 |
| Asthma-related symptoms (no. of days / last 2 wks) at randomization | ||
| Maximum symptom days | 2.1 ± 2.7 | 2.4 ± 3.0 |
| Days of wheeze, chest tightness or cough | 1.8 ± 2.7 | 2.2 ± 3.0 |
| Days of interference with activities | 1.2 ± 1.9 | 1.0 ± 1.7 |
| Nights of sleep disruption | 0.6 ± 1.5 | 0.6 ± 1.4 |
| School days missed | 0.2 ± 0.6 | 0.3 ± 1.0 |
| Lung function and exhaled nitric oxide level at randomization | ||
| FEV1 (% of predicted value) | 95.9 ± 15.5 | 95.7 ± 15.9 |
| FEV1/FVC | 79.8 ± 9.0 | 80.4 ± 8.3 |
| FENO (ppb) | 20.5 (11.5 - 45.3) | 19.7 (10.9 - 38.0) |
| Asthma-related health care use in the year prior to enrollment (%) | ||
| ≥ 1 Hospitalizations | 14.5 (40/276) | 14.8 (40/270) |
| ≥ 1 Unscheduled visits | 67.8 (187/276) | 69.6 (188/270) |
| ≥ 1 Prednisone courses | 52.2 (144/276) | 51.9 (140/270) |
| ≥ 1 Exacerbations | 79.3 (219/276) | 78.5 (212/270) |
Plus-minus values are means ± SD. Interquartile range is provided in parentheses with medians. Counts are provided in parentheses with percentages. Of the 546 study participants more than 491 (90%) responded for all characteristics except for the following: 468 for caretaker completed high school; 471 for duration of asthma; 381 for school days missed.
Treatment during the run-in resulted in an increased amount of controller medication compared to pre-study levels: 219 mcg change (95% CI: 199 – 238; P<0.0001) in inhaled corticosteroid (ICS) (fluticasone) dose and 6.04 mcg change (95% CI: 0.78 – 11.30; P=0.0243) in long-acting ß2-agonist (LABA) dose. This change led to a substantive improvement in asthma control with a reduction in maximum symptom days to 2.3 days per two weeks (mean within participant reduction: 3.4 days/2 weeks 95% CI: 3.0 – 3.8; P<0.0001; Figure 2a). Mean Asthma Control Test™ (ACT™) score also improved by 3.0 points (95% CI: 2.7 – 3.4; P<0.0001). At randomization, most participants (70.5%, 385/546) were at Control Level 1, however 12.4% (68/546) were poorly controlled at Levels 3 and 4. FEV1 % predicted improved (mean change: 3.3%, 95% CI: 2.4 – 4.2; P<0.0001; Figure 2b) as did FEV1/FVC ratios (2.2, 1.6 – 2.8; P<0.0001). FENO levels decreased to a median 20.1 ppb (IQR: 11.2 – 40.6; mean reduction: 12.9 ppb, 95% CI: 10.1-15.6; P<0.0001).
Figure 2. Asthma Outcomes and Medications by Study Visit*.
