Abstract
Pyrogultamylated arginine-phenylalanineamide peptide (QRFP) is strongly conserved across species and is a member of the family of RFamide-related peptides, with the motif Arg-Phe-NH2 at the C-terminal end. The precursor peptide for QRFP generates a 26-amino acid peptide (QRFP-26) and a 43-amino acid peptide (QRFP-43), both of which bind to the G protein-coupled receptor, GPR103. Recently, QRFP has been characterized in rats, mice and humans and has been reported to have orexigenic properties. In rodents, prepro-QRFP mRNA is expressed in localized regions of the mediobasal hypothalamus, a region implicated in feeding behavior. Increased intake of a high fat diet contributes to increased weight gain and obesity. Therefore, the current experiments investigated the effects of QRFP administration in rats and the effects of a high fat diet on prepro-QRFP mRNA and GPR103 receptor mRNA levels. Intracerebroventricular administration of QRFP-26 (3.0nM, 5.0nM) and QRFP-43 (1.0nM, 3.0nM) dose-dependently increased 1h, 2h, and 4h cumulative intake of high fat (55% fat), but not low fat (10% fat) diet. In Experiment 2, hypothalamic prepro-QRFP mRNA levels and GPR103 receptor mRNA levels were measured in rats fed a high fat or a low fat diet for 21 days. Prepro-QRFP mRNA was significantly increased in the ventromedial nucleus/arcuate nucleus of the hypothalamus of rats fed a high fat diet compared to those fed a low fat diet, while GPR103 mRNA levels were unchanged. These findings suggest that QRFP is a regulator of dietary fat intake and is influenced by the intake of a high fat diet.
Keywords: prepro-QRFP mRNA, GPR103 receptor, high fat diet, Real-time PCR
1.1 Introduction
RFamide-related peptides are a family of biologically active peptides that have the motif Arg-Phe-NH2 at their C-terminal end. A number of RFamide-related peptides have been characterized in invertebrates and vertebrates since the first member of this family was isolated from a venus clam. The members of this peptide family exert a large array of biological activities which include effects on analgesia, food intake, locomotor activity, blood pressure and hormone regulation [9,11]. Recently, a 26-amino acid peptide exhibiting the Arg-Phe-NH2 signature was isolated from frog brain, pyrogultamylated arginine-phenylalanineamide peptide (QRFP-26, also referred to as 26RFa) and the cDNA encoding QRFP-26 was characterized in rat, mouse, and human [4,5]. The structure of QRFP-26 appears to be strongly conserved across vertebrates, in particular the C-terminal octapeptide, suggesting that this region is crucial for the biological activity of the peptide. The QRFP-26 precursor has been shown to generate an N-terminal extended form of 43-amino acids (QRFP-43, also referred to as 43RFa). Both QRFP-26 and QRFP-43 are potent ligands for GPR103, a G protein-coupled receptor, and both peptides have been shown to increase food intake and locomotor activity in mice [5,8,22,33].
Distribution of prepro-QRFP mRNA in the rat central nervous system is localized in the hypothalamus, specifically the arcuate nucleus (ARC), retrochiasmatic area, lateral hypothalamus (LH) and ventromedial hypothalamus (VMH) [5,11]. These hypothalamic regions are important in the regulation of ingestive behaviors, particularly food intake, and are abundant in neurotransmitters, neuropeptides, and receptor systems that influence feeding (i.e. neuropeptide Y, agouti-related peptide, orexin, galanin, and melanin concentrating hormone) [2,3,7,10,13,15,16,23,28–32,34,36]. The receptor for QRFP-26 and QRFP-43, GPR103, is more widely expressed than the peptide and is found in the various regions of the central nervous system including the VMH, LH, cortex, ventral pallidum, amygdala, medial preoptic nucleus, suprachiasmatic nucleus, raphe nucleus, locus coeruleus, nucleus of the solitary tract [11]. Recently, Kampe and colleagues [14] have reported a second g- protein coupled receptor in the brain for QRFP-26 (QRFP-r2).
Previous research has shown that hypothalamic prepro-QRFP mRNA expression is up-regulated in 48h fasted mice and in obese ob/ob and db/db mice, which have a deficiency in leptin regulation [33]. Central administration of QRFP-26 in food restricted mice lead to a significant increase in food intake which peaked at 30 minutes and was diminished after 60 minutes [8,33]. Chronic administration of QRFP-43 increased body weight over 14 days while only increasing food intake for the first few days [22]. In mice fed a moderately fat diet (32.6% calories from fat), chronic administration of varying doses of QRFP-43 increased body weight, daily food intake, and percent body fat [22].
Few studies have been conducted to determine the effects of QRFP administration on food intake in rats and on the effects of dietary manipulation on prepro-QRFP mRNA expression in the rat brain. One such study, reported that intracerebroventricular administration of QRFP-26 moderately, though not significantly, increased standard chow intake in rats at 2h following administration [14]. Standard chow is a food source which is relatively low in dietary fat. Increased intake of foods high in dietary fat is thought to contribute to an increase in food intake and weight gain. Therefore, it is possible that the amount of fat in the diet is an important factor mediating the orexigenic actions of QRFP. The purpose of Experiment 1 was to investigate the effects of QRFP-26 and QRFP-43 on the consumption of a high fat (55% calories from fat) and a low fat (10% of calories from fat) diet in rats. It was hypothesized that intracerebroventricular administration of QRFP-26 and QRFP-43 would increase the intake of high fat food to a greater extent than low fat food. Experiment 2 was conducted to determine if the intake of a high fat or low fat diet would significantly alter prepro-QRFP mRNA levels and GPR103 receptor mRNA levels in the hypothalamus, specifically, the VMH/ARC and the LH. We hypothesized that high fat diet would significantly increase prepro-QRFP mRNA levels.
