Abstract
The mechanisms of action of gallium nitrate, an antineoplastic drug, are only partly understood. Using a DNA microarray to examine genes induced by gallium nitrate in CCRF-CEM cells, we found that gallium increased metallothionein-2A (MT2A) and heme oxygenase-1 (HO-1) gene expression and altered the levels of other stress-related genes. MT2A and HO-1 were increased after 6 and 16 h of incubation with gallium nitrate. An increase in oxidative stress, evidenced by a decrease in cellular GSH and GSH/GSSG ratio, and an increase in dichlorodihydrofluoroscein (DCF) fluorescence, was seen after 1 – 4 h incubation of cells with gallium nitrate. DCF fluorescence was blocked by the mitochondria-targeted antioxidant mitoquinone. N-acetyl-L-cysteine blocked gallium-induced MT2A and HO-1 expression and increased gallium’s cytotoxicity. Studies with a zinc-specific fluoroprobe suggested that gallium produced an expansion of an intracellular labile zinc pool, suggesting an action of gallium on zinc homeostasis. Gallium nitrate increased the phosphorylation of p38 mitogen-activated protein kinase and activated Nrf-2, a regulator of HO-1 gene transcription. Gallium-induced Nrf-2 activation and HO-1 expression were diminished by a p38 MAP kinase inhibitor. We conclude that gallium nitrate induces cellular oxidative stress as an early event which then triggers the expression of HO-1 and MT2A through different pathways.
Keywords: Gallium, oxidative stress, chemotherapy, lymphoma, metallothionein, zinc, heme oxygenase-1, p38 MAP kinase, Nrf2, antioxidants
Introduction
Gallium nitrate is metallodrug that has clinical activity in the treatment of non-Hodgkin’s lymphoma and urothelial malignancies [1–4]. Several clinical trials conducted over the past two decades as well as more recent studies conducted in patients with lymphoma have confirmed its antineoplastic activity when used as a single agent or in combination with other chemotherapeutic drugs [5–11].
Whereas gallium nitrate has demonstrated antineoplastic activity in the clinic, there remains only a partial understanding of its intracellular targets and the molecular determinants of tumor cell sensitivity to gallium compounds. Investigations directed at elucidating gallium’s mechanism of action are important since they may provide insight into tumor biology and may help identify subtypes of lymphomas and other malignancies more likely to respond to treatment with this agent. Prior studies have shown that gallium shares properties with iron in that it binds to transferrin in the circulation and can be incorporated into cells via transferrin receptor-mediated endocytosis [12–16]. Within the cell, gallium disrupts iron homeostasis and interferes with deoxyribonucleotide synthesis by inhibiting the activity of the iron-dependent R2 subunit of ribonucleotide reductase [15,17–19]. Lymphoma cells exposed to gallium nitrate undergo apoptosis through the “intrinsic” pathway that involves proapoptotic Bax, release of cytochrome c from mitochondria, and the activation of effector caspase-3 [20]. More recently, we reported that human lymphoma cells with acquired resistance to gallium nitrate displayed an increase in their steady-state expression of the genes for metallothionein-2A (MT2A) and zinc transporter-1 [21]. The upregulation of MT2A in gallium-resistant cells suggested that MT2A might play a role in modulating the cytotoxicity of gallium nitrate. In support of this was the finding that the induction of metallothionein gene expression by zinc sulfate led to a reduction in the growth-inhibitory action of gallium nitrate in CCRF-CEM cells; this protective effect was lost when metallothionein levels returned to baseline [21]. In addition, an examination of the effects of gallium nitrate in a panel of lymphoma cell lines revealed that its cytotoxicity inversely correlated with the level of endogenous MT2A expression in different cell lines; cells with the highest MT2A content were least sensitive to gallium nitrate [21].
Prior studies also revealed that in gallium-sensitive cells, gallium nitrate induced the expression of MT2A and ZnT-1 by increasing the binding of metal-responsive transcription factor-1 (MTF-1) to metal responsive elements (MREs) located on the promoter regions of these genes [21]. This finding suggests an effect of gallium nitrate on zinc pathways. In addition, whereas the triggering of apoptosis required at least 24– 30 h of exposure of cells to gallium nitrate, an increase in MT2A occurred at much earlier time-points thereby suggesting that there are cellular events that occur shortly after exposure of cells to gallium nitrate that have not been previously appreciated.
Although an increase in MTF-1 activity and MT2A gene expression has been demonstrated in cells exposed to gallium nitrate, the mechanisms underlying this effect and its significance with respect to gallium’s cytotoxicity in gallium-sensitive cells have not been elucidated. It is known that changes in the cellular zinc pool and oxidative stress may increase MTF-1 activity [22,23] and we therefore hypothesized that gallium might act through these pathways. In the present investigation, we show that the increase in cellular MT2A in cells exposed to gallium nitrate results from a gallium-induced production of reactive oxygen species (ROS) and is associated with a shift in intracellular zinc pools that are accessible to chelation. Moreover, we show that gallium nitrate induces the expression of heme oxygenase-1 (HO-1) via a pathway that involves signaling through p38 mitogen-activated protein (MAP) kinase and activation of transcription factor Nrf-2.
Materials and Methods
Materials
The GEArray Q Series Human Metal Transport and Homeostasis Gene Array and reagents required for its use were purchased from SuperArray Bioscience Corporation (Frederick, MD). Gallium nitrate was obtained from Genta Incorporated (Berkely Heights, NJ). Zinc sulfate, 2-methyl-8-[(4-methylphenyl)sulfonylamino]-6-(ethyloxycarbonylmethyloxy)quinoline (Zinquin), N,N′,N′-Tetrakis (2-pyridylmethyl)ethylenediamine (TPEN), 3-(4,5-dimethlythiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), N-acetyl-L-cysteine (NAC), MAP kinase inhibitors SB203580, SP600125, and PD98059 were purchased from Sigma Chemical Corporation (St. Louis, MO). 6-carboxy-2’,7’-dichlorodihydrofluoroscein diacetate, diacetoxymethyl ester (6-carboxy-DCF-AM) was purchased from Invitrogen (Carlsbad, CA). Murine monoclonal antibody E9 to metallothionein was purchased from DAKO Corporation (Carpinteria, CA). Antibody to HO-1 was purchased from Assay Designs (Ann Arbor, MI ), while murine MoAbs to EGR-1, Nrf2 and β-actin and goat antibody to p38 were obtained from Santa Cruz Biotechnology Inc (Santa Cruz, CA). Rabbit MoAb to phosphorylated p38 MAP kinase was purchased from Cell Signaling Technology (Danvers, MA). Alpha 32P-dCTP and γ-32P-ATP were obtained from Perkin Elmer Life Sciences (Boston, MA). Mitoquinone (mito-Q) was generously provided by Dr. B. Kalyanaraman (Medical College of Wisconsin).
Cell lines
Human leukemia/lymphoma CCRF-CEM cells were obtained from American Type Culture Collection (Manassas, VA) and maintained in culture as previously described [20,21].
Cell proliferation
Cells (0.4 × 106 cells/ml) were plated in complete medium in 1-ml wells. Gallium nitrate, NAC, or both gallium nitrate and NAC were added to the wells. Cell growth was determined after 48 h incubation by counting cells using a hemocytometer.
Caspase-3 assay
Cells were incubated with 0 – 300 µmol/L gallium nitrate in the absence or presence of NAC for 24 h and then analyzed for caspase-3 activity using a fluorescent Apo-One Homogeneous Caspase-3/7 assay (ProMega, Madison WI) according to the manufacturer’s protocol. Cells were assayed in microwell plates (10,000 cells per well) for assay; the fluorescence from each well was measured by spectrofluorometer at excitation wavelength of 485 nm and emission wavelength of 530 nm.
RNA Isolation
Total RNA was extracted from cells using Trizol reagent, as recommended by the manufacturer (Invitrogen, Carlsbad, CA). RNA was treated with RNase-free DNase I to remove any residual genomic DNA and the quality and integrity of the RNA was assessed by agarose gel electrophoresis before being used.
DNA Microarray Analysis
The effect of gallium nitrate on the differential expression of genes related to metal transport and homeostasis was examined by DNA microarray using a GEArray focused DNA microarray, as recommended by the manufacturer. Total RNA (5 µg) isolated from CCRF-CEM cells incubated with or without 250 µmol/L gallium nitrate was used as a template for the synthesis of corresponding biotin-16- dUTP-labeled cDNA probes. Hybridization of the denatured cDNA probes to the microarray membranes and analysis of the chemiluminescent array image was performed as previously described by us [21]. The signal intensity from each gene was normalized to the signal intensity of β-actin on the same membrane.
cDNA Probe and Northern Blotting
MT2A mRNA expression in cells was detected by Northern blotting using a 32P-labeled cDNA probe as previously described [21]. Membranes were stripped and reprobed with a 32P-labeled β-actin probe to confirm equal loading of RNA on the gel. Autoradiography of the membranes was done using BioMax X-ray film (Eastman Kodak, Rochester, NY).
