Abstract
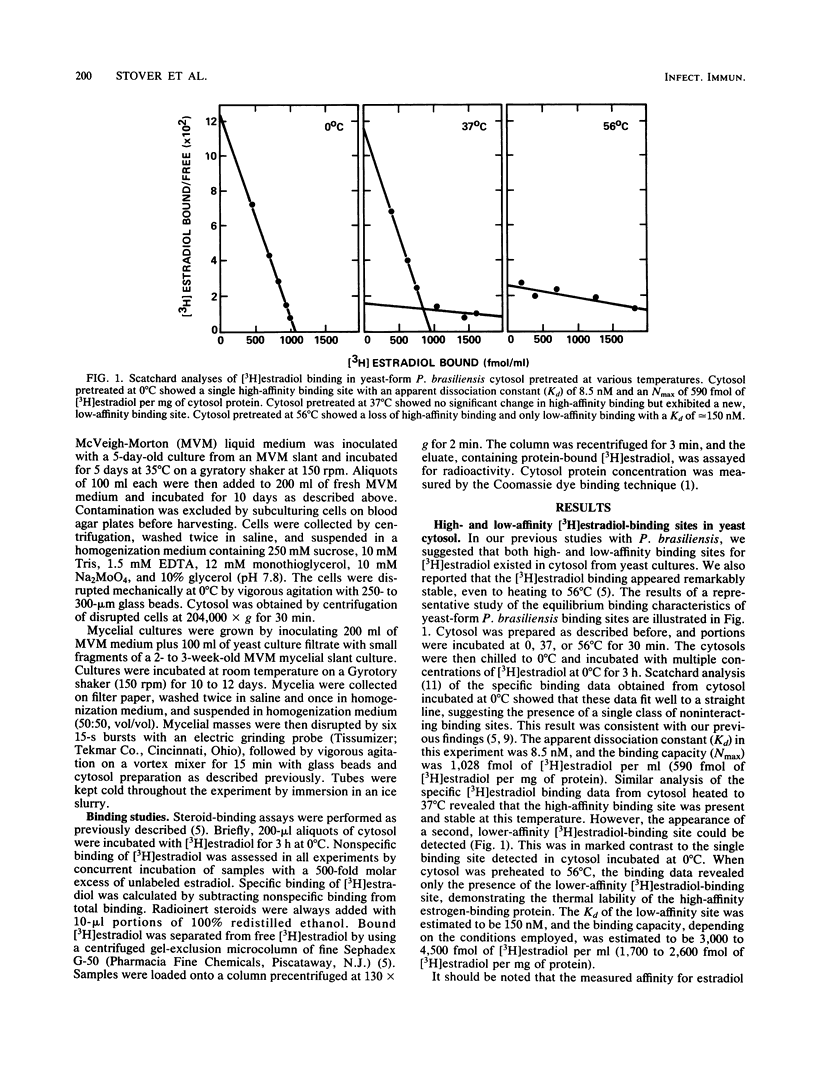
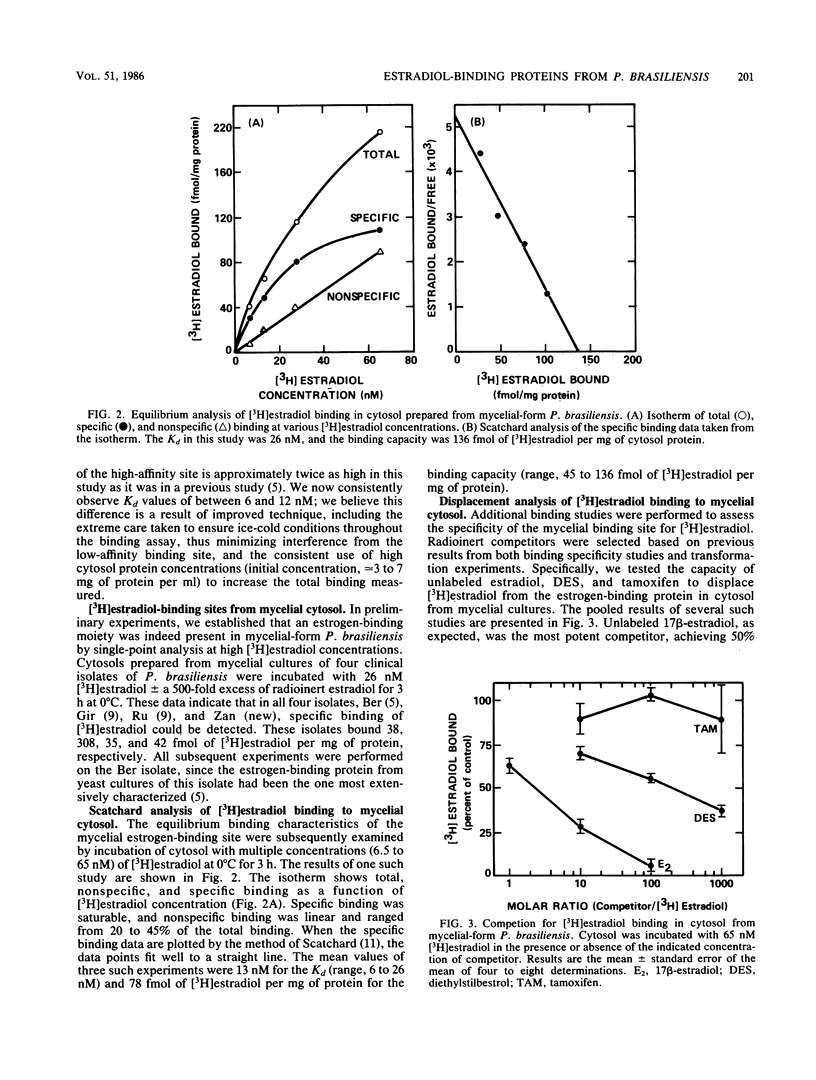
Paracoccidioides brasiliensis, the etiologic agent of paracoccidioidomycosis, causes disease much more frequently in men than it does in women, suggesting that the hormonal milieu of the host might influence P. brasiliensis pathogenicity. We recently demonstrated that cytosol from yeast cultures of P. brasiliensis contains a high-affinity, low-capacity, tritiated 17 beta-estradiol [( 3H]estradiol)-binding protein. Estradiol and, to a lesser degree, diethylstilbestrol (DES), inhibited the transformation of P. brasiliensis cultures from the mycelial to the yeast form, an event critical to the establishment of infection. Our current studies demonstrated a somewhat higher affinity (apparent dissociation constant [Kd], approximately equal to 6 to 12 nM) of the estrogen-binding protein for [3H]estradiol than was previously described for yeast cytosol. The presence of both high- and low-affinity estrogen-binding sites in yeast-form P. brasiliensis cytosol was detected after warming the cytosol to 37 degrees C. The high-affinity protein was labile to further heating (56 degrees C), although the low-affinity protein was stable. Additional experiments demonstrated the presence of an estrogen-binding protein in cytosol prepared from mycelial-form P. brasiliensis. This estrogen-binding protein had a slightly lower affinity for [3H]estradiol (Kd approximately equal to 13 nM), and its cytosol contained somewhat fewer binding sites (approximately equal to 78 fmol/mg of protein) than did yeast-form P. brasiliensis cytosol. Of particular interest was the finding that DES, a weak competitor for [3H]estradiol binding in yeast cytosol, displaced [3H]estradiol from the mycelial-form binding moiety. DES had a 50- to 100-fold-lower affinity for the [3H]estradiol-binding protein than did estradiol, consistent with its lower bioactivity in the mycelial-to-yeast-form transformation studies. The current results lend further support to our hypothesis that endogenous estrogens in the host, acting through the cytosol binding protein in the fungus, inhibit mycelial-to-yeast-form transformation, thus explaining the resistance of women to paracoccidioidomycosis.