2.1 Methods
2.1.1 Animals
Adult male Long Evans rats (Harlan, Inc., Indianapolis, IN) weighing between 175–200g upon arrival were used for all experiments. Rats were individually housed in an AAALAC approved animal facility on a 12/12h light/dark cycle (lights on at 0700) with food and water available ad libitum. All procedures were approved by the Pennington Biomedical Research Center Institutional Animal Care and Use Committee and followed the Principles for Care and Use of Laboratory Animals. One week following arrival to the Pennington Biomedical Research Center, rats were adapted to one of two diets (Research Diets, New Brunswick, NJ), a pelleted high fat (55%)/low carbohydrate (21%) diet or a pelleted low fat (10%)/high carbohydrate (66%) diet based on Lin et al. [20,27].
2.1.2 Experiment 1: Indwelling cannula surgery
After a two week diet adaptation period, all rats underwent surgery to implant a unilateral indwelling cannula into the lateral ventricle. Rats were anesthetized with ketamine cocktail (ketamine, 80mg/ml; acepromazine, 1.6mg/ml; xylazine, 5mg/ml, i.p) and their heads were shaved, cleaned and injected with a local anesthetic (bupivicane/lidocaine, 1mg/kg, s.c). Once anesthetized, rats were placed in a stereotaxic apparatus (David Kopf, Tujunga, CA). Following a midline incision, bregma was measured and a single hole was drilled into the skull to allow the implantation of the cannula. Unilateral 22-gauge stainless steel cannula, 7mm in length (Plastics One, Roanoke, VA) were implanted into the lateral ventricle, using the coordinates AP −0.9, LM −1.5, DV −3.0 [24] and anchored with orthodontic resin (Dentsply Caulk, Milford, DE). Following surgery, Carprofen (1.0mg/kg, s.c.) was given for postoperative analgesia.
2.1.3 Drug Treatment
QRFP-26 and QRFP-43 (Phoenix Pharmaceuticals, Inc, Burlingame, CA) were dissolved in vehicle (30% propylene glycol in 0.9% sterile saline). On test day, rats were injected with 5.0µl of QRFP-26 (0.3nM, 0.5nM, 1.0nM, 3.0nM, 5.0nM), QRFP-43 (0.3nM, 0.5nM, 1.0nM, 3.0nM) or vehicle using a 20-gauge injector (Plastics One), which extended 1mm beyond the guide cannula. Injections were made manually at a rate of 5.0µl/min. The injectors remained in the cannula for an additional minute to allow for diffusion.
2.1.4 Measurement of Food Intake
Testing began one week following cannula implantation surgery. QRFP-26, QRFP-43 and vehicle were administered using a Latin-square design to account for variation due to the duration of exposure to the high fat diet and/or carry-over effects from QRFP administration. Each rat received 5 separate injections, 4–7 days apart. Due to the number of doses and multiple drugs, this experiment was conducted in three successive groups of Long Evans rats that were treated similarly. On test day, fully satiated rats received intracerebroventricular injections of QRFP-26, QRFP-43 or vehicle and were immediately returned to their home cage and given pre-weighed fresh high fat or low fat food. All injections were made between 0930 and 1030. Food intake was measured at 1h, 2h, 4h and 24h post-injection.
2.1.5 Histology
Rats were sacrificed by CO2 asphyxiation; brains were removed and placed in 4% paraformaldehyde/0.1M sodium phosphate solution for 24 hours at 4°C. Brains were subsequently placed in a 15% sucrose/0.1M sodium phosphate buffer solution at 4°C until slicing (50µm sections) using a cryostat (HRM550, Microm, Int.). Sections were thaw mounted on slides, stained with cresyl violet histological stain, and examined under a low power microscope. Cannula placement was plotted using the rat atlas of Paxinos and Watson [24]. Five animals were removed from analyses due to improper cannula placement.
2.1.6 Experiment 2: Real-time Polymerase Chain Reaction (PCR)
Rats were placed on the pelleted, high fat or a low fat diet (as described in Experiment 1) for 3 weeks prior to sacrifice. Body weight was measured weekly and body fat was determined by measurement of the retroperitoneal and epididymal fat pads at the time of sacrifice.
2.1.7 Real-time PCR
Animals were euthanized by decapitation. Brains were removed and immediately frozen on dry ice and stored at −80°C until further processing. For gene expression analysis, bilateral 1mm diameter brain punches were taken from the VMH/ARC, LH, and the paraventricular nucleus of the hypothalamus (PVN). Coordinates for punches were based on the Rat Brain Atlas by Paxinos and Watson [24]. Brains were sliced using a freezing microtome (Microm HM400, Waldorf, Germany). Coordinates for VMH/ARC punches began at AP-2.3 and the punches were 1.2–1.5mm thick. Coordinates for the PVN punches began at AP-1.80 and punches were 0.4–0.6mm thick. The PVN is a region of the hypothalamus important in the regulation of food intake. However, previous reports suggest that there is no significant expression of prepro-QRFP mRNA in this brain region [5,11]. Therefore, we used the PVN as a control region. RNA was isolated from bilateral punches of the specific hypothalamic regions using Tri-Reagent (Molecular Research Ctr, Cincinnati, OH USA) and RNeasy Minikit procedures (Qiagen, Valencia, CA USA) and based on previous experiments by Primeaux et al. [25,26]. Briefly, thawed tissue was homogenized in Tri-Reagent using a motorized tissue homogenizer, chloroform was added to the lysate, and the mixture was centrifuged (12,000×g) in phase lock tubes to separate RNA. Ethanol (70%) was added to the upper aqueous phase, which was filtered by centrifugation (8000×g). Following multiple washes, samples were subjected to an elution step using RNAase-free water. Reverse transcription (RT) was conducted using the High-Capacity cDNA Reverse Transcriptase Kit (Applied Biosystems, Foster City, CA, USA). For RT, 1.5µg of RNA from each sample was added to random primers (10x), dNTP (25x), MultiScribe Reverse Transcriptase (50U/µl) and RT buffer (10x) and incubated in a thermal cycler (PTC-100, MJ Research, Inc, Watertown, MA, USA) for 10 min at 27°C, then for 120 min at 37°C. Taqman Gene Expression Assays (Applied Biosystems) were used to assess levels of prepro-QRFP, GPR103 and the housekeeping gene, cyclophilin. For Real-time PCR, Taqman Universal PCR Master Mix (Applied Biosystems), gene expression assay, and RT product (10ng) were added to a 384 well plate. The cycling parameters consisted of an initial 2 min incubation at 50 °C, followed by 10 min at 95 °C, then 15 sec at 95 °C, and a 1 min annealing/extension step at 60 °C (40 cycles). The quantity of prepro-QRFP mRNA and GPR103 mRNA levels were based on a standard curve using pooled cDNA from the VMH/ARC of all experimental samples and normalized to cyclophilin levels (ABI Prism 7900 Sequence Detection System, Applied Biosystems).