Preparation of Nuclear Extracts
Nuclear extracts from CCRF-CEM cells were prepared as described by Martin et.al [24]. Cells (107) were lysed in 20 mmol/L HEPES pH 7.9, 0.15 mmol/L EDTA, 0.015 mmol/L EGTA, 10 mmol/L KCl, buffer containing 1% Nonidet-40, and complete protease inhibitor cocktail (Roche Diagnostics, Indianapolis, IN) and the homogenate was centrifuged at 1000 g for 15 min at 4 °C. The nuclear pellet was resuspended in 10 mmol/L HEPES, pH 7.9, 25% glycerol, 0.1 mol/L NaCl, 0.1 mmol/L EDTA, buffer containing protease inhibitor cocktail. After centrifugation at 1000 g for 15 min at 4 °C, nuclei were resuspended in 200 µl of hypertonic cold buffer containing 10 mmol/L HEPES, pH 7.9, 25% glycerol, 0.4 mol/L NaCl, 0.1 mmol/L EDTA buffer with protease inhibitor cocktail and incubated for 30 min at 4 °C. Nuclear debris was removed by centrifugation of the sample at 9000 g for 15 min at 4 °C and the supernatant was analyzed for Nrf2 by immunoblotting.
Western Blotting
Cellular metallothionein and nuclear Nrf2 proteins were detected by Western blotting as previously described [21]. For detection of other proteins, cells were harvested, lysed in 50 mol/L Tris pH 7.4, 150 mmol/L NaCl, 1% deoxycholate, 1% Triton X-100, 0.1% SDS buffer and centrifuged to remove cellular debris. The protein content of supernatants was measured by bicinchoninic protein assay (Pierce, Rockford, IL). Samples were resolved by SDS-PAGE as described by Laemmli [25] and transferred onto a polyvinylidene difluoride membrane using a Transblot system (Bio-Rad, Richmond, CA). Membranes were incubated with specific primary antibody to HO-1, metallothionein, p38, phosphorylated p38, Nrf2, or β-actin (1:500 dilution). Washed membranes were incubated in appropriate secondary antibody conjugated to horseradish peroxidase and then immersed in an enhanced chemiluminescence detection solution (Amersham, Arlington Heights, IL) and exposed to XAR-5 film for autoradiography.
Measurement of GSH and GSSG levels
Reduced glutathione (GSH) and oxidized glutathione (GSSG) levels in cells treated with gallium nitrate for 1 – 6 h were measured using a glutathione assay kit from Cayman Chemicals (Ann Arbor, MI), according to the manufacturer’s instructions. Briefly, CCRF-CEM cells that had been incubated with gallium nitrate were washed with ice-cold PBS and then homogenized in cold phosphate buffer, pH 7.0, containing 1 mmol/L EDTA and deproteinated with 10% metaphosphoric acid and 0.2 mol/L triethanolamine. GSH was measured after the deproteination step. For GSSG measurement, deproteinated samples were further treated with 0.1 mol/L 2-vinylpyridine and mixed with assay cocktail containing NADP+, glucose-6-phosphate, glutathione reductase, glucose-6-phosphate dehydrogenase, and DTNB (5,5’ – dithiobis-2-nitrobenzoic acid) in MES buffer (0.2 mol/L 2-(N-morpholino)ethanesulphonic acid, 0.05 mol/L phosphate, and 2 mmol/L EDTA, pH 6.0), and then the mixtures were incubated at room temperature in the dark for 30 min. The absorbance was measured at 405 nm by spectrophotometer. Standard curves were generated using known concentrations of GSH or GSSG and were used to calculate the concentration of GSH and GSSG in the samples. Data were expressed as a GSH/GSSG ratio.
Measurement of reactive oxygen species (ROS) in cells
Intracellular ROS was measured as described [26]. CCRF-CEM cells (106 cells/mL) were first incubated for 1 h at 37°C with 10 µmol/L 6-carboxy-DCF-AM in tissue culture flasks to load them with this agent. Gallium nitrate (100 or 300 µM) was then added to each flask. In separate flasks, 10 µM mito-Q, a mitochondria-targeted antioxidant [27], was added to cells incubated with 300 µM gallium nitrate. After 1–4 h of incubation, 1 mL of cell suspension (106 cells) was removed and centrifuged. Cell pellets were resuspended in 400 µL PBS and transferred to 96-well assay plates. DCF fluorescence in each well was measured by spectrofluorometer using an excitation wavelength of 495 nm and an emission wavelength of 525 nm. Nonspecific (background) fluorescence values obtained in wells containing PBS only (without cells) were subtracted from the fluorescence values obtained from wells containing cells.
Measurement of intracellular labile zinc
Labile intracellular zinc was examined using Zinquin, a fluoroprobe which fluoresces upon binding to zinc [28,29]. Cells were plated in poly-L-lysine-coated 12-well plates (1.5 × 106cells/well) in 2 ml culture medium, and incubated for 16 h with 100 µM zinc sulfate or 100 – 300 µmol/L gallium nitrate. In additional wells, 50 µmol/L TPEN was added to cells that had been exposed to 300 µM gallium nitrate for 16 h and the incubation was continued for an additional 90 min. Cells were then washed with HBSS and incubated for 40 min at 37°C in 1 ml of HBSS containing 25 µmol/L Zinquin. Cells were washed three times with HBSS to remove extracellular Zinquin and maintained in HBSS. Zinquin fluorescence in cells was monitored by fluorescence microscopy at excitation and emission wavelengths of 365 nm and 420 nm, respectively. In separate experiments, the direct interaction of Zinquin with zinc and gallium was examined by incubating solutions of Zinquin (3 µmol/L) with zinc sulfate (0 – 3 µmol/L) or gallium nitrate (0, 3, 30, and 300 µmol/L) in a total volume of 100 µL in 96-well plates for 40 min at room temperature protected from light. The fluorescence in each well was measured by spectrofluorometer.
Results
Effect of gallium nitrate on the expression of metal-related genes in CCRF-CEM human lymphoma cells
Previously, we showed that 250 µmol/L gallium nitrate inhibits the growth of CCRF-CEM cells by 50% over 48 h of incubation [20]. In the present study therefore, cells were incubated with or without 250 µmol/L gallium nitrate for 24 h and analyzed for differential expression of metal-related genes using the GEArray Q series human metal transport and homeostasis gene array. The 96 genes represented on this array encode for proteins involved in the transport, metabolism, and storage or sequestration of iron, copper, zinc, and selenium. Included in this array are antioxidant enzymes whose prosthetic groups contain these metals and other metal-responsive stress proteins and transcription factors. cDNA probes prepared from total RNA extracted from CCRF-CEM cells incubated with or without gallium nitrate were each hybridized to DNA array membranes. The autoradiographs of these membranes are shown in Figure 1A and B. The intensity of the hybridization signal on each membrane reflects the expression level of different genes in the cells. Hybridization signals were quantified and normalized to the intensity of the β-actin gene signal in the respective membrane; the level of normalized gene expression in gallium-treated cells (Figure 1B) was compared to that of control cells incubated without gallium nitrate (Figure 1A) and was calculated as a gene expression ratio. Table 1 lists the differentially expressed genes in CCRF-CEM cells incubated without or with gallium nitrate. A 2-fold difference in gene expression was used as a threshold level to identify differentially expressed genes. Based on this criterion, 8 genes were found to be upregulated and 18 genes were downregulated following incubation of cells with gallium nitrate for 24 h (Table 1). Consistent with our recent report [21], an increase in MT2A gene expression was noted in cells exposed to gallium nitrate. Although an increase in metallothionein-3 (MT-3) in gallium-treated cells was also found on microarray analysis (Figure 1B and Table 1), this actually represents an increase in MT2A and not MT-3 since PCR analysis using stringent primer pairs previously revealed that only MT2A is expressed in CCRF-CEM cells [21]. MT2A and MT-3 share 65% nucleotide sequence homology which may explain the increased hybridization signal at the MT-3 position on the membrane [30]. Considering that MT-3 expression in gallium-treated cells in the microarray actually reflects MT2A, it can be concluded that MT2A expression in gallium-treated cells is increased by approximately 9-fold rather than by 2.8-fold (Table 1).
