Abstract
Background
Despite the significant weight losses and dramatic improvements in comorbidities associated with bariatric surgery, a significant minority of patients appear to experience suboptimal weight losses. The reasons for this are not well understood, but often attributed to preoperative psychosocial characteristics and/or eating behaviors as well as poor adherence to the recommended postoperative diet.
Objective
To investigate the relationship between preoperative eating behavior, postoperative dietary adherence and weight loss following gastric bypass surgery.
Setting
A major, urban medical center with a comprehensive bariatric surgery program.
Methods
A prospective investigation of 200 female and male patients studied preoperatively and 20, 40, 66 and 92 weeks postoperatively. All patients underwent either open or laparoscopic Roux-en-Y gastric bypass surgery. Measures were percent weight loss, macronutrient intake, dietary adherence, and eating behaviors.
Results
Gender, baseline cognitive restraint, and self-reported adherence to the postoperative diet at postoperative Week 20 were associated with percent weight loss at postoperative Week 92. Those high in dietary adherence lost 4.5% more weight at postoperative Week 92 compared to those low in dietary adherence.
Conclusions
Baseline cognitive restraint and adherence to the recommended postoperative diet were associated with percent weight loss following gastric bypass surgery. These results suggest the potential utility of pre- or postoperative dietary counseling interventions to improve postoperative outcomes.
Keywords: bariatric surgery, gastric bypass surgery, cognitive restraint, dietary adherence
Introduction
Bariatric surgery has surged in popularity over the past decade. Whereas approximately 13,000 procedures were performed in the United States in1998, an estimated 103,000 surgeries were completed in 2003.1 The most common bariatric procedure in the United States presently is the Roux-en-Y gastric bypass, which accounted for 88% of all procedures in 2002.1 Two years postoperatively, patients typically have lost 35% of their initial body weight with the bypass procedure and 25% with the restrictive banding procedures.2 These weight losses are significantly larger and more durable than weight losses typically achieved with behavior modification or pharmacotherapy.3
Despite these impressive weight losses, there appears to be significant patient-to-patient variability in postoperative outcomes. Reviews have concluded that between 10%–20% of patients fail to achieve these weight losses or experience premature and/or sizable weight regain.4–7 For example, 9% of gastric bypass patients and 25% of adjustable gastric banding patients failed to maintain at least a 5% reduction in initial weight 10 years postoperatively.8
The reasons for these suboptimal outcomes are not well understood. Some investigators have suggested that preoperative psychosocial status is associated with poor postoperative outcomes.4–7 The evidence supporting this hypothesis is inconclusive, with many studies of this issue suffering from methodological problems. The relationship between preoperative eating behavior and postoperative weight loss is similarly unclear. Several studies, 9–13 but not all, 14–15 have suggested that the presence of preoperative binge eating or excessive sweet eating is associated with suboptimal postoperative weight loss.
Adherence to the rigorous postoperative diet also is believed to contribute to postoperative outcomes. Caloric intake often increases significantly during the postoperative course.8,14–19 In the Swedish Obese Subjects trial,8 surgery patients consumed approximately 2900 kcal/d prior to surgery. Their intake decreased to approximately 1500 kcal/d 6 months after surgery, but increased to approximately 2000 kcal/d 10 years later, when patients had regained approximately 10% of their maximum weight loss. Thus, despite the impressive strength of bariatric surgery as a treatment for extreme obesity, psychosocial, eating behavior, and dietary variables may play an influential role in postoperative outcome.
The primary aim of this study was to investigate the relationship of 92 week postoperative weight losses to preoperative psychosocial variables (i.e., self-esteem and mood), preoperative eating behaviors (i.e., cognitive restraint, disinhibition, and hunger), dietary intake (i.e., daily kcals, % kcals from fat, protein, etc.) and self-reported adherence to the postoperative diet.
Materials and Methods
Participants
Study participants were 200 individuals who underwent Roux-en-Y gastric bypass surgery at the Hospital of the University of Pennsylvania between November 2001 and June 2004. The study was approved by the Institutional Review Board of the University of Pennsylvania; all participants provided informed consent prior to the study.
Measures
Approximately 4 weeks prior to surgery, participants completed a psychosocial/behavioral evaluation to assess their appropriateness for surgery.20 As part of this assessment, patients completed the Weight and Lifestyle Inventory (WALI),21 which provided information on race, employment, education, and self-reported height. Weight was confirmed with a digital scale at the onset of the evaluation.