2.1.8 Statistical Analysis
In Experiment 1, cumulative food intake in kilocalories (kcal) was assessed for each dietary condition and at each time point (1h, 2h, 4h, 24h) following QRFP-26 or vehicle injection or QRFP-43 or vehicle injection using a two-way ANOVA. Bonferroni post-hoc tests were used to compare vehicle to QRFP-26 and QRFP-43, when a significant main effect or interaction was detected. Bonferroni post-hoc analyses were also used to assess differences between high fat and low fat food intake for each dose of QRFP-26 or QRFP-43. In Experiment 2, diet-induced differences in body weight and percent body fat ((fat pad weight/body weight)*100) were assessed using a two-tailed between subjects t-test. For Real-Time PCR, a two-tailed between subjects t-test was used to compare prepro-QRFP mRNA levels in each hypothalamic region of high fat and low fat fed rats. A two-tailed between subjects t-test was also used to compare GPR103 receptor mRNA in each hypothalamic region of rats fed either a high fat or a low fat diet. A significance level of p<.05 was used for all tests.
3.1 Results
3.1.1 Experiment 1 QRFP-26 administration
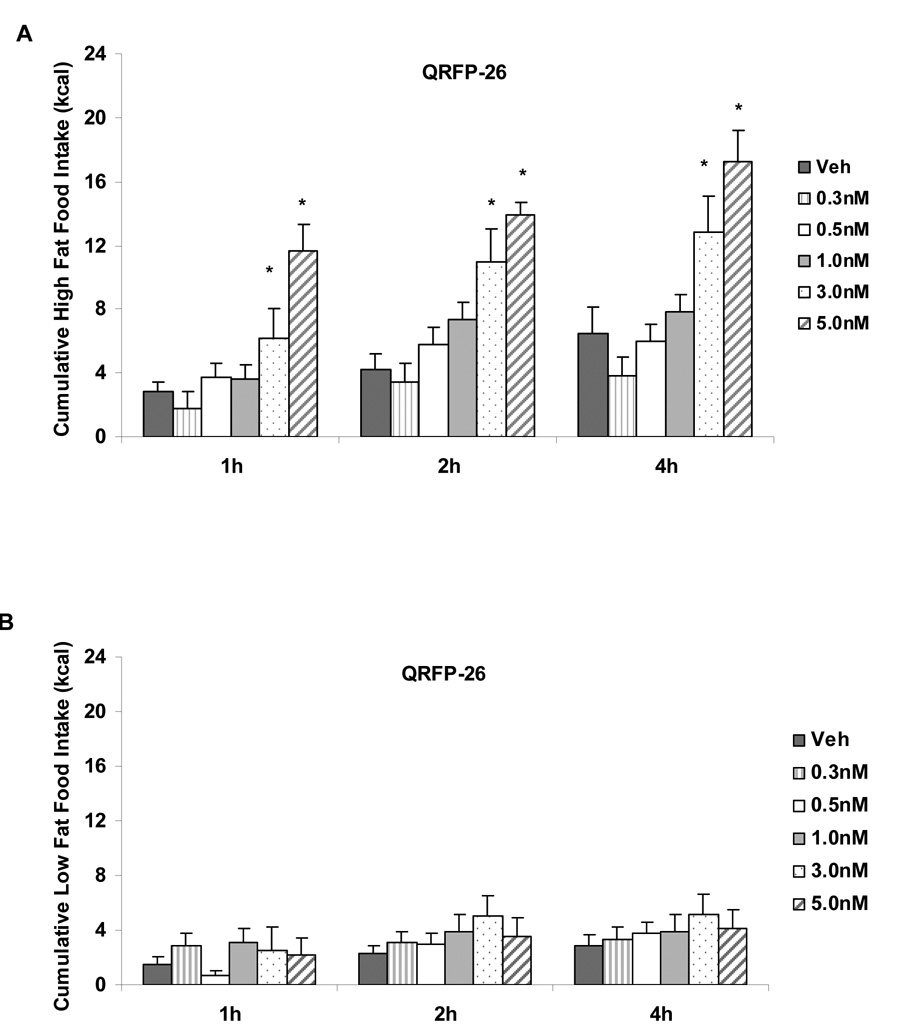
A significant diet by dose interaction was detected for the first hour of food intake following QRFP-26 administration (F(5,98)=4.79, p<.001). A significant interaction was also found in cumulative food intake at 2h and 4h following QRFP-26 administration (F(5,98)=3.33, p<.01 and F(5,98) = 3.61, p<.01, respectively). A significant main effect of diet was detected at 24h following the administration of QRFP-26 (F(1, 99)=10.40, p<.01). Post-hoc analyses revealed a significant increase in high fat food intake following the two highest doses of QRFP-26 (p<.05; 3.0nM and 5.0nM compared to vehicle administration; See Figure 1A). QRFP-26 administration into the lateral ventricles did not significantly alter cumulative low fat food intake at any time point (p>.05; See Figure 1B). Post-hoc analyses revealed significant increases in cumulative high fat food intake (kcal) compared to low fat food intake following 3.0nM QRFP-26 at 2h and 4h (p<.05) and 5.0nM QRFP-26 at 1h, 2h, 4h, and 24h, suggesting a increased sensitivity to the feeding effects of QRFP-26 in rats fed a high fat diet.