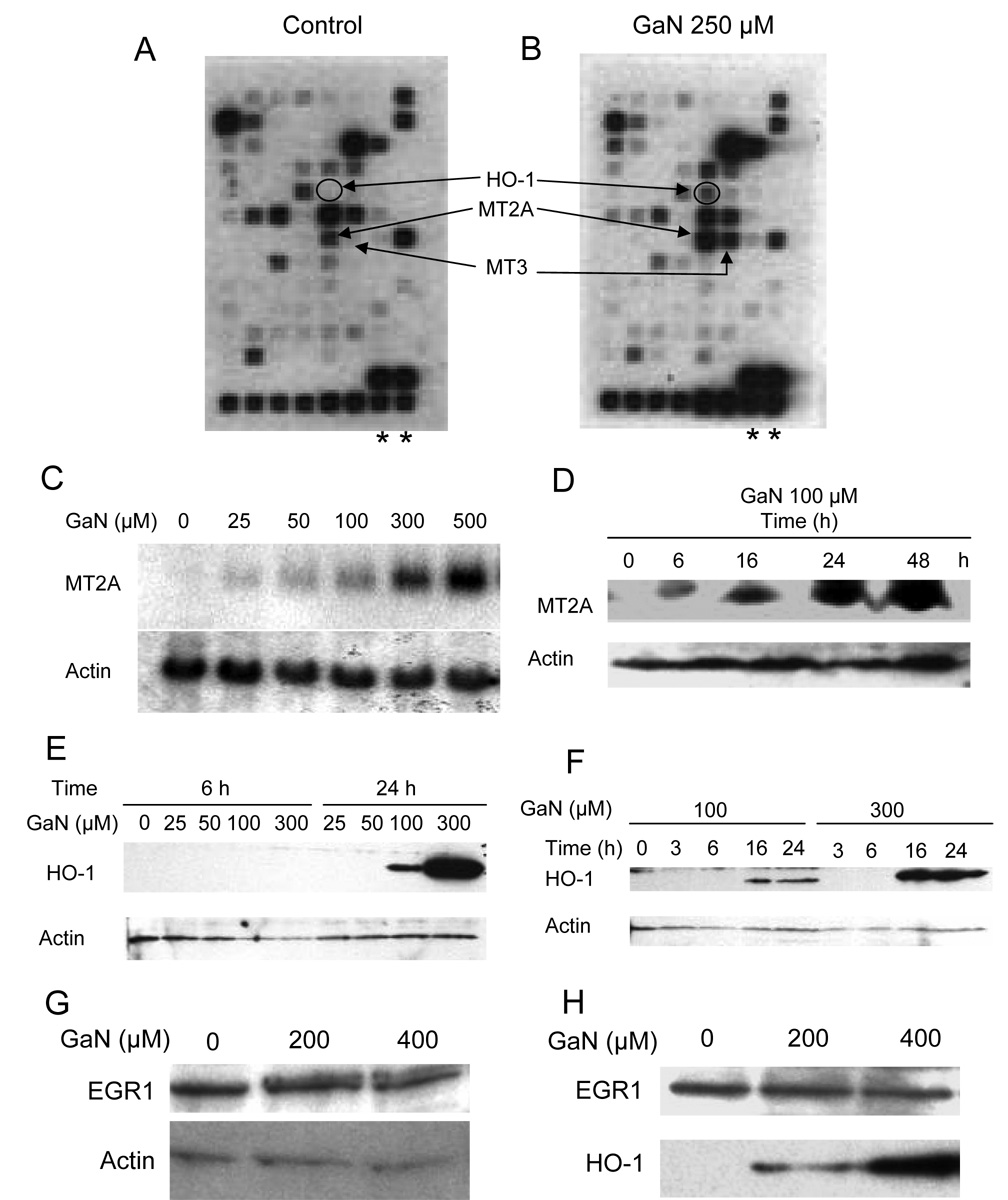
Figure 1. Gallium nitrate induces the expression of metallothionein and heme oxygenase-1 in CCRF-CEM cells.
A and B. DNA arrays showing induction of gene expression with gallium nitrate (GaN). Biotinylated cDNA probes prepared from CCRF-CEM cells incubated without (Control, A) or with 250 µmol/L gallium nitrate (B) for 24 h were hybridized to human metal transport and homeostasis gene array membranes and hybridization signals were detected as detailed under Methods. The positions of the HO-1 and metallothionein genes are shown. Asterisks mark the location of the beta-actin gene.
C and D. Verification that gallium nitrate increases metallothionein-2A (MT2A) expression. C. CCRF-CEM cells were incubated with increasing concentrations of gallium nitrate for 24 h and analyzed for the MT2A mRNA levels by Northern blotting. D. Time-dependent induction of MT2A expression by gallium nitrate. CCRF-CEM cells were incubated with 100 µmol/L gallium nitrate for the times shown and analyzed for MT2A protein levels by Western blotting.
E and F. Verification that gallium nitrate increases heme oxygenase-1 (HO-1) expression. E. Concentration-dependent upregulation of HO-1 expression by gallium nitrate. CCRF cells were incubated with increasing concentrations of gallium nitrate for 6 and 24 h and analyzed for HO-1 protein content by Western blotting; F. Time-dependent upregulation of HO-1 expression by gallium nitrate. CCRF-CEM cells were incubated with 100 or 300 µmol/L gallium nitrate for the times shown and analyzed for HO-1 protein content by Western blotting.
G and H. EGR1 protein expression is not increased by gallium nitrate. G. Western blot. Cells were incubated with gallium nitrate for 24 h at the concentrations shown and analyzed by Western blotting for EGR1. H. Western blot showing that HO-1 but not EGR1 is increased by gallium nitrate. Cells were incubated with gallium nitrate for 24 h. The membrane was immunoblotted sequentially with antibodies against EGR1 and HO-1.
Table 1.
Differentially expressed genes in CCRF-CEM incubated with or without gallium nitrate
| Gene | Gene symbol | Relative change in gene expression | |
|---|---|---|---|
| Gallium nitrate | |||
| 0 µM | 250 µM | ||
| Aconitase-1 | Aco1 | 1 | 2.6 |
| DnaJ (Hsp40) homolog, subfamily A, member1 | DNAJA1 | 1 | 4.2 |
| Early growth response 1 | EGR1 | 1 | 19 |
| Ferritin, light polypeptide | FTL | 1 | 3 |
| Heme oxygenase-1 | HMOX1 | 1 | 11 |
| Metallothionein-2A | MT2A | 1 | 2.8 |
| Metallothionein-3 | MT3 | 1 | 9 |
| Metal-regulatory transcription factor 1 | MTF1 | 1 | 5.7 |
| ATP-binding cassette, sub-family B, member 7 | ABCB7 | 1 | 0.48 |
| Beta-2-microglobulin | B2M | 1 | 0.19 |
| Calreticulin | CALR | 1 | 0.19 |
| Catalase | CAT | 1 | 0.36 |
| Glutathione peroxidase 4 | GPX4 | 1 | 0.21 |
| Hypoxia-inducible factor1, alpha subunit | HIF1A | 1 | 0.26 |
| Heat shock 70 kDa protein 5 | HSPA5 | 1 | 0.34 |
| Heat shock 70 kDa protein 9B | HSPA9B | 1 | 0.2 |
| Heat shock 27 kDa protein 1 | HSPB1 | 1 | 0.22 |
| Heat shock 60 kDa protein 1 | HSPD1 | 1 | 0.43 |
| V-myc myelocytomatosis viral oncogene homolog | c-MYC | 1 | 0.36 |
| Popeye domain containing 2 | POPDC2 | 1 | 0.33 |
| Peroxiredoxin 4 | PRDX4 | 1 | 0.27 |
| Solute carrier family 39 (zinc transporter) member 6 | SLC39A6 | 1 | 0.15 |
| Solute carrier family 40 (iron-regulated transporter) member 6 | SLC40A1 | 1 | 0.33 |
| Superoxide dismutase 1 | SOD1 | 1 | 0.44 |
| Superoxide dismutase 2 | SOD2 | 1 | 0.27 |
| Transferrin receptor (p90, CD71) | TFRC | 1 | 0.1 |
Cells were analyzed after 24 h of incubation without or with gallium nitrate. Genes with at least a 2-fold difference (>2 or <0.5) in expression after gallium treatment were considered significant.
As shown in Table 1, the other genes differentially expressed following exposure of cells to gallium nitrate include genes involved in cellular antioxidant function, stress response, and RNA transcription. Of the antioxidant genes, the expression of HO-1 was upregulated 11-fold whereas the expression of GPX4, PRDX4, SOD1, and SOD2 was downregulated. In addition, four stress protein genes (heat shock 70 kDa protein 5, heat shock 70 kDa protein 9B, heat shock 27 kDa protein 1, and heat shock 60 kDa protein 1) were downregulated. One of the transcription factor genes, the gene for early growth response 1 (EGR1), was upregulated significantly, while the genes for hypoxia-inducible factor-1 and V-myc myelocytomatomatosis viral oncogene homolog (c-Myc) were downregulated. Collectively, these findings suggest that gallium nitrate affects not only the expression of genes involved in the regulation of cellular zinc homeostasis (MT2A and MTF-1) but also genes involved in the cellular response to stress.
Confirmation that gallium nitrate induces an increase MT2A and HO-1 gene expression
Since the cDNA microarray studies suggested that the greatest level of gene induction by gallium nitrate occurred in the metallothionein, HO-1, and EGR1 genes, further studies were conducted to confirm this observation. As shown in Figure 1C, cells incubated with increasing concentrations of gallium nitrate displayed an increase in the expression of MT2A mRNA in a concentration-dependent manner. With 100 µmol/L gallium nitrate, an increase in MT2A protein was detected as early as 6 h of incubation; this increased further with longer incubation times (Figure 1D). The gallium-induced increase in cellular HO-1 protein levels is shown in Figures 1E and F. HO-1 expression was detected after a 24 h incubation of cells with 100 µmol/L and 300 µmol/L gallium nitrate, but not at lower gallium concentrations or after 6 h of incubation (Figure 1E). Further time-course analysis showed that HO-1 expression could be detected after 16 h of incubation with both 100 and 300 µmol/L gallium nitrate (Figure 1F). It should be noted that neither 100 µmol/L nor 300 µmol/L gallium nitrate were growth-inhibitory at 24 h; hence, the expression of both MT2A and HO-1 was not due to cytotoxic changes in cells.
In contrast to the positive effect of gallium nitrate on the expression of MT2A and HO-1 proteins, cells treated with gallium nitrate did not display an increase in EGR1 protein level (Figure 1G). This conclusion was further supported by an additional experiment in which the membrane from the Western blot analysis was sequentially immunoblotted with anti-EGR1 and HO-1 antibodies. As shown in Figure 1H, cells exposed to gallium nitrate for 24 h failed to show an increase in EGR1 protein above baseline levels whereas HO-1 protein was increased by gallium in a concentration-dependent manner. These results suggest that although EGR1 mRNA appears to be upregulated by gallium nitrate on DNA microarray analysis, this does not translate into increased EGR1 protein expression. Our subsequent studies therefore focused on gaining an understanding of the basis for the induction of MT2A and HO-1 expression by gallium nitrate.