After completing the psychosocial/behavioral evaluation, participants completed a packet of questionnaires. Participants were mailed these questionnaires again approximately 20, 40, 66 and 92 weeks following surgery and provided a postage-paid envelope to facilitate return. The packet included the following measures:
Rosenberg Self-Esteem Scale
Self-esteem was assessed by the Rosenberg Self-Esteem Scale (RSE). This 10-item scale provides a global measure of self-esteem. Lower scores indicate higher self-esteem.22
Beck Depression Inventory-II
The presence of depressive symptoms was measured with the Beck Depression Inventory-II (BDI-II), the most widely-used measure of depressive symptomatology. Higher scores reflect greater dysphoria.23
Positive and Negative Affect Scale
Mood state, both positive and negative, also was assessed with the Positive and Negative Affect Scale (PANAS). The measure has separate subscales for both positive and negative affect, with higher scores reflecting greater frequency of the identified mood.24
Eating Inventory
The Eating Inventory is a 51-item self-report inventory that assesses three-factors: 1) Cognitive Restraint; 2) Disinhibition; and 3) Hunger. This widely-used inventory measures the degree to which persons exert conscious control over food intake and the extent to which such control is disrupted by internal or external factors.25
Block 98 Food Frequency Questionnaire
This questionnaire provides estimates of daily calorie intake and macronutrient composition by asking respondents to describe their food intake over the past 2 months. The questionnaire has excellent reliability and has been validated against multiple 4-day and 7-day food records.26–27
Dietary Adherence
At each assessment point, participants were asked, “How well are you following the diet plan given to you by the dietitian?” They responded on a 9-point Likert scale that ranged from 1 (“not well at all”) to 9 (“very well”).
Weight
Weight was self-reported by participants at all postoperative assessments.
Preoperative Dietary Counseling
Approximately two weeks prior to surgery, study participants met with one of the program’s registered dietitians. In a group session, patients were instructed in the dietary and behavioral changes believed necessary for a successful postoperative outcome. They were instructed to begin with a liquid diet for the first two weeks after surgery. They then slowly progressed to small portions of pureed or soft foods over the next two to four weeks. At approximately 2 months postoperatively, patients typically returned to a diet of regular foods, consisting of several 1–2 oz meals totaling approximately 1000–1200 kcal/d. Patients also were encouraged to use a daily multivitamin, increase protein intake and avoid sweets, fats and other foods that may trigger vomiting and dumping.
Surgical Procedure
The gastric bypass surgery was performed as either open or laparoscopic procedure by one of two surgeons (SER and NNW). Both surgeons performed essentially the same operation, with the formation of a small gastric pouch with a capacity of approximately 30 ml. The second step of the operation was the formation of a Roux-en-Y jejunal limb of approximately 70 cm on average. Postoperative weight loss did not differ by surgeon or by technique (open or laparoscopic). Thus, results from both surgeons were combined in all analyses.
Statistical Analysis Plan
Descriptive statistics are presented as means ± standard deviations (SD) for continuous variables and as percentages for categorical variables.
All 200 participants completed a baseline packet and at least 1 postoperative packet. We received 198 packets at Week 20, 147 at Week 40, 92 at Week 66, and 112 at Week 92. The declining response occurred despite repeated mailings, telephone calls, and email contacts (when available). These assessment points were selected as this study was conducted in parallel with a study of behavioral treatment for obesity, the results of which are not presented here.
To compare the mean percent change in weight over the 4 time points (Weeks 20, 40, 66, and 92), repeated-measures analyses were conducted using a means model with the SAS Mixed procedure. An unstructured variance-covariance form in repeated measurements was assumed for each outcome, and estimates of the standard errors were used to conduct tests and construct 95% confidence intervals.
In the mixed model analyses, we considered the following variables as candidate predictors of percent change in weight loss: demographic variables—age, gender, and ethnicity; preoperative psychosocial variables—self-esteem, depressive symptoms, positive affect and negative affect; preoperative eating behavior—cognitive restraint, disinhibition, and hunger; preoperative baseline dietary intake—daily kcals, % kcal from fat, % kcals from protein, % kcals from carbohydrate, and % kcals from sweets/desserts; and self-reported dietary adherence. The outcome variable (percent weight loss) satisfied the normality assumption of the mixed models and residual analyses confirmed an appropriate fit of the mixed models.
To investigate how the behavioral variables changed over time, each variable was considered as the outcome in a mixed model analysis. Using a Bonferroni adjustment for the ten pairwise comparisons, these mixed model analyses also were used to identify significant within mean differences between time points for each variable.
In the mixed model analysis with postoperative dietary adherence as the outcome, each of the other behavioral variables was considered as independent predictor variable to assess its individual association with dietary adherence. Furthermore, postoperative dietary adherence at Week 20 was split at the median (as “high” and “low” dietary adherence) and entered into a mixed model as the independent variable to examine its contribution to change in weight loss from Week 20 to Week 92. We chose to split these groups at the median as there is no widely accepted standard of adherence to the postoperative diet. Unless otherwise noted, all statistical tests described were two-tailed with an alpha level ≤ 0.05 considered significant.