Figure 1.
A. Dose response curve of QRFP-26 administered into the lateral ventricles of fully satiated rats fed a high fat diet. QRFP-26 (3.0nM, 5.0nM) dose-dependently increased high fat food intake at 1h, 2h and 4h following administration. B. Dose response curve of QRFP-26 administered into the lateral ventricles of rats fed a low fat diet. QRFP-26 did not alter low fat food intake. Twenty-four hour intake was not altered by any QRFP-26 dose. Data shown as mean ± SEM, *p<.05.
3.1.2 QRFP-43 administration
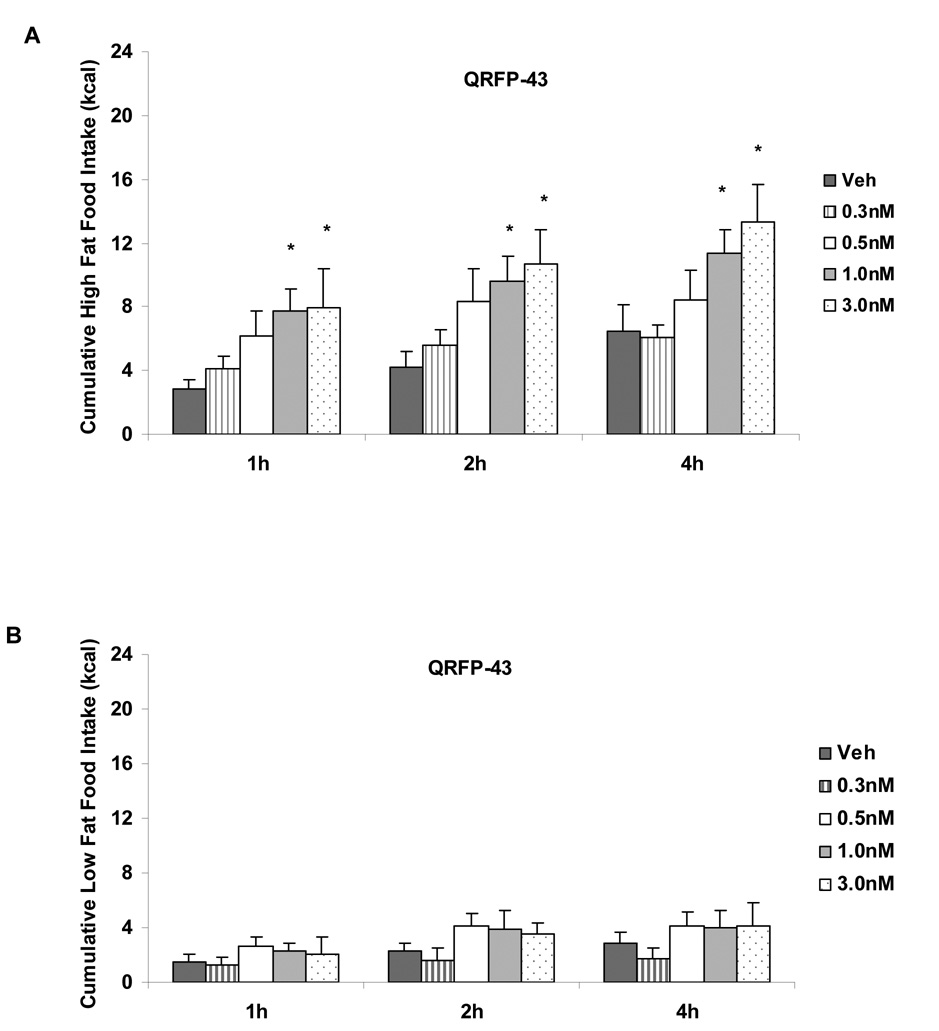
Significant main effects of diet (F(1,74)=26.55, p<.0001) and dose (F(4,74)=3.38, p<.02) were found at 1h following QRFP-43 administration (See Figure 2A). Significant main effects of diet and dose on cumulative food intake were also detected at 2h (F(1,74)=25.64, p<.0001 and F(4,74)=4.07, p<.01, respectively) and at 4h (F(1,73)=27.63, p<.0001 and F(4,73)=2.75, p<.05, respectively). A main effect of diet on cumulative food intake was detected at 24h following QRFP-43 administration (F(1,81)=17.16, p<.0001). Post-hoc analysis revealed a significant increase in high fat intake with the two highest doses of QRFP-43 (1.0nM and 3.0nM compared to vehicle; p< .05; See Figure 2).Administration of QRFP–43 did not significantly increase cumulative low fat food intake at any dose tested (See Figure 2B). Post-hoc analyses reveal a significant increase in cumulative high fat food intake compared to low fat intake following 0.3nM QRFP-43 administration at 1h, 2h, and 4h, following 0.5nM QRFP-43 administration at 24h, following 1.0nM QRFP-43 administration at 1h, 2h, 4h, and 24h, and following 3.0nM QRFP-43 at 1h, 2h and 4h (p<.05).
Figure 2.