Gallium nitrate increases reactive oxygen species (ROS) in cells
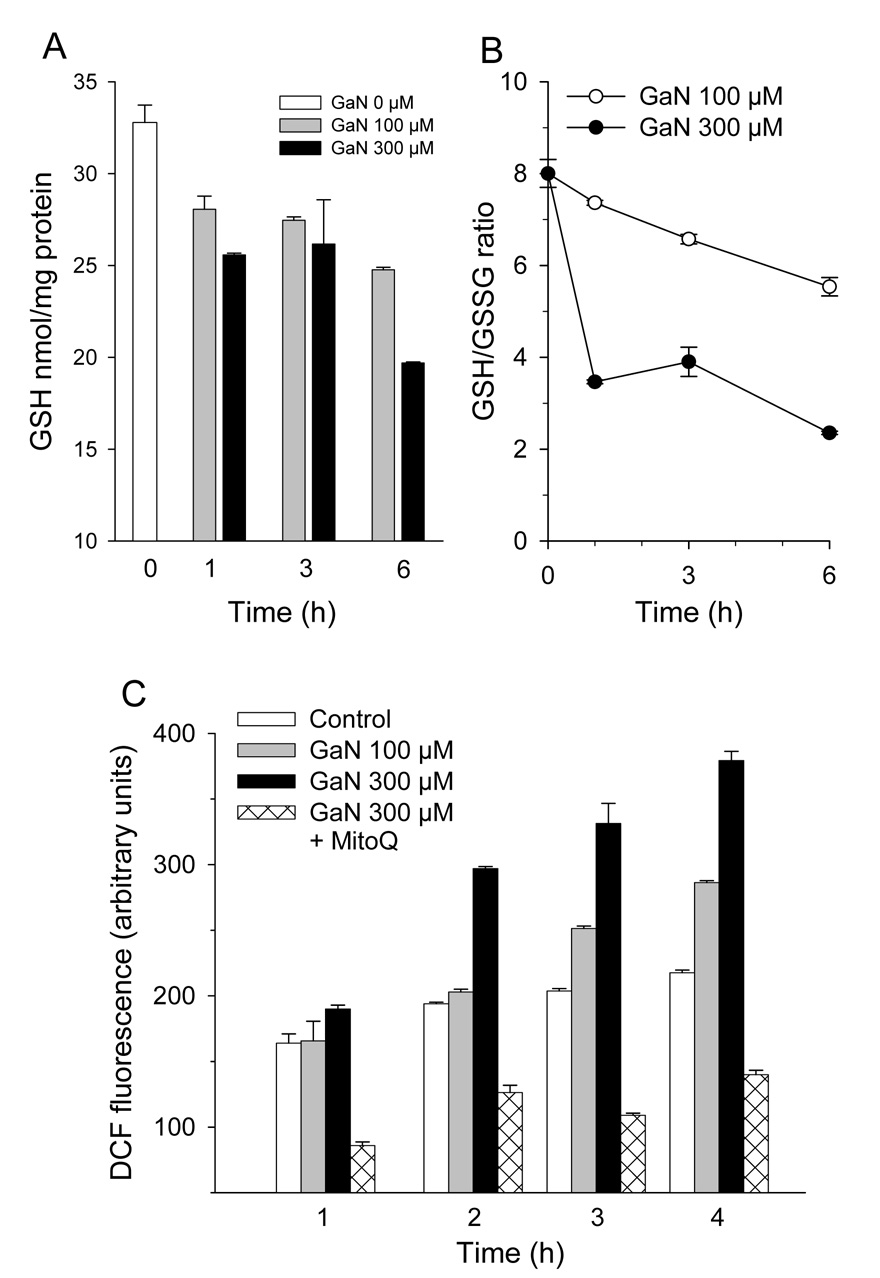
Previous studies have shown that oxidative stress induces the expression of metallothionein and HO-1 [23,31]. The upregulation of MT2A and HO-1 expression coupled with the downregulation of GPX4, PRDX4, SOD1 and SOD2 in CCRF-CEM cells incubated with gallium nitrate suggested that these cells might contain elevated levels of ROS. To examine this possibility, cells were incubated with gallium nitrate for various periods of time and analyzed for changes in intracellular GSH and GSSG. As shown in Figure 2A, consistent with an increase in intracellular ROS, cells exposed to gallium nitrate displayed a decrease in intracellular GSH. This decrease in GSH was noted within 1 h of incubation; by 6 h of incubation, GSH levels had decreased by 25% and 40% % in cells incubated with 100 µmol/L and 300 µmol/L gallium nitrate, respectively. Consistent with an increase in intracellular ROS, gallium-treated cells displayed a decrease in their intracellular GSH/GSSG ratio indicating that GSH had been oxidized to GSSG. After 6 h of incubation with 300 µmol/L gallium nitrate, the GSH/GSSG ratio in cells decreased by approximately 70% (Figure 2B).
Figure 2. Gallium nitrate induces oxidative stress in cells.
A and B. Effect of gallium nitrate on cellular GSH levels and GSH/GSSG ratio. CCRF-CEM cells were treated with 100 or 300 µmol/L gallium nitrate for the times shown and then analyzed for GSH and GSSG levels as described under Methods. Data represents means ±S.E. (n = 3). C. Gallium nitrate increases intracellular ROS. ROS production was detected in intact CCRF-CEM cells by DCF fluorescence. Cells were loaded with 10 µmol/L 6-carboxy-DCF-AM for 1 h, incubated with gallium nitrate for the specified times, and analyzed for DCF fluorescence by spectrofluorimeter as described under Methods. Values shown are means +/− S.E. of a representative experiment performed in triplicate.
The generation of oxidative stress by gallium nitrate was also examined by a fluorescent method based on the intracellular oxidation of nonfluorescent 6-carboxy-DCF-AM to fluorescent DCF by intracellular ROS. Cells loaded with 6-carboxy-DCF-AM were incubated with gallium nitrate for 1 – 4 h and then analyzed for DCF fluorescence by spectrofluorimetry. As shown in Figure 2C, control (untreated) cells had an intrinsic amount of DCF fluorescence that was consistent with a basal level of ROS production. Cells incubated with 300 µmol/L gallium nitrate displayed a marked increase in intracellular DCF fluorescence above control cells that was detected after 1 h and that increased progressively with the time of incubation. Differences in DCF fluorescence between control cells and cells incubated with 300 µmol/L gallium nitrate were highly significant (P = <0.001, 0.012, and 0.002 after 2, 3, and 4 h of incubation respectively, by t-test). Cells incubated with 100 µmol/L gallium nitrate also displayed greater DCF fluorescence than control cells but at a lower level than cells incubated with 300 µmol/L gallium nitrate (Figure 2C). The presence of the mitochondria-targeted antioxidant mito-Q produced a decrease in the level of endogenous ROS production and blocked the gallium-induced increase in ROS production (Figure 2C). Collectively, the experiments in Figure 2 indicate that gallium nitrate increases intracellular ROS and that the level of ROS production increases with gallium concentration and the duration of exposure of cells to this drug. Blockade of ROS by mito-Q suggests that the ROS generated in gallium-treated cells originates from the mitochondrion.