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chen T. L., Feldman D. Distinction between alpha-fetoprotein and intracellular estrogen receptors: evidence against the presence of estradiol receptors in rat bone. Endocrinology. 1978 Jan;102(1):236–244. doi: 10.1210/endo-102-1-236. [DOI] [PubMed] [Google Scholar]
- Jordan V. C., Collins M. M., Rowsby L., Prestwich G. A monohydroxylated metabolite of tamoxifen with potent antioestrogenic activity. J Endocrinol. 1977 Nov;75(2):305–316. doi: 10.1677/joe.0.0750305. [DOI] [PubMed] [Google Scholar]
- Loose D. S., Stover E. P., Restrepo A., Stevens D. A., Feldman D. Estradiol binds to a receptor-like cytosol binding protein and initiates a biological response in Paracoccidioides brasiliensis. Proc Natl Acad Sci U S A. 1983 Dec;80(24):7659–7663. doi: 10.1073/pnas.80.24.7659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radanyi C., Mercier-Bodard C., Secco-Millet C., Baulieu E. E., Richard-Foy H. alpha-Fetoprotein is not a component of the estradiol receptor of the rat uterus. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2269–2272. doi: 10.1073/pnas.74.6.2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Restrepo A., Robledo M., Ospina S., Restrepo M., Correa A. Distribution of paracoccidioidin sensitivity in Colombia. Am J Trop Med Hyg. 1968 Jan;17(1):25–37. [PubMed] [Google Scholar]
- Restrepo A., Salazar M. E., Cano L. E., Stover E. P., Feldman D., Stevens D. A. Estrogens inhibit mycelium-to-yeast transformation in the fungus Paracoccidioides brasiliensis: implications for resistance of females to paracoccidioidomycosis. Infect Immun. 1984 Nov;46(2):346–353. doi: 10.1128/iai.46.2.346-353.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rippon J. W. Dimorphism in pathogenic fungi. Crit Rev Microbiol. 1980;8(1):49–97. doi: 10.3109/10408418009085078. [DOI] [PubMed] [Google Scholar]