A. Dose response curve of QRFP-43 administered into the lateral ventricles of fully satiated rats fed a high fat diet. QRFP-43 (1.0nM, 3.0nM) dose-dependently increased high fat food intake at 1h, 2h and 4h following administration. B. Dose response curve of QRFP-43 administered into the lateral ventricles of rats fed a low fat diet. QRFP-43 did not alter low fat food intake. Twenty-four hour intake was not altered by any dose of QRFP-43. Data shown as mean ± SEM, *p<.05.
3.1.3 Experiment 2
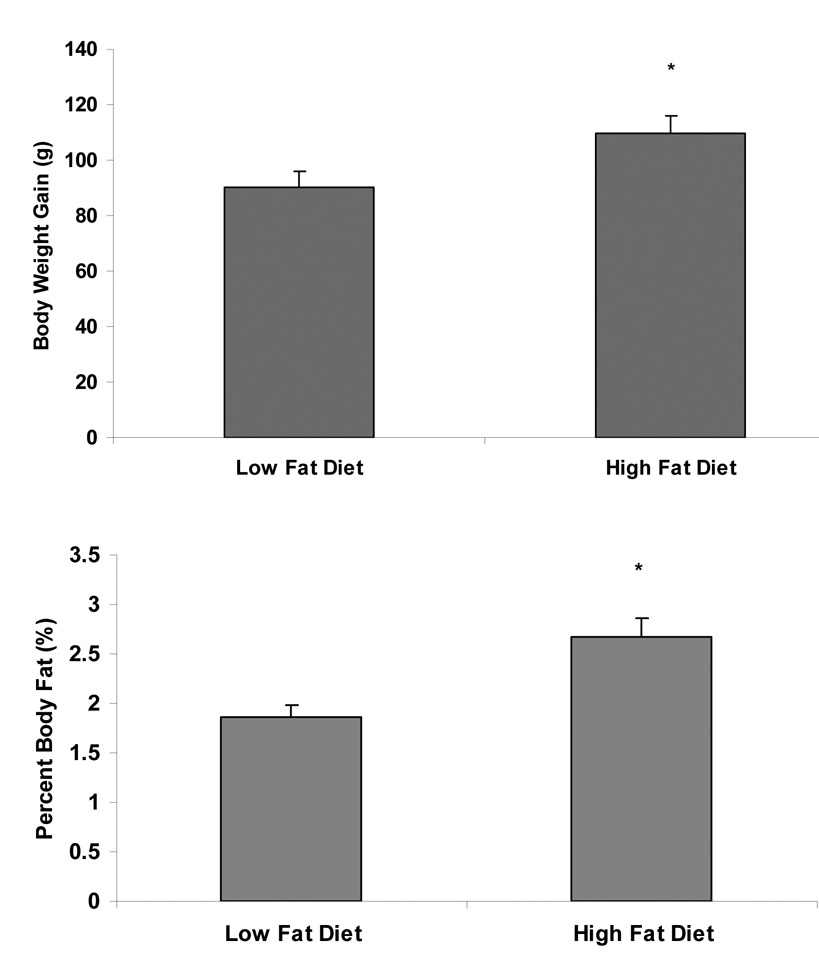
Rats were fed either a low fat or a high fat diet for 3 weeks. Animals fed a high fat diet gained significantly more body weight than those fed a low fat diet (t(13)=2.40, p<.05; See Figure 3A). Rats fed the high fat diet also gained more body fat ((retroperitoneal + epididymal fat pad weight/body weight)*100) than those fed a low fat diet (t(13)= 3.31, p<.01; See Figure 3B).
Figure 3.
A. Rats were fed either a low fat (10% calories from fat) or a high fat (55% calories from fat) diet for 3 weeks. Consumption of a high fat diet led to a significantly greater gain in body weight than consumption of a low fat diet. B. Percent body fat was measured by weighing retroperitoneal and epididymal fat pads at the time of sacrifice. High fat food intake led to an increase in body fat compared to low fat food intake. Data shown as mean ± SEM, *p<.05.
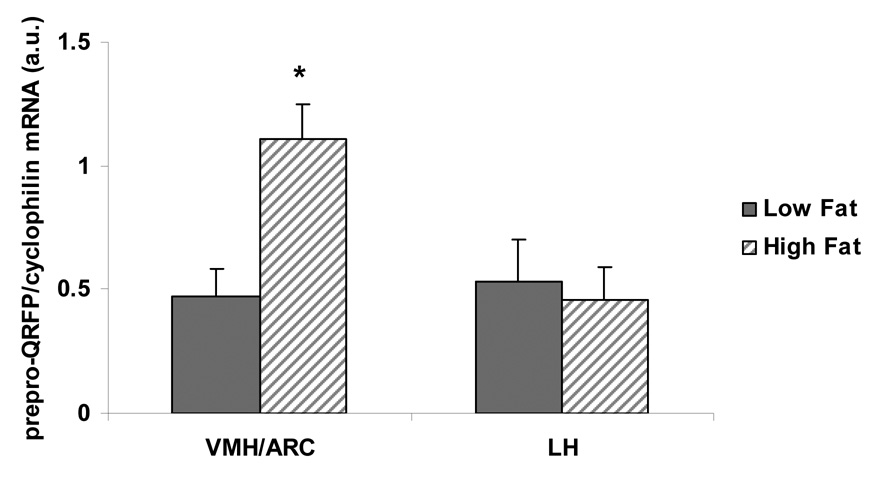
Prepro-QRFP mRNA levels in the VMH/ARC were significantly elevated following 3 weeks on a high fat diet (t(10)=3.30, p<.01; See Figure 4). In contrast, consuming a high fat diet did not alter prepro-QRFP mRNA levels in the LH, nor alter prepro-QRFP mRNA levels in the PVN. GPR103 receptor mRNA was not altered by high fat diet in the VMH/ARC, LH or the PVN (p>.05).
Figure 4.