Gallium-induced increase in MT2A and HO-1 results from an increase in ROS production
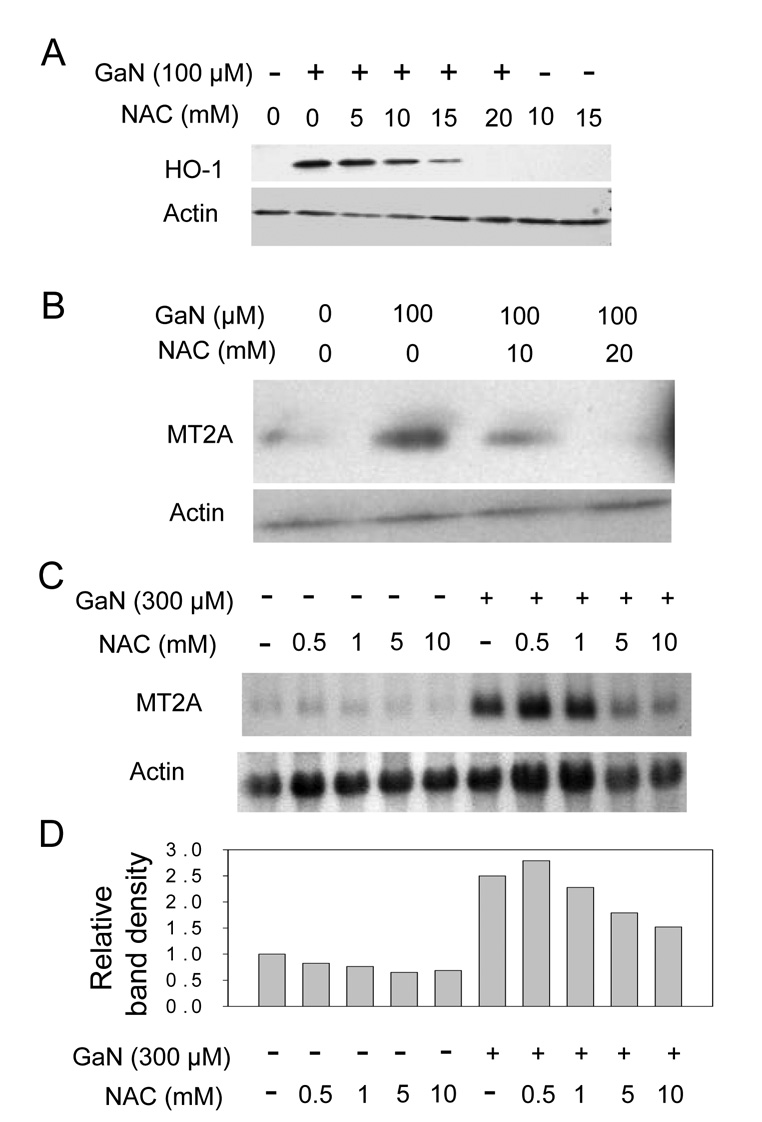
Since the above studies showed that cells incubated with gallium nitrate had an increase in intracellular ROS at time-points that preceded the increase in HO-1 and MT2A, we hypothesized that the upregulation of these proteins was triggered by gallium-induced oxidative stress. To test this hypothesis, cells were incubated with gallium nitrate in the presence or absence of N-acetylcysteine (NAC), a glutathione precursor and free radical scavenger, and then analyzed for HO-1 and MT2A levels. These experiments revealed that NAC inhibited the increase in HO-1 and MT2A in cells exposed to gallium nitrate. As shown in Figure 3A and B, NAC diminished gallium-induced HO-1 and MT2A protein expression in a dose-dependent manner. The effect of NAC on MT2A mRNA expression was also evaluated in cells incubated with 300 µmol/L gallium nitrate. With this higher concentration of gallium nitrate, the induction of MT2A expression is significantly greater than with 100 µM gallium nitrate (as shown in Figure 1C).
Figure 3. Effect of NAC on gallium-induced upregulation of MT2A and HO-1.
A – D. NAC blocks the increase in MT2A and HO-1 expression by gallium nitrate. CCRF-CEM cells were preincubated for 2 h with or without NAC and then incubated with or without gallium nitrate for 16 h at the concentrations shown. In coincubations, cells were exposed to both gallium nitrate and NAC simultaneously for 16 h.
A and B. Western blots showing HO-1 (panel A) and MT2A (panel B) protein levels in cells. C. Northern blot showing steady-state MT2A mRNA levels in cells. D. Quantification of the MT2A band densities from the Northern blot in Figure 3C normalized to the corresponding β-actin level.
Even under these conditions of stronger induction, MT2A mRNA expression was suppressed, although not completely blocked, by NAC. As shown in Figure 3 C and D, as expected, MT2A mRNA levels were increased in cells incubated with 300 µmol/L gallium nitrate; in the presence of 1, 5, and 10 mmol/L NAC, MT2A mRNA levels in gallium-treated cells were diminished by 13%, 47%, and 67% respectively compared with cells incubated with gallium nitrate alone. Taken together, the above data suggest that the gallium-induced increase in HO-1 and MT2A is the result of intracellular ROS production by gallium nitrate.
Inhibition of gallium-induced ROS production modulates the cytotoxicity of gallium nitrate
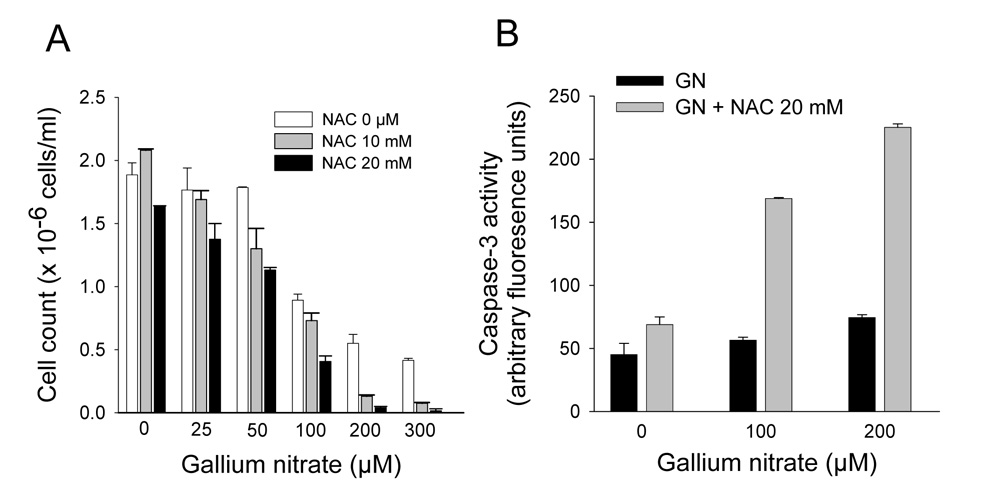
In prior studies, we showed that a zinc-induced increase in metallothionein conferred partial protection against the growth-inhibitory effects of gallium nitrate in CCRF-CEM cells and that this protection was lost when metallothionein levels decreased to baseline [21]. Others have shown that HO-1 may also protect cells against a variety of stresses [31]. Hence, we considered whether decreasing cellular MT2A and HO-1 levels by blocking ROS production would impact on the cytotoxicity of gallium nitrate. To examine this, cells were incubated with gallium nitrate in the presence or absence of 10 or 20 mmol/L NAC and the effects on cell growth were determined after 48 h of incubation. As shown in Figure 4A, gallium nitrate alone inhibited the growth of CCRF-CEM cells in a dose-dependent manner; however, the presence of NAC resulted in further inhibition of cell growth. With 50 µmol/L gallium nitrate cell proliferation was inhibited by 9%, however, in the presence of 20 mM NAC, it was inhibited by 44%. With 100 µmol/L and 200 µmol/L gallium nitrate cell proliferation was inhibited by 55% and 70%, respectively. However, when 20 mmol/L NAC was added to cells incubated with gallium nitrate, the corresponding inhibition of cell growth was 80% and 98%, respectively (Figure 4A).
Figure 4. Effect of NAC on the cytotoxicity of gallium nitrate.
A. NAC increases the antiproliferative effects of gallium nitrate cells. CCRF-CEM cells were pretreated with 10 or 20 mol/L of NAC for 2 h and then incubated with increasing concentrations of gallium nitrate plus NAC for 48 h. Cell growth was determined by counting the number of cells using a hemocytometer. B. NAC increases gallium-induced activation of caspase-3. CCRF-CEM cells were preincubated with 20 mmol/L NAC for 2 h and then incubated with increasing concentrations of gallium nitrate plus NAC for 24 h. Caspase-3 activity in intact cells was assayed as described under Methods. Values shown represent the mean ± S.E. (n = 3).
The effect of NAC on gallium-induced cell death was examined by measuring caspase-3 activity in cells. Consistent with our recent report [32], 100 µM gallium nitrate produced only a minor increase in caspase-3 activity when compared with control cells incubated without gallium nitrate. With the addition of NAC to the incubation there was an approximately 3-fold increase in caspase-3 activity (Figure 4B). Collectively, these studies demonstrate that the inhibition of ROS production by NAC results in an increase in gallium-induced cell death in CCRF-CEM cells and they suggest that the upregulation of MT2A or HO-1 or both represents a cellular response that provides partial protection against the cytotoxicity of gallium nitrate.