Results
Participants’ Characteristics
Demographic and descriptive variables are presented in Table 1. The majority (n = 164, 82%) were women; they had a mean preoperative age of 42.6 ± 9.9 yrs, weight of 139.4 ± 25.6 kg, and BMI of 51.4 ± 9.0 kg/m2. Men (n = 36) had a mean preoperative age of 45.7 ± 9.4 yrs, weight of 181.3 ± 37.2 kg, and BMI of 55.5 ± 10.0 kg/m2. Approximately 87% of all participants were European-American, while 9% were African-American, and the remainder were of other ethnic origin. Participants reported 14.0 ± 2.3 years of education. Just under half (49.5%) reported being married, 34.5% were single, and the remainder were separated, divorced, or widowed. Eighty three percent indicated that they were employed and 6.5% were on disability. The remaining 9.5% indicated that they were unemployed or retired.
Table 1.
Participant Characteristics
| Total Sample N = 200 % or Mean ± SD | Men N = 36 % or Mean ± SD | Women N = 164 % or Mean ± SD | |
|---|---|---|---|
| Ethnicity | |||
| European-American | 87.5% | 97.2% | 85.4% |
| African-American | 9.5% | 2.8% | 11.0% |
| Other | 2.0% | 0.0% | 2.4% |
| Currently Employed | 82.7% | 80.0% | 83.3% |
| Currently Married | 49.5% | 63.9% | 46.3% |
| Mean Years of Education | 14.0 ± 2.3 | 14.6 ± 2.7 | 13.9 ± 2.2 |
| Mean Age at Surgery (yrs) | 43.2 ± 9.8 | 45.7 ± 9.4 | 42.6 ± 9.9 |
| Mean Baseline Height (cm) | 167.6 ± 9.5 | 180.7 ± 8.4 | 164.7 ± 6.9 |
| Mean Baseline Weight (kg) | 146.9 ± 32.3 | 181.3 ± 37.2 | 139.4 ± 25.6 |
| Mean Baseline BMI (kg/m2) | 52.1 ± 9.3 | 55.5 ± 10.0 | 51.4 ± 9.0 |
Changes in Weight
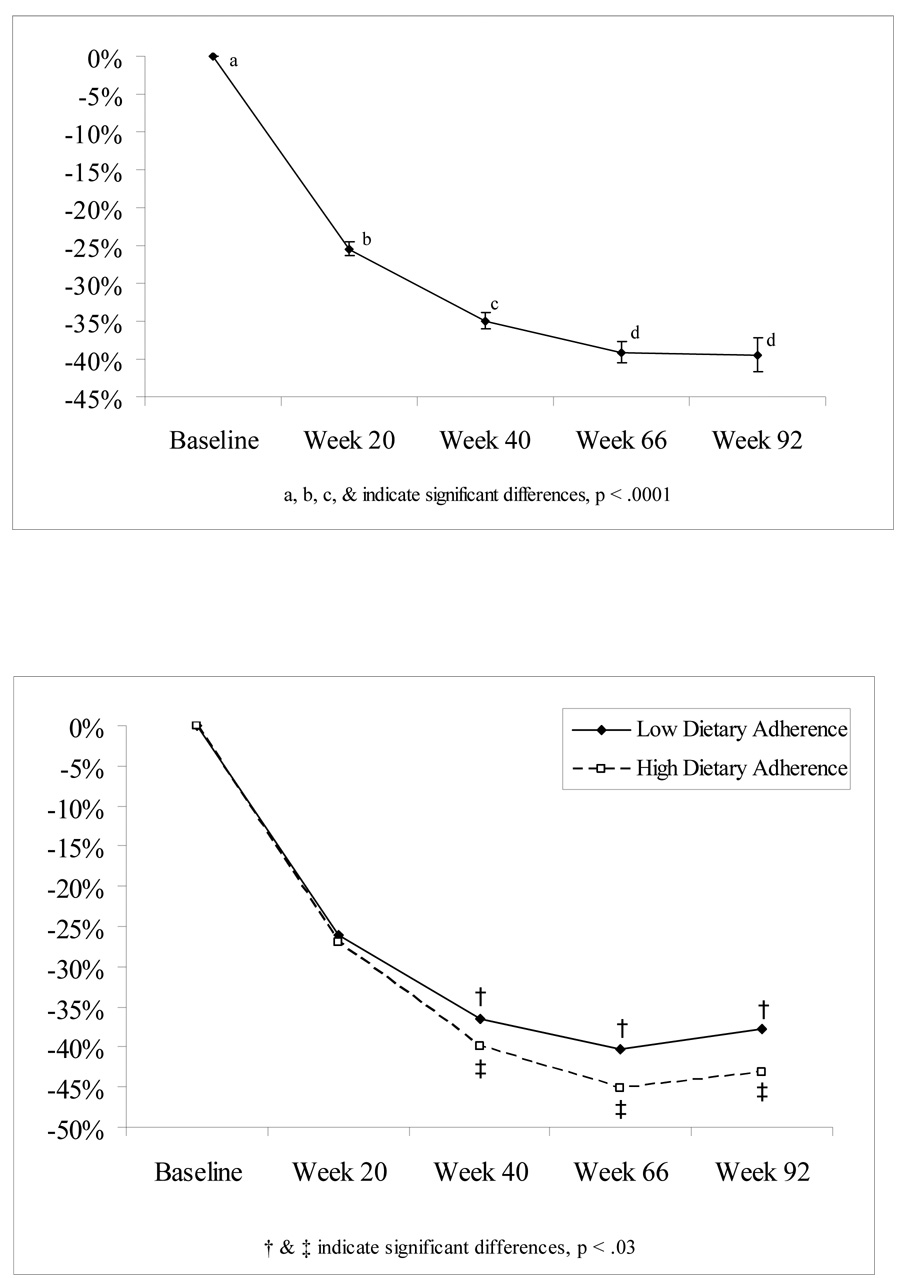
Based on the mixed model analysis that used all available data at each time point, mean percent weight loss significantly changed over time (See Figure 1a, Overall Time F = 223, p <0.0001). Participants, on average, lost approximately 25.5% (95% CI: 24.6, 26.3) of their body weight 20 weeks after surgery; 35.0% (CI: 33.8, 36.1) at 40 weeks; 39.1% (CI: 37.7, 40.6) at 66 weeks; and 39.4% (95% CI: 37.2, 41.7) at 92 weeks. Mean percent change in weight loss at Week 20 differed from all other time points and all other pairwise means were significantly different except for Week 66 vs. Week 92 (p < 0.0001 for all significant pairwise comparisons).
Figure 1.
Figure 1a: Model based Mean % Weight Loss Over Time
Figure 1b: Model based Mean % Weight Loss Over Time by Dietary Adherence Group.
Changes in Behavioral Variables