Following 3 weeks of high fat or low fat food intake, prepro-QRFP mRNA levels were measured in the VMH/ARC and LH. High fat food intake increased prepro-QRFP mRNA levels in the VMH/ARC, but not the LH. Data shown as mean ± SEM, *p<.05.
4.1 Discussion
QRFP-26, a 26-amino acid peptide exhibiting the Arg-Phe-NH2 signature has recently been characterized in rat, mouse, and human [4,5] and has been reported to have orexigenic properties. Distribution of prepro-QRFP mRNA in the rat central nervous system is localized in specific regions of the hypothalamus [5,11] which are important in the regulation of food intake [21,30,36]. Few studies have been conducted to determine the effects of QRFP administration on food intake in rats and on the effects of dietary manipulations on prepro-QRRP or GPR103 receptor gene expression in the rat brain. Therefore, the current experiments were conducted to investigate the role of QRFP-26 and QRFP-43 on the intake of a low fat and a high fat diet and to determine if the consumption of a high fat diet alters the gene expression of prepro-QRFP and/or GPR103 receptors in the hypothalamus.
Experiment 1 demonstrates that QRFP is a mediator of high fat food intake. QRFP-26 and QRFP-43 dose-dependently increases cumulative high fat food intake in rats at 1h, 2h, and 4h, following intracerebroventricular administration (See Figure 1 and Figure 2) . No differences in high fat food intake were detected 24 hours following administration of either QRFP-26 or QRFP-43, compared to vehicle treated controls. Similar to the study by Kampe and colleagues [14], QRFP-26 administration did not significantly increase the intake of a diet which had a low fat content (standard chow). In the current study, neither QRFP-26 nor QRFP-43 altered low fat food intake at any time point or at any dose (See Figure 1B and Figure 2B).
Experiment 2 demonstrates that QRFP expression in the VMH/ARC is influenced by a diet high in fat. This experiment investigated the effects of a high fat and a low fat diet on prepro-QRFP mRNA and GPR103 receptor mRNA levels in the VMH/ARC, LH and PVN of rats. Rats were given access to the high fat or the low fat diet for 3 weeks prior to assessing alterations in gene expression. Prepro-QRFP mRNA levels were significantly increased in the VMH/ARC, but not LH of rats fed a high fat diet, compared to rats fed a low fat diet. As previously reported [5,11], there was insignificant expression of prepro-QRFP mRNA in the PVN. GPR103 receptor mRNA level was not altered by dietary manipulations in these hypothalamic regions. The increase in prepro-QRFP mRNA in the VMH/ARC, without a compensatory decrease in GPR103 receptor mRNA may present a possible mechanism by which QRFP increases high fat food intake. It should be noted that recent evidence suggests that there may be multiple receptors for QRFP-26 (GPR103 and QRFP-r2) [14]. Therefore, it is possible that high fat food intake alters the mRNA levels of QRFP-r2 in the hypothalamus and that QRFP-r2 is involved in the effects of QRFP-26 and QRFP-43 on high fat food intake. Future studies should address these possibilities.
The mechanism by which QRFP increases food intake, specifically high fat food intake is not currently known. As mentioned, the precursor peptide is expressed in specific regions of the hypothalamus (VMH, ARC, LH), which are important for the regulation of food intake and which contain various neurotransmitter, neuropeptide, and receptor systems that have been implicated in feeding behaviors [21,30,36]. Several of these systems are thought to be important for the intake of a high fat diet (i.e. agouti-related peptide, galanin, mu opioid receptors) [1,3,6,12,17–19,23,35]. In congruence with the current findings regarding QRRP mRNA levels, mu opioid receptor mRNA and galanin mRNA levels are increased in the hypothalamus of rats fed a high fat diet [1,10,18]. Though not currently known, it is possible that in the ARC, QRFP expressing neurons may co-express agouti-related peptide or neuropeptide Y or their respective receptors. It is also possible that in the LH, QRFP expressing neurons colocalize with melanin concentrating hormone or orexin producing neurons. The release and action of QRFP may be mediated by one or more of the neurotransmitter systems or QRFP may be the mediator. Another possibility is that QRFP expressing neurons project to regions of the brain outside of the hypothalamus, which may be relevant for feeding behaviors (i.e. amygdala, nucleus accumbens).
There have been a few studies which have investigated the effects of QRFP in relation to other orexigenic peptides. One study reported that a QRFP-43-induced increase in food intake was not abolished in orexin knockout mice, suggesting that orexin and QRFP have independent effects on food intake [33]. A separate study investigated the effects of the neuropeptide Y Y1 receptor antagonist, BIBP3226, on QRFP-43-induced feeding in mice [33]. In this study, QRFP-43 (10nM) significantly increased the intake of a standard low fat chow diet in mice. These results differ from the results reported in the current experiments in that the intake of low fat diet was not increased by QRFP-43 administration. Takayasu et al. [33] reported that the effects of QRFP-43 on chow intake were attenuated by BIBP3226 and suggested a relationship between the two peptide systems. Neuropeptide Y administration significantly increases the intake of the animal’s preferred diet [31]. Therefore, by antagonizing neuropeptide Y with BIBP3226, we would expect that given a single diet choice, BIBP3226 would significantly attenuate the effects of QRFP on high fat food intake. Future experiments should investigate interactions between QRFP and neuropeptide Y and attempt to elucidate the projection patterns of QRFP in the brain.
The results of the current experiments provide evidence that QRFP (both QRFP-26 and QRFP-43) may be an important regulator of high fat food intake. The high fat-induced increase in prepro-QRFP mRNA in the VMH/ARC, but not the LH provides insight into the neuronal populations that may either be mediated by QRFP or which may mediate QRFP’s effects on dietary fat. The neurotransmitter, neuropeptide and receptor systems in the VMH/ARC are potential targets for the actions of QRFP and have yet to be determined.