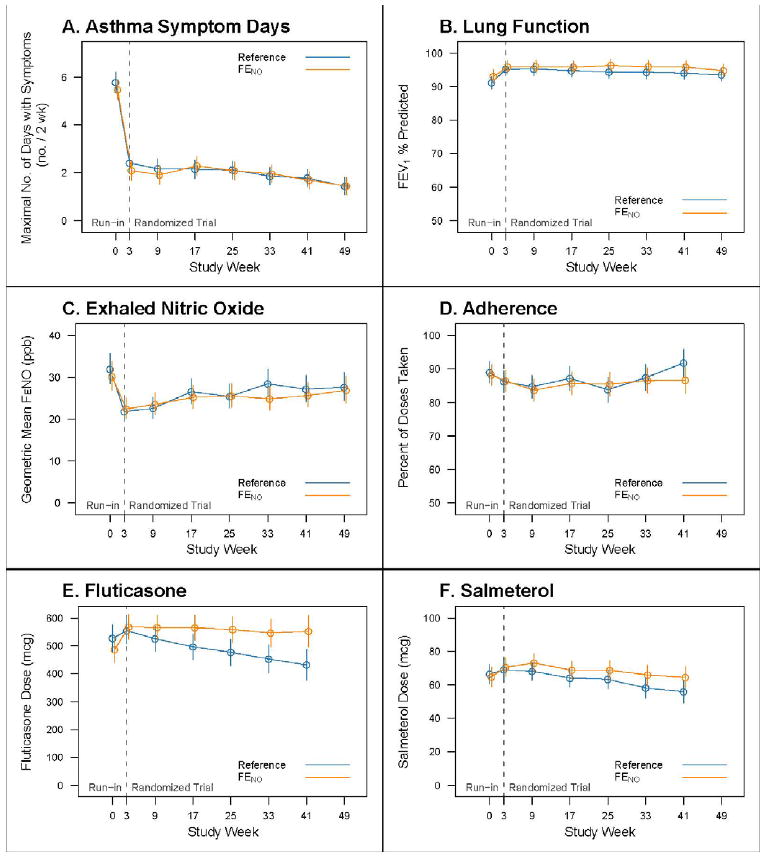
Mean values and 95% confidence intervals for asthma outcomes, pulmonary function and medications burden through the course of the study with maximum symptom days (Panel A), FEV1 % predicted (Panel B), exhaled nitric oxide levels (Panel C), adherence by percent of doses taken (Panel D), inhaled corticosteroid dose in mcg/day (Panel E), and long-acting ß2-agonist therapy in mcg/day (Panel F). The first three weeks constitute the run-in period.
* Data for treatment related variables (Panels D,E and F) is presented through the final treatment assessment at week 41, whereas follow-up data panels (Panels A,B and C) continue through week 49, the end of the study period
Response to Intervention
Intent-to-treat analysis demonstrated no differences between groups for maximum symptom days, other asthma symptoms, or ACT™ scores over the study period (Table 3). Following randomization, asthma symptoms remained low in both groups (Figure 2A). Control levels were not different between groups over the study with 57.3% (306/534) well controlled for at least 80% of visits. Only 22.8% (122/534) demonstrated poor control (Level 3 or 4) for at least 20% of visits (FENO Group: 22.1%, 59/267; Reference Group: 23.6%, 63/267; χ2=0.17, P=0.6801). Spirometry (Figure 2B), FENO (Figure 2C), and adherence (Figure 2D) were not significantly different between groups during the study; however, despite the level of control achieved, only 35.6% (190/534) of all participants had FENO levels <20 ppb on at least 80% of post-randomization visits. Medication adherence averaged 86.6% (SD: 27.7) during the study. FENO was significantly lower when adherence was ≥50% (Geometric mean of 23.9 vs. 30.8 ppb for adherence <50%; Ratio of means: 1.28, 95% CI: 1.24 – 1.34; P<0.0001).
Table 3.
Effect of Intervention on Asthma Symptoms and Health Care Use During 46 Weeks of Follow-up
| FENO n=276 |
Reference
n=270 |
Diff | P Value | |
|---|---|---|---|---|
| Asthma-related symptoms (no. of days / last 2 wks) | ||||
| Maximum symptom days | 1.93 ± 0.09 | 1.89 ± 0.09 | 0.04 (-0.22 – 0.29) | 0.7796 |
| Days of wheeze | 1.71 ± 0.09 | 1.69 ± 0.09 | 0.03 (-0.21 – 0.26) | 0.8291 |
| Days of activity interference | 0.87 ± 0.07 | 0.95 ± 0.07 | -0.08 (-0.26 – 0.10) | 0.3817 |
| Nights of sleep disruption | 0.52 ± 0.05 | 0.50 ± 0.05 | 0.03 (-0.11 – 0.16) | 0.7054 |
| School days missed | 0.19 ± 0.03 | 0.23 ± 0.03 | -0.04 (-0.12 – 0.05) | 0.3846 |
| Asthma Control Test™ | ||||
| ACT™ score in the last month | 21.89 ± 0.12 21.83 ± 0.12 | 0.06 (-0.28 – 0.40) | 0.7212 | |
| Lung function | ||||
| FEV1 (% of predicted value) | 96.3 ± 0.5 | 95.5 ± 0.5 | 0.8 (-0.51 – 2.07) | 0.2338 |
| FEV1/FVC | 80.3 ± 0.3 | 79.7 ± 0.3 | 0.6 (-0.13 – 1.34) | 0.1055 |
| Asthma-related health care use (%) | ||||
| ≥ 1 Hospitalizations * | 3.3 ± 1.1 | 4.1 ± 1.2 | -0.8 (-4.0 – 2.3) | 0.6136 |
| ≥ 1 Unscheduled visits | 21.3 ± 2.7 | 22.7 ± 2.7 | -1.4 (-9.3 – 6.7) | 0.7427 |
| ≥ 1 Prednisone courses | 32.1 ± 2.9 | 42.0 ± 3.1 | -10.3 (-18.5 – -2.2) | 0.0137 |
| ≥ 1 Exacerbations | 37.0 ± 2.7 | 43.6 ± 2.1 | -6.5 (-14.4 – 1.4) | 0.1068 |
Plus-minus values are means ± SE or difference (95% CI). Values are adjusted for study site and levels at randomization unless noted.