Table 2 displays the mixed model based means ± SE of the behavioral variables of interest at baseline (preoperatively) and at the four postoperative assessment points. Consistent with previous investigations, participants reported significant improvements in self-esteem and depressive symptoms as well as positive and negative affect following surgery (by postoperative Week 20, all p’s < 0.001). These improvements remained significantly different from baseline at postoperative Week 92 (all p’s < 0.001). Cognitive restraint increased significantly at postoperative Week 20 (p < .001) and remained significantly different at Week 92 (p < .001).Self-reports of hunger and disinhibition significantly decreased from baseline to postoperative Week 20 (p < .001) and also remained significantly different from baseline at Week 92 (p <.001).
Table 2.
Postoperative Changes in Dietary Adherence, Psychosocial Variables, Macronutrient Intake.
| Baseline Mean ± SE | Postoperative Week 20 Mean ± SE | Postoperative Week 40 Mean ± SE | Postoperative Week 66 Mean ± SE | Postoperative Week 92 Mean ± SE | |
|---|---|---|---|---|---|
| Dietary Adherence | ---- | 6.5 ± 2.0 | 5.7 ± 2.2 | 5.6 ± 2.5 | 5.4 ± 2.2 |
| Psychosocial Variables | |||||
| BDI | 12.3 ± 0.6a | 6.0 ± 0.52b | 7.0 ± 0.7b | 7.1 ± 0.7b | 8.0 ± .8b |
| RSE | 20.5 ± 0.4a | 17.6 ± 0.4b | 16.9 ± 0.4b | 16.7 ± 0.5b | 16.7 ± 0.5b |
| PANAS (+) | 30.4 ± 0.6a | 34.2 ± 0.6b | 34.4 ± 0.7 b | 34.4 ± 0.8b | 33.7 ± .8b |
| PANAS (−) | 21.7 ± 0.6a | 16.8 ± 0.5b | 17.9 ± 0.7b | 17.9 ± 0.8b | 18.1 ± 0.7 b |
| Eating Inventory Subscales | |||||
| Cognitive Restraint | 8.3 ± 0.3a | 13.4 ± 0.3b | 12.9 ± 0.4bc | 12.4 ± 0.4c | 12.3 ± 0.4bc |
| Disinhibition | 10.0 ± 0.3a | 5.2 ± 0.2b | 5.1 ± 0.3b | 5.2 ± 0.3b | 5.5 ± 0.3b |
| Hunger | 7.1 ± 0.3a | 3.1 ± 0.2b | 3.4 ± 0.3bc | 3.8 ± 0.3c | 3.7 ± 0.3bc |
| Macronutrient Intake | |||||
| Daily Kcals | 2390.9 ± 99.0a | 1172.9 ± 46.5b | 1189.5 ± 54.2b | 12 ± 629.2bc | 1358.1 ± 60.5c |
| % of Kcal from fat | 41.3 ± 0.6a | 39.9 ± 0.7a | 40.2 ± 0.8a | 42.0 ± 0.9a | 41.6 ± 0.9a |
| % of Kcal from protein | 15.2 ± 0.3abc | 16.5 ± 0.3bc | 16.0 ± 0.3abc | 15.6 ± 0.4abc | 14.9 ± 0.3a |
| % of Kcal from carbohydrates | 44.1 ± 0.7a | 44.8 ± 0.7a | 44.6 ± 0.9a | 43.2 ± 0.9a | 43.4 ± 0.9a |
| % of Kcal from sweets | 18.5 ± 0.9a | 9.3 ± 0.7b | 11.7 ± 0.8c | 13.3 ± 0.9c | 13.3 ± 0.9c |
a, b, and c indicate significant differences across time at the p = .005 significance level.
Total caloric intake decreased by over 50% by postoperative Week 20 (p < .001), the first assessment point at which participants had returned to a diet of regular foods. Total daily calories, however, increased significantly by approximately 150 kcal/d from Week 20 to Week 92 (p < .001). The percentage of calories from sweets/desserts followed a similar pattern, with a sizable decrease in the first few months after surgery (p < .001) and a small, but significant (p < .001) increase from Week 20 to Week 92. Percentage of calories from protein increased significantly from baseline to postoperative Week 20 (p < .001), but returned to near baseline levels by postoperative Week 92. There were no significant changes in the percentage of calories from fat and carbohydrates following surgery.