Gallium induced upregulation of MT2A involves changes in intracellular zinc homeostasis
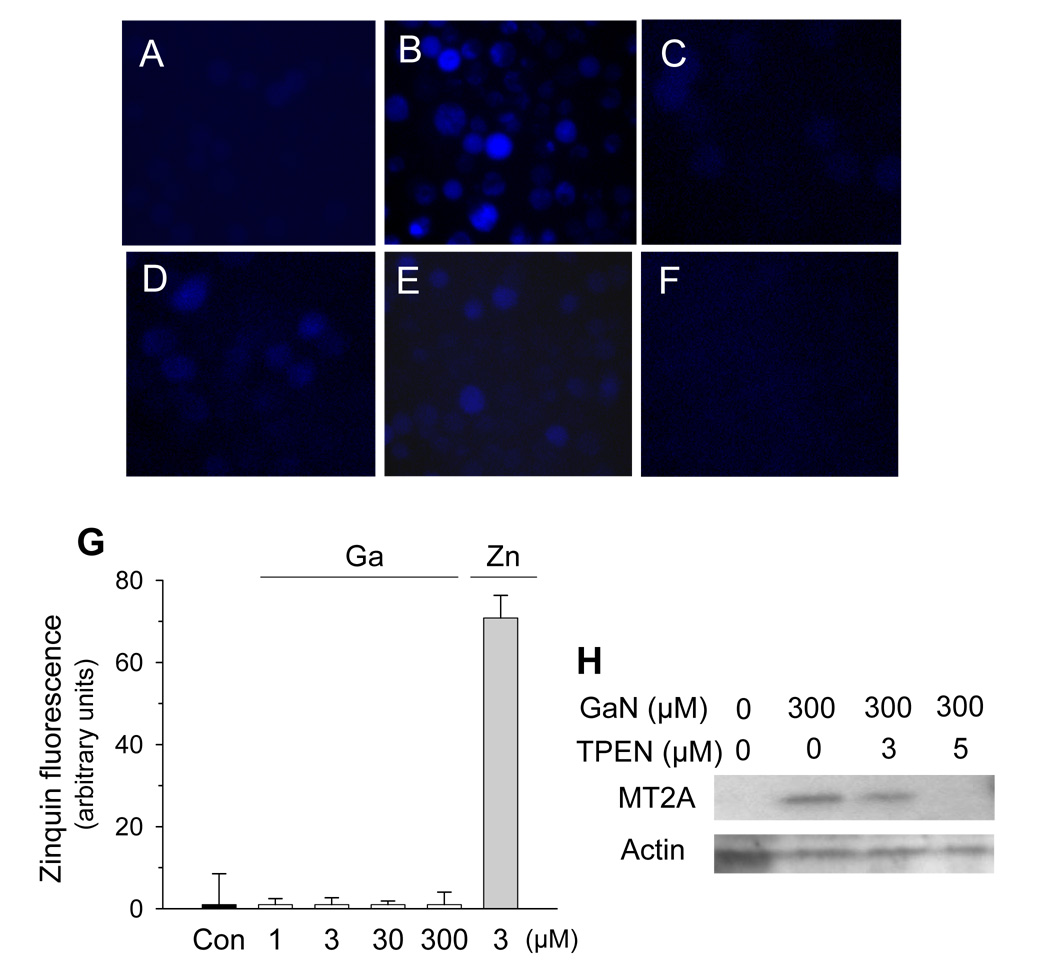
It is well known that an increase in cellular zinc content may upregulate metallothionein gene expression by increasing the binding of MTF-1, a zinc finger transcription factor, to MREs present on the promoter region of the metallothionein gene [33,34]. To determine whether gallium produced changes in cellular zinc homeostasis, cells exposed to zinc sulfate or gallium nitrate were allowed to incorporate Zinquin, a zinc-specific fluorophore that fluoresces upon binding to zinc, and then examined by fluorescence microscopy. As shown in Figure 5A, no fluorescence was noted in control cells that had been incubated without additives. As expected, cells incubated with 100 µmol/L zinc sulfate displayed Zinquin fluorescence (Figure 5B). Cells incubated with gallium nitrate also displayed Zinquin fluorescence, albeit at a lower intensity than those exposed to zinc sulfate. As shown in Figure 5 C, D, and E, Zinquin fluorescence increased with the concentration of gallium and was greatest with 300 µmol/L gallium nitrate (Figure 5E). This fluorescence in gallium-treated cells was completely quenched by the zinc-specific chelator TPEN (Figure 5F).
Figure 5. Gallium-induced increase in MT2A expression involves changes in cellular zinc homeostasis.
A – F. Zinquin fluorescence. CCRF-CEM cells were incubated for 16 h without additives (A), or with 100 µM ZnSO4 (B), 100 µmol/L gallium nitrate (C), 200 µmol/L gallium nitrate (D), or 300 µmol/L gallium nitrate (E and F). Cells were then allowed to take up Zinquin for 40 min, as described under Methods. In panel F, prior to the addition of Zinquin, 50 µM TPEN was added for 90 minutes to cells that had incubated with 300 µmol/L of gallium nitrate. Cells were examined by fluorescence microscope. G. Zinquin fluorescence is produced by zinc and not by gallium. Gallium nitrate or zinc sulfate solutions at the concentrations shown were incubated with 3 µmol/L Zinquin for 40 min and then analyzed for fluorescence by spectrofluorimetry. H. Gallium-induced MT2A protein expression is reduced by TPEN. CCRF cells were incubated for 16 h with 300 µmol/L gallium nitrate, TPEN, or both agents in the combinations shown. MT2A and β-actin levels were measured by Western blotting.
To exclude the possibility that Zinquin fluorescence in gallium-treated cells was the result of a direct interaction of gallium with Zinquin, gallium nitrate and zinc sulfate solutions were incubated with a Zinquin solution under cell-free conditions and examined for fluorescence. As shown in Figure 5G, the mixture of Zinquin and gallium nitrate over a wide range of gallium concentrations did not produce fluorescence above background (control). In contrast, zinc sulfate produced strong fluorescence when incubated with Zinquin (Figure 5G).
The absence of fluorescence in solutions containing gallium nitrate and Zinquin suggests strongly that this fluoroprobe does not interact directly with gallium and that the fluorescence in cells incubated with gallium nitrate shown in Figure 5 C – E was related to a change in intracellular zinc status. Furthermore, the ability of the zinc-specific chelator TPEN to quench Zinquin fluorescence in gallium-treated cells (Figure 5F) supports this notion and suggests that this fluorescence was due to an increase in a chelator- accessible intracellular zinc pool. The possibility that the quenching of Zinquin fluorescence by TPEN in gallium-treated cells was the result of gallium chelation by TPEN was excluded by separate experiments which demonstrated that gallium is not chelated by TPEN (data not shown). In the next experiment shown in Figure 5H, we examined whether the gallium-induced increase in MT2A was related to a change in intracellular zinc homeostasis. Cells were incubated with gallium nitrate in the presence or absence of TPEN and then analyzed for MT2A protein expression. In this experiment, cells were first preincubated with 3 – 5 µM TPEN for 2 h followed by coincubation with both gallium nitrate and TPEN for 16 h. As expected, gallium nitrate alone increased MT2A expression (Figure 5H). However, this increase in MT2A expression was diminished when cells were coincubated with gallium nitrate and TPEN (Figure 5H). Collectively, the experiments shown in Figure 5 suggest that the gallium-induced increase in MT2A expression is mediated, at least in part, by an increase in a labile intracellular zinc pool.
Gallium-induced upregulation of HO-1 involves MAP kinase p38 and the activation of transcription factor Nrf2
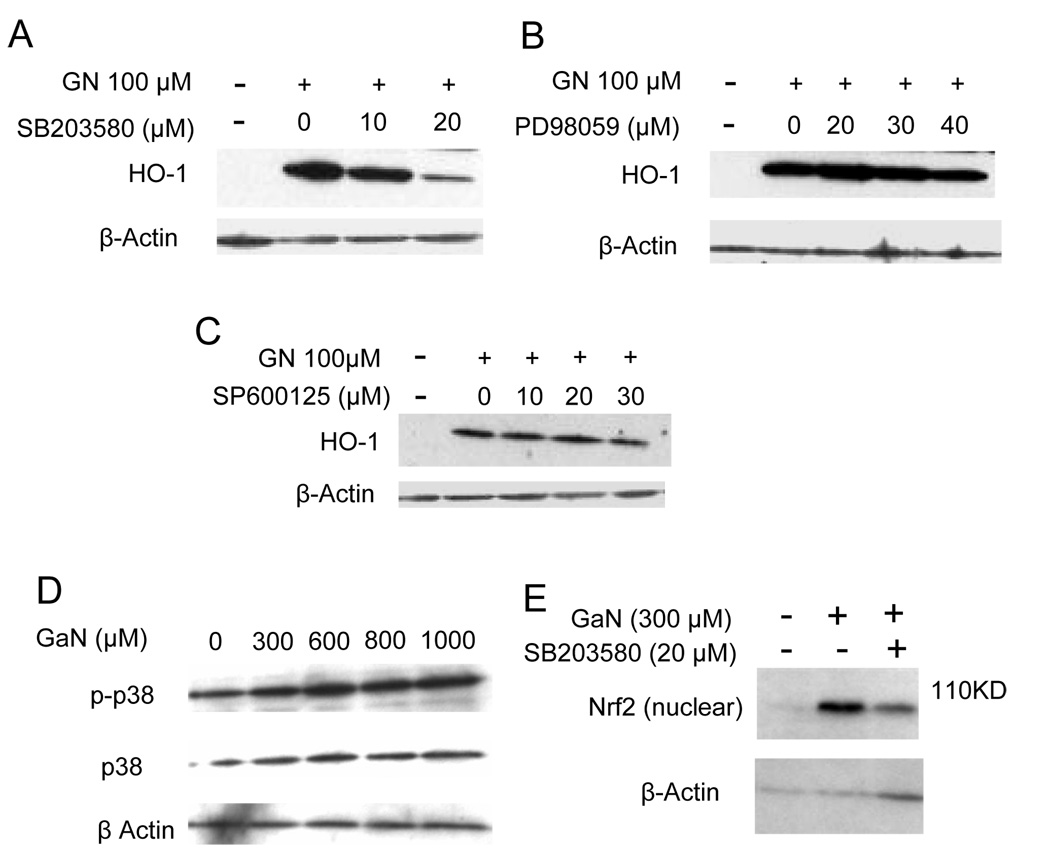
Prior studies by a number of investigators have demonstrated a role for signaling pathways in the regulation of HO-1 gene transcription [31]. MAP kinases consist of the extracellular signal-regulated kinases (ERKs) involved in the regulation of cell division, c-Jun amino-terminal kinases (JNKs) involved in transcriptional regulation, and p38 MAP kinase involved in the response to inflammatory cytokines and stress; all have been shown to play roles in HO-1 gene expression [31]. MAP kinases can be activated by oxidative stress [35] and since gallium nitrate increased intracellular ROS, we examined whether these signaling pathways were involved in the upregulation of HO-1 by gallium nitrate. CCRF-CEM cells were treated with a p38 MAP kinase inhibitor (SB203580), an ERK inhibitor (PD98059), or a JNK inhibitor (SP600125) and then incubated with gallium nitrate and analyzed for HO-1 protein expression. As shown in Figure 6A, gallium nitrate alone increased cellular HO-1 expression; however, this was slightly reduced by 10 µM SB203580 and significantly diminished by 20 µM SB203580. In contrast, PD98059 and SP600125 did not have any effect on the gallium-induced increase in HO-1 expression (Figure 6 B and C). To confirm the involvement of p38 MAP kinase in gallium-induced HO-1 expression, cells were examined for changes in p38 phosphorylation. As shown in Figure 6D, cells exposed to gallium nitrate displayed an increase in phosphorylated p38 (p-p38) consistent with the activation of this pathway by gallium.