Unadjusted due to sparse data.
More participants in the Reference Group had at least one prednisone course (FENO 32.1%, 95% CI: 25.3 – 36.7 vs. Reference 42.0%, 95%CI: 35.1 – 47.4; Mean Difference: 10.3, 95% CI: 2.1 – 18.54; P=0.137; Table 3); however, there was no difference in the mean number of courses per year between groups (FENO 0.66, SE: 0.085 vs. Reference 0.84, SE: 0.085, Mean Difference:0.17, 95% CI: -0.08 - 0.41; P=0.14). Overall healthcare utilization rates were low (mean 0.04 hospitalizations per participant year, SD: 0.25). There were no significant differences between groups for hospitalizations, unscheduled visits, or exacerbations (Table 3). These exacerbation measures were remarkably lower in both groups when compared with the year prior to the study (Table 3 versus Table 2, respectively). Missed school days and caretaker disruption were not different between groups.
To explore whether the intervention could prove effective for some subgroups, a series of post-hoc, exploratory analyses were performed. Testing for heterogeneity of treatment effects across levels of 9 pre-randomization characteristics showed that the effect of the intervention varied with levels of BMI (BMI ≥ 30, Interaction P= 0.0117; BMI percentile > 97; Interaction P=0.0291), number of positive skin tests (≥ 10 positive tests, Interaction P=0.0170) and serum IgE levels (>460 kU/L, Interaction P=0.0072). The intervention was effective in these groups. For example, among participants with BMI ≥ 30, the treatment group had 0.60 fewer maximum symptom days per 2 weeks than the control group (95% CI: 0.08-1.13, P=0.0245). A similar treatment effect was found for those with a high number of positive skin tests (0.84, 0.11-1.58, P=0.0243) and among those with high serum IgE (0.51, 0.05-0.96, P=0.0296). Characteristics, such as age, gender and pre-randomization asthma severity, lung function and FENO were not associated with differences between study groups.
Medication Burden
The FENO Group received supplemental treatment due to elevated FENO at 405 (26%) of the 1,558 visits. The rate of reduction in ICS use was greater in the Reference Group than the FENO Group (P=0.0054 for difference in slope), resulting in a difference of 118.9 mcg of inhaled fluticasone per day by the final visit (95% CI: 48.5-189.3; P=0.0010; Figure 2E). By study conclusion, 52.1% (139/267)of the Reference Group had at least a one step reduction as compared to 39.3% (105/267) in the FENO Group (χ2=8.723, P=0.0031 ). Although the rate of reduction in LABA dose was not different between groups, 56.3% (SE: 3.1) of the Reference Group were on LABA at the end of the study as compared to 64.8% (SE: 3.0) in the FENO Group (Mean Difference: 8.5, 95% CI: 0.04 – 16.93; P=0.0490; Figure 2F).