The mean self-reported dietary adherence level at Week 20 was 6.44 (95% CI: 6.16, 6.73). Self-reported dietary adherence decreased significantly at the subsequent postoperative weeks (5.74 at Week 40, 5.54 at Week 66, and 5.36 at Week 92; p<0.0004). Baseline BDI, RSE, PANAS(−), daily Kcals, and % Kcal from sweets were negatively correlated with changes in postoperative dietary adherence through week 92, while PANAS (+), Cognitive Restraint, and % Kcal from protein were positively associated with changes in postoperative dietary adherence (p < 0.03 for all). The change in these variables over time, however, was not associated with change in postoperative dietary adherence from Week 20 to Week 92. Change in weight loss from week 20 through week 66 was significantly associated with change in postoperative dietary adherence (p < 0.02).
Predictors of Weight Loss
Among the predictor variables of interest, only three—gender (F = 6.4, p = 0.01), baseline cognitive restraint (F = 8.8, p = 0.003), self-reported dietary adherence at postoperative week 20 (F = 9.3, p = 0.003)—were significantly associated with postoperative weight loss. To examine how percent weight loss over time differed based on levels of self-reported dietary adherence at postoperative Week 20, we split dietary adherence at the median (score = 7). At Week 20, the low adherence group had lost 26% of their initial weight and the high dietary had lost 27% of their initial weight. A mixed model analysis was then conducted to compare the low adherence group and the high adherence group on percent weight loss at weeks 40, 66, and 92. From Week 20 to Week 40 those high in dietary adherence lost an additional 13.0% (CI: 11.7, 14.4) of their weight, which was significantly greater than the additional 10.6% (CI: 9.2, 12.0) lost by those with poorer dietary adherence (p = 0.02). At Week 66, weight losses for the two groups, measured from Week 20, were 18.1% (CI: 16.0, 20.1) and 14.3% (CI: 12.1, 16.5), respectively (p = 0.01 for between group differences). By Week 92, both groups had regained some weight but the high adherence group still lost significantly more weight than those in low adherence group at Week 92. Those high in dietary adherence had lost 16.2% (CI: 13.7, 18.7) of their weight since Week 20, compared to only 11.7% (CI: 9.0, 14.4) for those low in dietary adherence (p = 0.02), representing a 28% difference in weight loss between the two groups (Figure 1b).