Figure 6. Involvement of p38 MAP kinase and Nrf2 in the induction of HO-1 gene expression by gallium nitrate.
A – C. Effects of MAP kinase inhibitors on HO-1 expression. CCRF-CEM cells were incubated for 16 h with 100 µmol/L gallium nitrate in the absence or presence of: A. p38 kinase inhibitor SB203580; B. ERK inhibitor PD98059; and C. JNK inhibitor SP600125 at the concentrations shown. Cellular HO-1 level was determined by Western blotting. D. Gallium nitrate increases the phosphorylation of p38 MAP kinase. Cells were incubated for 6 h with increasing concentrations of gallium nitrate. Cell lysates were analyzed by Western blotting for unphosphorylated (p38) and phosphorylated forms (p-p38) of p38 MAP kinase. E. Gallium nitrate increases Nrf-2 levels in the nucleus. Cells were incubated with or without SB203580 for 2 h. Following this, gallium nitrate was added to cells as shown and the incubation continued for an additional 6 h. Nuclear proteins were extracted as described under Methods and analyzed for Nrf-2 levels by Western blotting.
Studies have indicated that the induction of HO-1 gene expression by MAP kinases is regulated through the activity of Nrf2, a basic leucine zipper transcription factor that interacts with antioxidant response elements (AREs) in the promoter region of the HO-1 gene [31,36]. Under basal conditions, Nrf2 is sequestered in the cytoplasm bound to KEAP-1 (Kelch-like ECH-associating protein 1) which targets it for proteasomal degradation [36]. In the presence of appropriate signals however, Nrf2 translocates from the cytoplasm to the nucleus to bind to ARE sequences resulting in transcription of the HO-1 gene [31,36]. To determine whether Nrf2 plays a role in gallium-induced HO-1 expression, cells were examined for changes in nuclear Nrf2 levels following exposure to gallium nitrate. As shown Figure 6E, a minimal level of nuclear Nrf2 was detected in control cells whereas a marked increase in nuclear Nrf2 was seen in cells incubated with gallium nitrate. The level of nuclear Nrf2 in gallium-treated cells was decreased by SB203580. Collectively, these experiments suggest that exposure of cells to gallium nitrate triggers signaling through the p38 MAP kinase pathway which leads to the activation of Nrf2 and transcription of the HO-1 gene.
Since blockade of ROS by NAC enhanced the antiproliferative effects of gallium nitrate (Figure 4), additional experiments were conducted to examine whether inhibition of HO-1 induction via p38 MAP kinase would impact on gallium-induced cell death. However, an increase in the growth-inhibitory effects of gallium nitrate was not seen when p38 kinase and HO-1 induction were inhibited by SB203580 (data not shown).
Discussion
The present study represents an important continuation of our earlier investigation into the action of gallium on biologic systems. There is very limited information regarding the effects of gallium nitrate on metallothionein gene expression and there are no prior studies of gallium’s impact on HO-1 expression. We now show for the first time that human lymphoma/leukemia CCRF-CEM cells upregulate their levels of MT2A and HO-1 in response to gallium nitrate and that the initiating event in this process appears to be an increase in intracellular ROS that occurs within a short period of incubation of cells with this metal salt and precedes the upregulation of MT2A and HO-1 gene expression. The generation of ROS by gallium nitrate was noted within 1 h of exposure of cells to gallium nitrate and was manifested as a decrease in intracellular GSH and GSH/GSSG ratio and an increase in intracellular DCF fluorescence. Evidence for the important role of ROS in gallium’s effect was provided by the demonstration that the gallium-induced increase in MT2A and HO-1 could be blocked by the antioxidant NAC. Moreover, NAC, by inhibiting ROS production, increased the level of cell growth inhibition and caspase-3 activation by gallium nitrate. These results indicate that ROS production appears to be central to triggering MT2A and HO-1 expression by gallium nitrate. Furthermore, since blockade of ROS produced a downregulation of MT2A and HO-1 expression and enhanced the cytotoxicity of gallium, it is reasonable to conclude that one or both of these proteins can modulate gallium-induced cell death and that their expression is part of a cytoprotective response against this gallium nitrate.
The mechanism by which gallium nitrate produces an increase in MT2A gene expression appears to be complex. In addition to a role for ROS in this process, the upregulation of MT2A by gallium nitrate likely involves an interaction of gallium with zinc pathways. Recently, we showed that incubation of cells with gallium nitrate leads to an increase in the binding of MTF-1 to MREs present on the promoter region of the metallothionein gene, thereby indicating that gallium affects metallothionein gene expression at the level of mRNA transcription [21]. However, it is unlikely that the increase in MTF-1-MRE binding in cells exposed to gallium nitrate is directly facilitated by gallium. Bittel et al have shown that zinc, but not a variety of other metals, can activate MTF-1-MRE binding in vitro [33]. Based on those studies, we considered the possibility that MTF-1 activation in gallium-treated cells involved changes in cellular zinc homeostasis. Using the zinc-specific fluoroprobe Zinquin, we showed that cells incubated with gallium nitrate displayed an increase in intracellular Zinquin fluorescence that could be blocked by TPEN, a zinc specific chelator. Moreover, TPEN diminished the gallium-induced increase in MT2A expression. These results suggest that exposure of cells to gallium nitrate results in an expansion of an intracellular labile zinc pool which could lead to an increase in MTF-1-MRE binding activity and metallothionein expression. The source of zinc contributing to the increased intracellular zinc pool remains to be determined; but, potential mechanisms involved in this process can be considered. One possible explanation is that gallium may increase the cellular uptake of zinc thereby expanding the labile zinc pool. Another explanation is that zinc may be mobilized from endogenous intracellular zinc-metallothionein by gallium-generated ROS. It is likely that the latter process is at play since others have shown that zinc can be released from metallothionein under conditions of cellular oxidative stress [22,37,38]. Regardless of the process involved, our present study suggests that an increase in the intracellular zinc pool contributes to the upregulation of MT2A expression in cells exposed to gallium nitrate. Further studies are planned to elucidate the interaction of gallium with cellular zinc metabolism.
In addition to its function in the intracellular metabolism and storage of divalent metals, primarily zinc, metallothionein protects cells against oxidants and electrophiles [22]. Indeed, metallothionein and HO-1 are among a group of Phase II detoxification enzymes and antioxidant proteins that function as a cellular defense mechanism against oxidative stress [39,40]; the expression of HO-1 in response to ROS is regulated by the interaction of transcription factor Nrf-2 with ARE sequences in the promoter regions of their respective genes [36]. Since MAP kinases are known to be involved in the activation of Nrf-2 [31], we examined whether gallium nitrate was signaling through this pathway. Our results suggest that the induction of HO-1 by gallium nitrate is mediated, at least in part, through p38 MAP kinase with downstream activation of Nrf-2. Evidence for the involvement of this pathway was provided by the demonstration that an inhibitor of this pathway significantly diminished the upregulation of HO-1 by gallium nitrate and reduced the gallium-induced translocation of Nrf-2 to the nucleus.
ROS production is felt play a role in the cytotoxicity of many antineoplastic drugs [41]; however, this does not appear to be the case with gallium nitrate. If ROS production was important in gallium nitrate-induced cell death, we would have expected NAC to protect against rather than enhance gallium-induced apoptosis. Although NAC blocked the induction of both MT2A and HO-1 by gallium nitrate and increased its cytotoxicity, inhibition of HO-1 induction by a p38 MAP kinase inhibitor did not impact on the growth-inhibitory effects of gallium nitrate. While these results may suggest that MT2A rather than HO-1 is the predominant cytoprotective protein against gallium, the evidence for this is indirect; future studies will determine the relative contributions of MT2A and HO-1 in protecting cells against gallium-induced apoptosis. We also appreciate though that our studies utilized a 96-gene focused DNA array to identify genes affected by gallium nitrate. Hence our analysis of gallium-induced genes was limited to those represented in this array. It is possible that there are additional genes that may have been blocked by NAC that could modulate or protect against gallium’s cytotoxicity. Further studies are in progress to elucidate these potential additional mechanisms.
The ability of NAC to increase gallium-induced cell death may have therapeutic relevance. NAC is approved for clinical use as a mucolytic agent in pulmonary disease and for treatment of hepatic damage from acetaminophen overdose [42,43]. That NAC might be used in conjunction with gallium nitrate as a means to enhance its antineoplastic activity is an intriguing possibility that warrants further study. Continued investigation into the mechanisms of action of gallium nitrate will undoubtedly yield additional information regarding the cellular processes affected by this interesting metallodrug.
Acknowledgments
Supported by USPHS RO1 CA109518 (to CRC).