Adverse Events
The four most common adverse events in ACE were upper respiratory tract infections (331 total events; 37.5% [205/546] of population with at least one event), headaches (242; 27.2% [149/546]), white blood cell abnormalities (235; 27.1% [148/546]) and upper respiratory signs and symptoms (191; 21.6% [118/546]). These events were distributed evenly between treatment groups.
Interpretation
Our study applied a guidelines-based asthma treatment approach1, 2 and sought to determine whether measurement of FENO added value to commonly used control measures. Whereas prior studies had typically replaced symptom and pulmonary function with a measure of FENO as the basis for determining asthma treatment, our study was designed to evaluate the utility of FENO in combination with standard symptom-based approaches to treatment. We believe this study design more realistically reflects the management approach in which the clinician would employ a measure of airway inflammation, as reflected by exhaled nitric oxide, as an adjunct to symptoms and pulmonary function rather than as a replacement.
This study provides several important observations. First, the application of current asthma treatment guidelines leads to good asthma control in the majority of inner-city adolescents and young adults. Second, the addition of FENO in guiding asthma therapy maintained a higher dose of ICS and LABA therapy. This FENO effect had a small impact on the need for prednisone bursts, but did not produce an overall improvement in asthma symptoms, lung function and health care utilization.
The theoretic basis of our algorithm was that an elevated FENO level would identify those patients with continuing airway inflammation who need increased controller medications. Therefore it was not unexpected that the FENO group received higher amounts of medication over the course of the study. This increase in treatment, however, did not result in any clinical important outcomes. Four other small clinical trials, two in adults24, 25 and two in children22, 23 have used FENO in asthma managaement. Two general approaches were used in these studies either using FENO as a guide for steroid reduction, or using FENO in conjunction with symptoms to guide therapy. Pulmonary function were used to influence therapy in some but not all of the studies.
Petsky et al36 published a meta-analysis involving these four studies that concluded there was no difference between the FENO and non- FENO guided groups in asthma exacerbations, symptoms, or spirometry. The decreased steroid use reported among adults whose treatment was guided by FENO was discounted as the finding was based on a post-hoc study analysis and not replicated in other studies. A major limitation of the studies included in the meta-analysis was their small size, single location, and varying outcomes. The ACE study addressed many of these concerns with its multi-site, large sample size, and standardized measures. The ACE study findings clearly demonstrate that the lack of effect of FENO in asthma management was not due to the aforementioned design problems with the previous studies.
It may appear unusual to employ symptoms both as a measure for determining treatment and as the primary outcome. However, for management purposes, the control levels which determined treatment included a range of symptom days, as well as pulmonary function measures. For example, control level 1 included the range 0 to 3 days of symptoms over the prior 2 weeks. Therefore, for asthma management purposes, a person with 0 symptom days would be treated the same as a person with 3 symptom days. For our outcome, symptom days were used as a continuous variable and the study was powered to detect a change of 0.70 days between groups. Although symptoms were employed to determine treatment, the analytic approach examined symptoms two months subsequent to the treatment adjustment to assess the effect of FENO. Therefore the use of symptoms as both the main outcome of the study and one of several criteria used to adjust therapy does not bias the study against finding a difference.
It is possible that the applied FENO cut-points were too high and that lower cut-points, especially those identifying good control (less than 20 ppb), should have been used. However, lower cut-points would have lead to even higher dose of ICS with no guarantee of clinical benefit. Further, while the 4 studies included in the review by Petsky et al36 used a single FENO cut-point ranging from 15 to 35 ppb, the ACE algorithm used 4 cut-points ranging from 20 to 40 ppb. The use of multiple cut-points over this extended range would increase the potential for FENO to influence therapy regardless of baseline level of severity. FENO resulted in therapeutic changes in approximately 26% of the study visits indicating FENO cut points were operational.
The FENO Group experienced a significant reduction in the risk of requiring at least one prednisone course for asthma exacerbations. Since the risk of asthma exacerbation is not tightly correlated with ongoing asthma symptoms and pulmonary function, titration of treatment according to FENO may have greater potential to reduce exacerbations than to improve day to day control. However, measures of asthma exacerbations, such as unscheduled visits and hospitalizations, did not differ between groups (Table 3).