Changes in Dietary Adherence
We also were interested in whether dietary adherence changed over time. At Week 20, the low adherence group had a mean adherence level of 4.59 (CI: 4.36, 4.82). This did not significantly change in weeks 40, 66, and 92 (mean level= 4.56, 4.64, 4.05 respectively). At Week 20, the high adherence group had a mean adherence level of 8.00 (CI: 7.80, 8.22) which was significantly different from the remaining weeks (6.79 at Week 40, 6.34 at Week 66, 6.52 at Week 92). At each week, the two adherence group’s mean adherence levels differed significantly from each other (p<0.005 for between differences at Week 20, 40, 66, 92).
Discussion
Results of the present study add to a growing body of literature on the characteristics associated with weight loss following bariatric surgery. Participants lost approximately 25% of their preoperative body weight within the first 20 weeks of gastric bypass surgery. They lost another 10% over the next 20 weeks and almost 40% of their initial body weight by the second postoperative year. Of the potential predictor variables of interest investigated, gender, baseline cognitive restraint, and self-reported dietary adherence at postoperative Week 20, when participants had returned to regular food, were significant predictors of percent weight loss over time.
Men who underwent bariatric surgery lost significantly more weight over time than did women. This result is not particularly surprising, as men have been found to lose more weight than women in other studies of bariatric surgery as well as studies of behavioral and pharmacological treatments for obesity.28 This difference is typically attributed to metabolic differences between the genders, although obese men, and their response to weight loss, surgical or otherwise, are studied far less frequently.
The contributions of baseline cognitive restraint and adherence to the postoperative diet represent novel and interesting findings. Those individuals who reported higher levels of cognitive restraint at baseline experienced greater weight losses postoperatively. Cognitive restraint is typically characterized as an individual’s ability to intentionally limit food intake, typically to prevent weight gain.25 Given the positive association between baseline cognitive restraint and dietary adherence found in this study, it may be that the ability to restrict food intake prior to surgery predicts adherence to the rigorous postoperative diet. As a whole, individuals in the present study reported relatively low levels of cognitive restraint preoperatively and could be described as “unrestrained eaters.” However, we found an association between baseline cognitive restraint and the % kcal/d from sweets (r = −0.30, p = 0.0003), % kcal/d from fat (r = −0.21, p = 0.01) and % kcal/d from protein (r = 0.20, p = 0.01), suggesting that those with higher levels of dietary restraint consumed comparatively healthier diets prior to surgery.
Self-reported adherence to the postoperative diet at postoperative week 20, when participants had returned to eating regular foods, also was associated with larger postoperative weight losses. Those individuals above the median in dietary adherence, as compared to those below the median, experienced a weight loss 2.4% percentage points greater at postoperative Week 40 and 3.8% percentage points greater at Week 66. By Week 92, both groups had regained some weight. However, those high in self-reported dietary adherence achieved a weight loss that was 4.5% percentage points greater than those who reported less adherence to the postoperative diet, representing a 28% greater weight loss. Interestingly, those individuals initially categorized as high in dietary adherence reported a significant deterioration in their adherence to the postoperative diet over the course of the study, although their adherence remained significantly greater at Week 92 than those initially categorized as low in dietary adherence.
Presently, there are no accepted standards for the postoperative diet following bariatric surgery. Adherence to the dramatically reduced portion sizes is believed to be a significant challenge for many patients. This study, like others,8,14–19 suggests that while patients often are able to decrease their caloric intake within the first postoperative year, caloric intake increases over time. The present study is the first, however, to provide evidence that adherence to the postoperative diet is predictive of postoperative changes in weight.
Baseline self-esteem and depressive symptoms, as well as positive and negative affect, were not associated with postoperative weight loss. These characteristics were, however, associated with changes dietary adherence during the postoperative period. In general, studies that have investigated the relationship between baseline psychosocial characteristics and/or psychopathology following bariatric surgery have been contradictory.4–7 This lack of conclusive association has led some to question the value of the assessment of preoperative psychosocial status. These evaluations typically assess the presence of formal psychopathology that may contraindicate bariatric surgery but also evaluate the environmental influences and psychosocial factors that may have contributed to the development of extreme obesity, and, perhaps most importantly, their potential relationship with postoperative outcome.20 Results of the present study underscore the importance of a preoperative assessment of eating behaviors, whether conducted by a mental health professional or dietitian.
Consistent with other investigations,4–6 participants experienced improvements in psychosocial status postoperatively. Self-esteem improved, positive affect increased, and negative affect and depressive symptoms decreased. Participants also experienced changes in their eating behavior. Dietary restraint increased, while hunger and disinhibition decreased 20 weeks after surgery and remained at these levels through the second postoperative year. Participants also reported a postoperative reduction in total caloric intake, as well as the percentage of kcals/d from sweets and desserts. These findings are likely related to the restrictive aspects of gastric bypass surgery, as well as concerns about the “dumping syndrome” following consumption of sugar. There was a significant increase in percentage of kcal/d from protein 20 weeks after surgery, but by 92 weeks protein intake returned to baseline levels. However, the overall change from baseline was modest and its clinical significance unclear. The lack of lasting change in protein is somewhat concerning, given that patients were explicitly instructed during their preoperative nutrition counseling session to increase the amount of protein they consume postoperatively.