Acknowledgements
This research was supported by NIDDK 32089 to G. A. Bray. The authors would like to thank the Pennington Biomedical Research Center’s Clinical Nutrition Research Unit Animal Models and Phenotyping Core and Molecular Mechanisms Core for their assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Barnes MJ, Holmes G, Primeaux SD, York DA, Bray GA. Increased expression of mu opioid receptors in animals susceptible to diet-induced obesity. Peptides. 2006;27(12):3292–3298. doi: 10.1016/j.peptides.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 2.Barsh GS, Schwartz MW. Genetic approaches to studying energy balance: perception and integration. Nat Rev Genet. 2002;3(8):589–600. doi: 10.1038/nrg862. [DOI] [PubMed] [Google Scholar]
- 3.Bray GA. Peptides affect the intake of specific nutrients and the sympathetic nervous system. Am J Clin Nutr. 1992;55(1 Suppl):265S–271S. doi: 10.1093/ajcn/55.1.265s. [DOI] [PubMed] [Google Scholar]
- 4.Chartrel N, Bruzzone F, Dujardin C, Leprince J, Tollemer H, Anouar Y, Vallarino M, Costentin J, Vaudry H. Identification of 26RFa from frog brain: a novel hypothalamic neuropeptide with orexigenic activity in mammals. Ann N Y Acad Sci. 2005;1040:80–83. doi: 10.1196/annals.1327.009. [DOI] [PubMed] [Google Scholar]
- 5.Chartrel N, Dujardin C, Anouar Y, Leprince J, Decker A, Clerens S, Do-Rego JC, Vandesande F, Llorens-Cortes C, Costentin J, Beauvillain JC, Vaudry H. Identification of 26RFa, a hypothalamic neuropeptide of the RFamide peptide family with orexigenic activity. Proc Natl Acad Sci U S A. 2003;100(25):15247–15252. doi: 10.1073/pnas.2434676100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheng X, Broberger C, Tong Y, Yongtau X, Ju G, Zhang X, Hokfelt T. Regulation of expression of neuropeptide Y Y1 and Y2 receptors in the arcuate nucleus of fasted rats. Brain Res. 1998;792(1):89–96. doi: 10.1016/s0006-8993(97)01468-6. [DOI] [PubMed] [Google Scholar]
- 7.Clark JT, Kalra PS, Crowley WR, Kalra SP. Neuropeptide Y and human pancreatic polypeptide stimulate feeding behavior in rats. Endocrinol. 1984;115:427–429. doi: 10.1210/endo-115-1-427. [DOI] [PubMed] [Google Scholar]
- 8.do Rego JC, Leprince J, Chartrel N, Vaudry H, Costentin J. Behavioral effects of 26RFamide and related peptides. Peptides. 2006;27(11):2715–2721. doi: 10.1016/j.peptides.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 9.Dockray GJ. The expanding family of -RFamide peptides and their effects on feeding behaviour. Exp Physiol. 2004;89(3):229–235. doi: 10.1113/expphysiol.2004.027169. [DOI] [PubMed] [Google Scholar]
- 10.Dourmashkin JT, Chang GQ, Gayles EC, Hill JO, Fried SK, Julien C, Leibowitz SF. Different forms of obesity as a function of diet composition. Int.J Obes. 2005;29(11):1368–1378. doi: 10.1038/sj.ijo.0803017. [DOI] [PubMed] [Google Scholar]
- 11.Fukusumi S, Fujii R, Hinuma S. Recent advances in mammalian RFamide peptides: the discovery and functional analyses of PrRP, RFRPs and QRFP. Peptides. 2006;27(5):1073–1086. doi: 10.1016/j.peptides.2005.06.031. [DOI] [PubMed] [Google Scholar]
- 12.Hagan MM, Rushing PA, Benoit SC, Woods SC, Seeley RJ. Opioid receptor involvement in the effect of AgRP- (83–132) on food intake and food selection. Am J Physiol Regul Integr Comp Physiol. 2001;280(3):R814–R821. doi: 10.1152/ajpregu.2001.280.3.R814. [DOI] [PubMed] [Google Scholar]
- 13.Heinrichs SC, Menzaghi F, Pich EM, Hauger RL, Koob GF. Corticotropin-releasing factor in the paraventricular nucleus modulates feeding induced by neuropeptide Y. Brain Res. 1993;611:18–24. doi: 10.1016/0006-8993(93)91771-j. [DOI] [PubMed] [Google Scholar]
- 14.Kampe J, Wiedmer P, Pfluger PT, Castaneda TR, Burget L, Mondala H, Kerr J, Liaw C, Oldfield BJ, Tschop MH, Bagnol D. Effect of central administration of QRFP(26) peptide on energy balance and characterization of a second QRFP receptor in rat. Brain Res. 2006;1119(1):133–149. doi: 10.1016/j.brainres.2006.08.055. [DOI] [PubMed] [Google Scholar]
- 15.Konturek PC, Konturek JW, Czesnikiewicz-Guzik M, Brzozowski T, Sito E, Konturek SJ. Neuro-hormonal control of food intake: basic mechanisms and clinical implications. J Physiol Pharmacol. 2005;56 Suppl 6:5–25. [PubMed] [Google Scholar]
- 16.Kyrkouli SE, Stanley BG, Seirafi RD, Leibowitz SF. Stimulation of feeding by galanin: anatomical localization and behavioral specificity of this peptide's effects in the brain. Peptides. 1990;11(5):995–1001. doi: 10.1016/0196-9781(90)90023-x. [DOI] [PubMed] [Google Scholar]
- 17.Kyrkouli SE, Strubbe JH, Scheurink AJ. Galanin in the PVN increases nutrient intake and changes peripheral hormone levels in the rat. Physiol Behav. 2006;89(1):103–109. doi: 10.1016/j.physbeh.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 18.Leibowitz SF, Akabayashi A, Wang J. Obesity on a high-fat diet: role of hypothalamic galanin in neurons of the anterior paraventricular nucleus projecting to the median eminence. J Neurosci. 1998;18(7):2709–2719. doi: 10.1523/JNEUROSCI.18-07-02709.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levine AS, Kotz CM, Gosnell BA. Sugars and fats: the neurobiology of preference. J Nutr. 2003;133(3):831S–834S. doi: 10.1093/jn/133.3.831S. [DOI] [PubMed] [Google Scholar]