The post-hoc analyses of intervention effects within various sample strata suggest that FENO-guided treatment may offer benefits in subsets of inner-city asthmatics. Among those subgroups of participants with greater obesity, higher blood eosinophil count, and greater atopy, the FENO Group showed a larger decrease in asthma symptom days. FENO measurements may be particularly helpful in obese patients because symptoms related to dyspnea may be difficult to interpret for assessing asthma control37. In addition, obesity, elevated blood eosinophils, and a high degree of atopy may be associated with airway inflammation that makes the measurement of FENO more germane to the assessment of asthma control. These post-hoc subgroup findings are intriguing but should be interpreted with caution.
In summary, in treating inner-city adolescents and young adults with asthma to achieve greater control, FENO measurements along with symptoms and spirometry did not reduce asthma impairment as compared to titrating therapy according to symptoms and spirometry alone. FENO monitoring slowed the rate the clinician could lower the inhaled steroid dose. The observed decrease in the percent of participants requiring ≥1 prednisone bursts is of questionable clinical significance as other indicators of exacerbation did not change. Therefore, in the context of our study, measurements of FENO add limited benefit to a carefully applied guidelines approach to asthma management.
Supplementary Material
Acknowledgments
This project has been funded by the National Institute of Allergy and Infectious Diseases, National Institutes of Health, under Contracts number NO1-AI-25496 and NO1-AI-25482, and from the National Center for Research Resources, National Institutes of Health, under grant M01 RR00533.
C.A. Sorkness receives research support from GSK, Schering and Pharmaxis and is a consultant for GSK. W.J. Morgan is a consultant to Genentech and was an invited speaker at the European Respiratory Society for Aerocrine Inc. No other conflicts of interested were reported.
Abbreviations
- eNO
exhaled nitric oxide
- NHLBI
National Heart Lung and Blood Institute
- NAEPP
National Asthma Education and Prevention Program
- NIAID
National Institute of Allergy and Infectious Diseases
- FENO
fraction of exhaled nitric oxide in parts per billion (ppb)
- ICS
inhaled corticosteroid
- ACE
Asthma Control Evaluation
- IQR
Interquartile range
- FEV1
forced expiratory volume in 1 second
- FEV1/FVC
ratio of FEV1 and forced vital capacity
- SD
Standard Deviation
- IgE
immunoglobulin E
- LABA
long-acting ß2-agonist
- ACT™
Asthma Control Test™
- BMI
Body Mass Index
Appendix 1
List of ACE investigators and institutions and acknowledgements
The Asthma Control Evaluation was a collaboration of the following institutions and investigators (principal investigators are indicated by asterisks):
Johns Hopkins University, Baltimore, MD—P. Eggleston*, E. Matsui, R. Wood; Boston University School of Medicine, Boston, MA—G. O’Connor*, S. Steinbach, N. Kozlowski, K. Burkart; Children’s Memorial Hospital, Chicago, IL—J. Pongracic*, R. Kumar, J. S. Kim, R. Story; Case Western Reserve University School of Medicine, Cleveland, OH—C. Kercsmar*, J. Chmiel, M. Hart, K. Ross; UT Southwestern Medical Center at Dallas, TX—R. Gruchalla*, V. Gan, W. Neaville; National Jewish Medical and Research Center, Denver, CO—S. Szefler*, A. Liu*, M. Gleason, R. Covar, J Spahn; Mount Sinai School of Medicine, New York, NY—M. Kattan*, H. Sampson, C. Lamm, A. Ting, E. Sembrano, L. Peters; Washington University School of Medicine, St Louis, MO—G. Bloomberg*, R. Strunk, L. Bacharier; The University of Arizona College of Medicine, Tucson, AZ—W. Morgan*, M. Brown, T. Guilbert; Children’s National Medical Center, Washington, DC—S. Teach*, K. Stone; Statistical and Clinical Coordinating Center—Rho, Inc, Chapel Hill, NC—H. Mitchell*, B. Shaw, A. Calatroni; Scientific Coordination and Administrative Center—University of Wisconsin, Madison, WI—W. Busse*, C. Sorkness, P. Heinritz; National Institute of Allergy and Infectious Diseases, Bethesda, MD—P. Gergen, E. Smartt.