While providing important new information, the present study has a number of limitations. Perhaps most critically, we experienced significant attrition. Only 56% of participants completed the final assessment, despite repeated mailings and phone calls and/or emails encouraging them to complete the study. High rates of attrition are a relatively common experience in obesity studies and represent a significant threat to the integrity of treatment outcomes studies. However, in a post-hoc analysis, we found no significant differences in the baseline variables of interest between those individuals who did and did not remain in the study. While the mixed model used in the present study is a recommended analytic method to handle missing data, it is not a perfect substitute for lower attrition rates.
Our reliance on self-report measures is another limitation. The self-reported weights used in the present study are likely to have overstated participants’ weight losses by 2 to 3 kg.29 Postoperative weight losses, however, remained substantial even with this likely overestimation. While self-report measures of psychological symptoms and eating behaviors are widely-used, they are not the ideal measures of psychopathology or dietary intake. Future studies should strive to augment these assessments with validated clinical interviews. While results of the present study suggest that cognitive restraint and adherence to the postoperative diet are related to change in weight in the first two postoperative years, their relationship to longer term weight losses is unknown. Regardless, these findings suggest the possible utility of pre- or postoperative dietary counseling interventions to improve dietary adherence and optimize postoperative outcomes.
ACKNOWLEDGMENTS
Work on this paper was supported, in part, by the National Institute of Diabetes and Digestive and Kidney Diseases (Grants K23-DK60023 to Dr. Sarwer and K24-DK065018 to Dr. Wadden).
Dr. Sarwer had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURE
Authors have no conflict of interest to disclose
References
- 1.Santry HP, Gillen DL, Lauderdale DS. Trends in bariatric surgical procedures. JAMA. 2005;294:1909–1917. doi: 10.1001/jama.294.15.1909. [DOI] [PubMed] [Google Scholar]
- 2.Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292:1724–1734. doi: 10.1001/jama.292.14.1724. [DOI] [PubMed] [Google Scholar]
- 3.Sarwer DB, Foster GD, Wadden TA. Treatment of obesity I: adult obesity. In: Thompson JK, editor. Handbook of eating disorders and obesity. Hoboken, NJ: John Wiley & Sons; 2004. pp. 421–442. [Google Scholar]
- 4.Bocchieri LE, Meana M, Fisher BL. A review of psychosocial outcomes of surgery for morbid obesity. J Psychosom Res. 2002;52:155–165. doi: 10.1016/s0022-3999(01)00241-0. [DOI] [PubMed] [Google Scholar]
- 5.Herpertz S, Kielmann R, Wolf AM, Langkafel M, Senf W, Hebebrand J. Does obesity surgery improve psychosocial functioning? A systematic review. Int J Obes Relat Metab Disord. 2003;27:1300–1314. doi: 10.1038/sj.ijo.0802410. [DOI] [PubMed] [Google Scholar]
- 6.Sarwer DB, Wadden TA, Fabricatore AN. Psychosocial and behavioral aspects of bariatric surgery. Obes Res. 2005;13:639–648. doi: 10.1038/oby.2005.71. [DOI] [PubMed] [Google Scholar]
- 7.van Hout GC, van Oudheusden I, van Heck GL. Psychological profile of the morbidly obese. Obes Surg. 2004;14:479–488. doi: 10.1381/096089204323093336. [DOI] [PubMed] [Google Scholar]
- 8.Sjostrom L, Lindroos AK, Peltonen M, et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med. 2004;351:2683–2693. doi: 10.1056/NEJMoa035622. [DOI] [PubMed] [Google Scholar]
- 9.Hsu LK, Benotti PN, Dwyer J, et al. Nonsurgical factors that influence the outcome of bariatric surgery. Psychosom Med. 1998;60:338–346. doi: 10.1097/00006842-199805000-00021. [DOI] [PubMed] [Google Scholar]
- 10.Hsu LK, Mulliken B, McDonagh B, et al. Binge eating disorder in extreme obesity. Int J Obes Relat Metab Disord. 2002;26:1398–1403. doi: 10.1038/sj.ijo.0802081. [DOI] [PubMed] [Google Scholar]