- 20.Lin L, Chen J, York DA. Chronic ICV enterostatin preferentially reduced fat intake and lowered body weight. Peptides. 1997;18(5):657–661. doi: 10.1016/s0196-9781(97)00128-9. [DOI] [PubMed] [Google Scholar]
- 21.Meister B. Neurotransmitters in key neurons of the hypothalamus that regulate feeding behavior and body weight. Physiol Behav. 2007;92(1–2):263–271. doi: 10.1016/j.physbeh.2007.05.021. [DOI] [PubMed] [Google Scholar]
- 22.Moriya R, Sano H, Umeda T, Ito M, Takahashi Y, Matsuda M, Ishihara A, Kanatani A, Iwaasa H. RFamide peptide QRFP43 causes obesity with hyperphagia and reduced thermogenesis in mice. Endocrinol. 2006;147(6):2916–2922. doi: 10.1210/en.2005-1580. [DOI] [PubMed] [Google Scholar]
- 23.Nagase H, Nakajima A, Sekihara H, York DA, Bray GA. Regulation of feeding behavior, gastric emptying, and sympathetic nerve activity to interscapular brown adipose tissue by galanin and enterostatin: the involvement of vagal-central nervous system interactions. J Gastroenterol. 2002;37 Suppl 14:118–127. doi: 10.1007/BF03326430. [DOI] [PubMed] [Google Scholar]
- 24.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. San Diego: Academic Press; 1997. [Google Scholar]
- 25.Primeaux SD, Barnes MJ, Bray GA. Olfactory bulbectomy increases food intake and hypothalamic neuropeptide Y in obesity-prone but not obesity-resistant rats. Behav Brain Res. 2007;180(2):190–196. doi: 10.1016/j.bbr.2007.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Primeaux SD, Wilson SP, Bray GA, York DA, Wilson MA. Overexpression of neuropeptide Y in the central nucleus of the amygdala decreases ethanol self-administration in "anxious" rats. Alcohol Clin Exp Res. 2006;30(5):791–801. doi: 10.1111/j.1530-0277.2006.00092.x. [DOI] [PubMed] [Google Scholar]
- 27.Primeaux SD, York DA, Bray GA. Neuropeptide Y administration into the amygdala alters high fat food intake. Peptides. 2006;27(7):1644–1651. doi: 10.1016/j.peptides.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 28.Qu D, Ludwig DS, Gammeltoft S, Piper M, Pelleymounter MA, Cullen MJ, Mathes WF, Przypek R, Kanarek R, Maratos-Flier E. A role for melanin-concentrating hormone in the central regulation of feeding behaviour. Nature. 1996;380(6571):243–247. doi: 10.1038/380243a0. [DOI] [PubMed] [Google Scholar]
- 29.Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richardson JA, Kozlowski GP, Wilson S, Arch JR, Buckingham RE, Haynes AC, Carr SA, Annan RS, McNulty DE, Liu WS, Terrett JA, Elshourbagy NA, Bergsma DJ, Yanagisawa M. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92(4):573–585. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- 30.Schwartz MW, Woods SC, Porte JD, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature. 2000;404:661–671. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- 31.Smith BK, Berthoud HR, York DA, Bray GA. Differential effects of baseline macronutrient preferences on macronutrient selection after galanin, NPY, and an overnight fast. Peptides. 1997;18(2):207–211. doi: 10.1016/s0196-9781(96)00318-x. [DOI] [PubMed] [Google Scholar]
- 32.Smith BK, York DA, Bray GA. Effects of dietary preference and galanin administration in the paraventricular or amygdaloid nucleus on diet self-selection. Brain Res Bull. 1996;39(3):149–154. doi: 10.1016/0361-9230(95)02086-1. [DOI] [PubMed] [Google Scholar]
- 33.Takayasu S, Sakurai T, Iwasaki S, Teranishi H, Yamanaka A, Williams SC, Iguchi H, Kawasawa YI, Ikeda Y, Sakakibara I, Ohno K, Ioka RX, Murakami S, Dohmae N, Xie J, Suda T, Motoike T, Ohuchi T, Yanagisawa M, Sakai JA. Neuropeptide ligand of the G protein-coupled receptor GPR103 regulates feeding, behavioral arousal, and blood pressure in mice. Proc Natl Acad Sci U S A. 2006;103(19):7438–7443. doi: 10.1073/pnas.0602371103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tiesjema B, la Fleur SE, Luijendijk MC, Brans MA, Lin EJ, During MJ, Adan RA. Viral mediated neuropeptide Y expression in the rat paraventricular nucleus results in obesity. Obesity. 2007;15(10):2424–2435. doi: 10.1038/oby.2007.288. [DOI] [PubMed] [Google Scholar]
- 35.Will MJ, Pratt WE, Kelley AE. Pharmacological characterization of high-fat feeding induced by opioid stimulation of the ventral striatum. Physiol Behav. 2006;89(2):226–234. doi: 10.1016/j.physbeh.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 36.Woods SC, Figlewicz DP, Madden LJ, Porte D, Sipols AJ, Seeley RJ. NPY and food intake: Discrepancies in the model. Regul Pept. 1998;75–76:403–408. doi: 10.1016/s0167-0115(98)00095-0. [DOI] [PubMed] [Google Scholar]