The study gratefully acknowledges all study staff and consultants. For a list of these study personnel, please refer to www.icacweb.org. The study also gratefully acknowledges receiving donated product from GlaxoSmithKline (study drugs) and Lincoln Diagnostics, Inc. (skin testing materials). Finally, we express our sincere appreciation to the many study participants and their families.
Appendix 2
Author contributions and conflict of interest
All authors contributed to the design and conduct of the study. The writing group for this manuscript included S.J. Szefler, P. Gergen, M. Kattan, H. Mitchell, W.J. Morgan, G.T. O’Connor, J.A. Pongracic, C.A. Sorkness, and S.J. Teach. All other authors reviewed the manuscript and provided substantial feedback. J.J. Wildfire and H. Mitchell were responsible for conducting all analyses.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.National Asthma Education and Prevention Program. Expert Panel Report 3: Guidelines for the diagnosis and management of asthma. Bethesda, MD: National Institutes of Health, National Heart, Lung, and Blood Institute; 2007. Report No.: Publication no. 08-4051.1. [Google Scholar]
- 2.Expert Panel Report 3 (EPR-3): Guidelines for the diagnosis and management of asthma-summary report 2007. J Allergy Clin Immunol. 2007;120:S94–138. doi: 10.1016/j.jaci.2007.09.043. [DOI] [PubMed] [Google Scholar]
- 3.Silkoff PE, Carlson M, Bourke T, Katial R, Ogren E, Szefler SJ. The Aerocrine exhaled nitric oxide monitoring system Niox is cleared by the US food and drug administration for monitoring therapy in asthma. J Allergy Clin Immunol. 2004;114(5):1241–56. doi: 10.1016/j.jaci.2004.08.042. [DOI] [PubMed] [Google Scholar]
- 4.Massaro AF, Gaston B, Kita D, Fanta C, Stamler JS, Drazen JM. Expired nitric oxide levels during treatment of acute asthma. Am J Respir Crit Care Med. 1995;152(2):800–3. doi: 10.1164/ajrccm.152.2.7633745. [DOI] [PubMed] [Google Scholar]
- 5.Stirling RG, Kharitonov SA, Campbell D, et al. Increase in exhaled nitric oxide levels in patients with difficult asthma and correlation with symptoms and disease severity despite treatment with oral and inhaled corticosteroids. Asthma and Allergy Group. Thorax. 1998;53(12):1030–4. doi: 10.1136/thx.53.12.1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Covar RA, Szefler SJ, Martin RJ, et al. Relations between exhaled nitric oxide and measures of disease activity among children with mild-to-moderate asthma. J Pediatr. 2003;142(5):469–75. doi: 10.1067/mpd.2003.187. [DOI] [PubMed] [Google Scholar]
- 7.Crater SE, Peters EJ, Martin ML, Murphy AW, Platts-Mills TA. Expired nitric oxide and airway obstruction in asthma patients with an acute exacerbation. Am J Respir Crit Care Med. 1999;159(3):806–11. doi: 10.1164/ajrccm.159.3.9805103. [DOI] [PubMed] [Google Scholar]
- 8.Fitzpatrick AM, Gaston BM, Erzurum SC, Teague WG. Features of severe asthma in school-age children: atopy and increased exhaled nitric oxide. J Allergy Clin Immunol. 2006;118(6):1218–25. doi: 10.1016/j.jaci.2006.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jones SL, Kittelson J, Cowan JO, et al. The predictive value of exhaled nitric oxide measurements in assessing changes in asthma control. Am J Respir Crit Care Med. 2001;164(5):738–43. doi: 10.1164/ajrccm.164.5.2012125. [DOI] [PubMed] [Google Scholar]
- 10.Meyts I, Proesmans M, De Boeck K. Exhaled nitric oxide corresponds with office evaluation of asthma control. Pediatr Pulmonol. 2003;36(4):283–9. doi: 10.1002/ppul.10317. [DOI] [PubMed] [Google Scholar]