- 11.Kalarchian MA, Marcus MD, Wilson GT, Labouvie EW, Brolin RE, LaMarca LB. Binge Eating among gastric bypass patients: a long-term follow-up. Obes Res. 2002;12:270–275. doi: 10.1381/096089202762552494. [DOI] [PubMed] [Google Scholar]
- 12.Sugerman HJ, Starkey JV, Birkenhauer R. A randomized prospective trial of gastric bypass versus vertical banded gastroplasty for morbid obesity and their effects on sweet versus non-seets eaters. Ann Surg. 1987;205:613–624. doi: 10.1097/00000658-198706000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sugerman HJ, Londrey GL, Kellum JM. Weight loss with vertical banded gastroplasty and Roux-en-Y gastric bypass with selective vs. random assignment. Am J Surg. 1989;157:93–102. doi: 10.1016/0002-9610(89)90427-3. [DOI] [PubMed] [Google Scholar]
- 14.Lindroos AK, Lissner L, Sjöstrom L. Weight change in relation to intake of sugar and sweet foods before and after weight reducing gastric surgery. Int J Obes Relat Metab Disord. 1996;20:634–643. [PubMed] [Google Scholar]
- 15.Malone M, Alger-Mayer S. Binge status and quality of life after gastric bypass surgery: a one-year study. Obes Res. 2004;12:473–481. doi: 10.1038/oby.2004.53. [DOI] [PubMed] [Google Scholar]
- 16.Anderson T, Larsen U. Dietary outcome in obese patients treated with a gastroplasty program. Am J Clin Nutr. 1989;50:1328–1340. doi: 10.1093/ajcn/50.6.1328. [DOI] [PubMed] [Google Scholar]
- 17.MacLean LD, Rhode BM, Shizgal HM. Nutrition following gastric operations for morbid obesity. Ann Surg. 1983;198:347–355. doi: 10.1097/00000658-198309000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miskowiak J, Honore K, Larsen L, Andersen B. Food intake before and after gastroplasty for morbid obesity. Scand J Gastroenterol. 1985;20:925–928. doi: 10.3109/00365528509088848. [DOI] [PubMed] [Google Scholar]
- 19.Naslund I, Jarnmark I, Anderson H. Dietary intake before and after gastric bypass and gastroplasty for morbid obesity in women. Int J Obes Relat Metab Disord. 1988;12:503–513. [PubMed] [Google Scholar]
- 20.Wadden TA, Sarwer DB. Behavioral assessment of candidates for bariatric surgery: a patient-oriented approach. Obesity. 2006;14 Suppl 2:51S–52S. doi: 10.1016/j.soard.2006.03.011. Note: Co-publication in Surgery for Obesity and Related Disorders 2006;2:171–179. [DOI] [PubMed] [Google Scholar]
- 21.Wadden TA, Foster GD. Weight and Lifestyle Inventory (WALI) Obesity (Silver Spring) 2006;14 Suppl 2:99S–118S. doi: 10.1038/oby.2006.289. Note: Co-publication in Surgery for Obesity and Related Disorders 2006;2:180–199. [DOI] [PubMed] [Google Scholar]
- 22.Rosenberg M. Conceiving the self. York: Basic Books; 1979. [Google Scholar]
- 23.Beck AT, Steer RA. BDI Beck Depression Inventory Manual. San Antonio: Harcourt Brace & Company; 1993. [Google Scholar]
- 24.Watson D, Clark LA. Development and validation of brief measures of positive and negative affect: The PANAS subscales. Journal of Personality and Social Psychology. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- 25.Stunkard AJ, Messick S. Eating inventory manual. San Antonio: Harcourt Brace Jovanovitch, Inc; 1988. [Google Scholar]
- 26.Block G, Woods M, Potosky A, Clifford C. Validation of a self-administered diet history questionnaire using multiple diet records. J Clin Epidemiol. 1990;43:1327–1335. doi: 10.1016/0895-4356(90)90099-b. [DOI] [PubMed] [Google Scholar]
- 27.Sorbell J, Block G, Koslowe R, Tobin J, Andres R. Validation of retrospective questionnaire assessing diet 10–15 years ago. Am J Epidemiol. 1989;130:173–187. doi: 10.1093/oxfordjournals.aje.a115310. [DOI] [PubMed] [Google Scholar]
- 28.Wadden TA, Foster GD, Letizia KA, Stunkard AJ. A multicenter evaluation of a proprietary weight reduction program for the treatment of marked obesity. Arch Int Med. 1992;152:961–966. [PubMed] [Google Scholar]
- 29.Tell GS, Jeffery RW, Kramer FM, Snell MK. Can self-reported body weight be used to evaluate long-term follow-up of a weight loss program? J Am Diet Assoc. 1987;87:1198–1201. [PubMed] [Google Scholar]