- 11.Sippel JM, Holden WE, Tilles SA, et al. Exhaled nitric oxide levels correlate with measures of disease control in asthma. J Allergy Clin Immunol. 2000;106(4):645–50. doi: 10.1067/mai.2000.109618. [DOI] [PubMed] [Google Scholar]
- 12.Strunk RC, Szefler SJ, Phillips BR, et al. Relationship of exhaled nitric oxide to clinical and inflammatory markers of persistent asthma in children. J Allergy Clin Immunol. 2003;112(5):883–92. doi: 10.1016/j.jaci.2003.08.014. [DOI] [PubMed] [Google Scholar]
- 13.Bratton DL, Lanz MJ, Miyazawa N, White CW, Silkoff PE. Exhaled nitric oxide before and after montelukast sodium therapy in school-age children with chronic asthma: a preliminary study. Ped Pulmonol. 1999;28(6):402–7. doi: 10.1002/(sici)1099-0496(199912)28:6<402::aid-ppul3>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 14.Zacharasiewicz A, Wilson N, Lex C, et al. Clinical use of noninvasive measurements of airway inflammation in steroid reduction in children. Am J Respir Crit Care Med. 2005;171(10):1077–82. doi: 10.1164/rccm.200409-1242OC. [DOI] [PubMed] [Google Scholar]
- 15.Jones SL, Herbison P, Cowan JO, et al. Exhaled NO and assessment of anti-inflammatory effects of inhaled steroid: dose-response relationship. Eur Respir J. 2002;20(3):601–8. doi: 10.1183/09031936.02.00285302. [DOI] [PubMed] [Google Scholar]
- 16.Kharitonov SA, Yates DH, Barnes PJ. Inhaled glucocorticoids decrease nitric oxide in exhaled air of asthmatic patients. Am J Respir Crit Care Med. 1996;153(1):454–7. doi: 10.1164/ajrccm.153.1.8542158. [DOI] [PubMed] [Google Scholar]
- 17.Kharitonov SA, Yates DH, Chung KF, Barnes PJ. Changes in the dose of inhaled steroid affect exhaled nitric oxide levels in asthmatic patients. Eur Respir J. 1996;9(2):196–201. doi: 10.1183/09031936.96.09020196. [DOI] [PubMed] [Google Scholar]
- 18.Silkoff PE, McClean P, Spino M, Erlich L, Slutsky AS, Zamel N. Dose-response relationship and reproducibility of the fall in exhaled nitric oxide after inhaled beclomethasone dipropionate therapy in asthma patients. Chest. 2001;119(5):1322–8. doi: 10.1378/chest.119.5.1322. [DOI] [PubMed] [Google Scholar]
- 19.Szefler SJ, Phillips BR, Martinez FD, et al. Characterization of within-subject responses to fluticasone and montelukast in childhood asthma. J Allergy Clin Immunol. 2005;115(2):233–42. doi: 10.1016/j.jaci.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 20.van Rensen EL, Straathof KC, Veselic-Charvat MA, Zwinderman AH, Bel EH, Sterk PJ. Effect of inhaled steroids on airway hyperresponsiveness, sputum eosinophils, and exhaled nitric oxide levels in patients with asthma. Thorax. 1999;54(5):403–8. doi: 10.1136/thx.54.5.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Payne DN, Adcock IM, Wilson NM, Oates T, Scallan M, Bush A. Relationship between exhaled nitric oxide and mucosal eosinophilic inflammation in children with difficult asthma, after treatment with oral prednisolone. Am J Respir Crit Care Med. 2001;164(8 Pt 1):1376–81. doi: 10.1164/ajrccm.164.8.2101145. [DOI] [PubMed] [Google Scholar]
- 22.Fritsch M, Uxa S, Horak F, Jr, et al. Exhaled nitric oxide in the management of childhood asthma: a prospective 6-months study. Pediatr Pulmonol. 2006;41(9):855–62. doi: 10.1002/ppul.20455. [DOI] [PubMed] [Google Scholar]
- 23.Pijnenburg MW, Bakker EM, Hop WC, De Jongste JC. Titrating steroids on exhaled nitric oxide in children with asthma: a randomized controlled trial. Am J Respir Crit Care Med. 2005;172(7):831–6. doi: 10.1164/rccm.200503-458OC. [DOI] [PubMed] [Google Scholar]
- 24.Shaw DE, Berry MA, Thomas M, et al. The use of exhaled nitric oxide to guide asthma management: a randomized controlled trial. Am J Respir Crit Care Med. 2007;176(3):231–7. doi: 10.1164/rccm.200610-1427OC. [DOI] [PubMed] [Google Scholar]
- 25.Smith AD, Cowan JO, Brassett KP, Herbison GP, Taylor DR. Use of exhaled nitric oxide measurements to guide treatment in chronic asthma. N Engl J Med. 2005;352(21):2163–73. doi: 10.1056/NEJMoa043596. [DOI] [PubMed] [Google Scholar]
- 26.Rosenstreich DL, Eggleston P, Kattan M, et al. The role of cockroach allergy and exposure to cockroach allergen in causing morbidity among inner-city children with asthma. N Engl J Med. 1997;336:1356–63. doi: 10.1056/NEJM199705083361904. [DOI] [PubMed] [Google Scholar]
- 27.Akinbami LJ, Schoendorf KC. Trends in childhood asthma: prevalence, health care utilization, and mortality. Pediatrics. 2002;110(2 Pt 1):315–22. doi: 10.1542/peds.110.2.315. [DOI] [PubMed] [Google Scholar]
- 28.Akinbami L. The state of childhood asthma, United States,1980-2005. Adv Data. 2006;381:1–24. [PubMed] [Google Scholar]
- 29.Cloutier MM, Wakefield DB, Hall CB, Bailit HL. Childhood asthma in an urban community: prevalence, care system, and treatment. Chest. 2002;122(5):1571–9. doi: 10.1378/chest.122.5.1571. [DOI] [PubMed] [Google Scholar]
- 30.Jones CA, Clement LT, Morphew T, et al. Achieving and maintaining asthma control in an urban pediatric disease management program: The Breathmobile Program. J Allergy Clin Immunol. 2007;119(6):1445–53. doi: 10.1016/j.jaci.2007.02.031. [DOI] [PubMed] [Google Scholar]
- 31.American Thoracic Society. ATS/ERS Recommendations for standardized procedures for the on-line and off-line measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide in adults and children-2005. Am J Respir Crit Care Med. 2005;171:912–30. doi: 10.1164/rccm.200406-710ST. [DOI] [PubMed] [Google Scholar]
- 32.Evans R, Gergen PJ, Mitchell H, et al. A randomized clinical trial to reduce asthma morbidity among inner-city children: results of the National Cooperative Inner-City Asthma Study. J Pediatr. 1999;135:332–8. doi: 10.1016/s0022-3476(99)70130-7. [DOI] [PubMed] [Google Scholar]
- 33.Morgan WJ, Crain EF, Gruchalla RS, et al. Results of a home-based environmental intervention among urban children with asthma. N Engl J Med. 2004;351:1068–80. doi: 10.1056/NEJMoa032097. [DOI] [PubMed] [Google Scholar]
- 34.Wang R, Lagakos SW, Ware JH, Hunter DJ, Drazen JM. Statistics in medicine—reporting of subgroup analyses in clinical trials. NEJM. 2007;357(21):2189–94. doi: 10.1056/NEJMsr077003. [DOI] [PubMed] [Google Scholar]
- 35.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. AM J Respir Crit Care Med. 1999;159:179–187. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 36.Petsky HL, Cates CJ, Li AM, Kynaston JA, Turner C, Chang AB. Tailored interventions based on exhaled nitric oxide versus clinical symptoms for asthma in children and adults (review) [June 12,2008];The Cochrane Collaboration. The Cochrane Library. 2008 (2) doi: 10.1002/14651858.CD006340.pub2. http://www.thecochranelibrary.com. [DOI] [PubMed]
- 37.Maniscalco M, de Laurentiis G, Zedda A, et al. Exhaled nitric oxide in severe obesity: effect of weight loss. Respir Physiol Neurobiol. 2007;156(3):370–3. doi: 10.1016/j.resp.2006.10.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.