List of Abbreviations
- MT2A
metallothionein-2A
- HO-1
heme oxygenase-1
- ROS
reactive oxygen species
- NAC
N-acetyl-L-cysteine
- MAP
mitogen-activated protein
- DCF
dichlorodihydrofluoroscein
- Mito-Q
mitoquinone
- TPEN
N,N′,N′-Tetrakis (2-pyridylmethyl)ethylenediamine.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Collery P, Keppler B, Madoulet C, Desoize B. Gallium in cancer treatment. Crit Rev. Oncol. Hematol. 2002;42:283–296. doi: 10.1016/s1040-8428(01)00225-6. [DOI] [PubMed] [Google Scholar]
- 2.Chitambar CR. Gallium compounds as antineoplastic agents. Curr. Opin. Oncol. 2004;16:547–552. doi: 10.1097/01.cco.0000142071.22226.d2. [DOI] [PubMed] [Google Scholar]
- 3.Straus DJ. Gallium nitrate in the treatment of lymphoma. Semin. Oncol. 2003;30:25–33. doi: 10.1016/s0093-7754(03)00173-8. [DOI] [PubMed] [Google Scholar]
- 4.Einhorn L. Gallium nitrate in the treatment of bladder cancer. Semin. Oncol. 2003;30:34–41. doi: 10.1016/s0093-7754(03)00174-x. [DOI] [PubMed] [Google Scholar]
- 5.Keller J, Bartolucci A, Carpenter JT, Jr, Feagler J. Phase II evaluation of bolus gallium nitrate in lymphoproliferative disorders: A Southeastern Cancer Study Group trial. Cancer Treat. Rep. 1986;70:1221–1223. [PubMed] [Google Scholar]
- 6.Weick JK, Stephens RL, Baker LH, Jones SE. Gallium nitrate in malignant lymphoma: A Southwest Oncology Group study. Cancer Treat. Rep. 1983;67:823–825. [PubMed] [Google Scholar]
- 7.Warrell RP, Jr, Coonley CJ, Straus DJ, Young CW. Treatment of patients with advanced malignant lymphoma using gallium nitrate administered as a seven-day continuous infusion. Cancer. 1983;51:1982–1987. doi: 10.1002/1097-0142(19830601)51:11<1982::aid-cncr2820511104>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 8.Chitambar CR, Zahir SA, Ritch PS, Anderson T. Evaluation of continuous-infusion gallium nitrate and hydroxyurea in combination for the treatment of refractory non-Hodgkin's lymphoma. Am. J. Clin. Oncol. 1997;20:173–178. doi: 10.1097/00000421-199704000-00015. [DOI] [PubMed] [Google Scholar]
- 9.Huang Z, Higgins B, Foss F. Activity of gallium nitrate in refractory peripheral T-cell lymphoma. Clin. Lymphoma. 2005;6:43–45. doi: 10.3816/clm.2005.n.026. [DOI] [PubMed] [Google Scholar]
- 10.Pro B, Bociek RG, Chitambar CR, Gregory SA, Leonard JP, Smith S, Novick S. Phase 2 multicenter trial of gallium nitrate in patients with advanced non-Hodgkin's lymphoma (NHL) Blood. 2004;104:682A. [Google Scholar]
- 11.Smith SE, Wren K, Stiff PJ, Toor A, Rodriguez T, van Gestel D. Gallium, rituximab, and dexamethasone for relapsed NHL. J. Clin. Oncol. 2007;25:8079. [Google Scholar]
- 12.Harris WR, Pecoraro VL. Thermodynamic binding constants for gallium transferrin. Biochemistry. 1983;22:292–299. doi: 10.1021/bi00271a010. [DOI] [PubMed] [Google Scholar]
- 13.Larson SM, Rasey JS, Allen DR, Nelson NJ, Grunbaum Z, Harp GD, Williams DL. Common pathway for tumor cell uptake of Gallium-67 and Iron-59 via a transferrin receptor. J. Natl. Cancer Inst. 1980;64:41–53. [PubMed] [Google Scholar]
- 14.Rasey JS, Nelson NJ, Larson SM. Tumor cell toxicity of stable gallium nitrate: Enhancement by transferrin and protection by iron. Eur. J. Cancer Clin. Oncol. 1982;l8:66l–668. doi: 10.1016/0277-5379(82)90212-7. [DOI] [PubMed] [Google Scholar]
- 15.Chitambar CR, Seligman PA. Effects of different transferrin forms on transferring receptor expression, iron uptake and cellular proliferation of human leukemic HL60 cells: Mechanisms responsible for the specific cytotoxicity of transferrin-gallium. J. Clin. Invest. 1986;78:1538–1546. doi: 10.1172/JCI112746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chitambar CR, Zivkovic Z. Uptake of gallium-67 by human leukemic cells: Demonstration of transferrin receptor-dependent and transferrin-independent mechanisms. Cancer Res. 1987;47:3929–3934. [PubMed] [Google Scholar]
- 17.Chitambar CR, Matthaeus WG, Antholine WE, Graff K, O'Brien WJ. Inhibition of leukemic HL60 cell growth by transferrin-gallium: Effects on ribonucleotide reductase and demonstration of drug synergy with hydroxyurea. Blood. 1988;72:1930–1936. [PubMed] [Google Scholar]
- 18.Chitambar CR, Narasimhan J, Guy J, Sem DS, O'Brien WJ. Inhibition of ribonucleotide reductase by gallium in murine leukemic L1210 cells. Cancer Res. 1991;51:6199–6201. [PubMed] [Google Scholar]
- 19.Narasimhan J, Antholine WE, Chitambar CR. Effect of gallium on the tyrosyl radical of the iron-dependent M2 subunit of ribonucleotide reductase. Biochem. Pharmacol. 1992;44:2403–2408. doi: 10.1016/0006-2952(92)90686-d. [DOI] [PubMed] [Google Scholar]
- 20.Chitambar CR, Wereley JP, Matsuyama S. Gallium-induced cell death in lymphoma: role of transferrin receptor cycling, involvement of Bax and the mitochondria, and effects of proteasome inhibition. Mol. Cancer Ther. 2006;5:2834–2843. doi: 10.1158/1535-7163.MCT-06-0285. [DOI] [PubMed] [Google Scholar]
- 21.Yang M, Kroft SH, Chitambar CR. Gene expression analysis of gallium-resistant and gallium-sensitive lymphoma cells reveals a role for metal-responsive transcription factor-1, metallothionein-2A, and zinc transporter-1 in modulating the antineoplastic activity of gallium nitrate. Mol. Cancer Ther. 2007;6:633–643. doi: 10.1158/1535-7163.MCT-06-0557. [DOI] [PubMed] [Google Scholar]
- 22.Davis SR, Cousins RJ. Metallothionein expression in animals: a physiological perspective on function. J. Nutr. 2000;130:1085–1088. doi: 10.1093/jn/130.5.1085. [DOI] [PubMed] [Google Scholar]
- 23.Andrews GK. Regulation of metallothionein gene expression by oxidative stress and metal ions. Biochem. Pharmacol. 2000;59:95–104. doi: 10.1016/s0006-2952(99)00301-9. [DOI] [PubMed] [Google Scholar]
- 24.Martin D, Rojo AI, Salinas M, Diaz R, Gallardo G, Alam J, De Galarreta CM, Cuadrado A. Regulation of heme oxygenase-1 expression through the phosphatidylinositol 3-kinase/Akt pathway and the Nrf2 transcription factor in response to the antioxidant phytochemical carnosol. J. Biol. Chem. 2004;279:8919–8929. doi: 10.1074/jbc.M309660200. [DOI] [PubMed] [Google Scholar]
- 25.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 26.Said AM, Hung WY, Zu JS, Hockberger P, Siddique T. Increased reactive oxygen species in familial amyotrophic lateral sclerosis with mutations in SOD1. J. Neurol. Sci. 2000;176:88–94. doi: 10.1016/s0022-510x(00)00317-8. [DOI] [PubMed] [Google Scholar]
- 27.Kelso GF, Porteous CM, Coulter CV, Hughes G, Porteous WK, Ledgerwood EC, Smith RA, Murphy MP. Selective targeting of a redox-active ubiquinone to mitochondria within cells: antioxidant and antiapoptotic properties. J. Biol. Chem. 2001;276:4588–4596. doi: 10.1074/jbc.M009093200. [DOI] [PubMed] [Google Scholar]
- 28.Zalewski PD, Forbes IJ, Betts WH. Correlation of apoptosis with change in intracellular labile Zn(II) using zinquin [(2-methyl-8-p-toluenesulphonamido-6-quinolyloxy)acetic acid], a new specific fluorescent probe for Zn(II) Biochem. J. 1993;296(Pt 2):403–408. doi: 10.1042/bj2960403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zalewski P, Truong-Tran A, Lincoln S, Ward D, Shankar A, Coyle P, Jayaram L, Copley A, Grosser D, Murgia C, Lang C, Ruffin R. Use of a zinc fluorophore to measure labile pools of zinc in body fluids and cell-conditioned media. BioTechniques. 2006;40:509–520. doi: 10.2144/06404RR02. [DOI] [PubMed] [Google Scholar]
- 30.Palmiter RD, Findley SD, Whitmore TE, Durnam DM. MT-III, a brain-specific member of the metallothionein gene family. Proc. Natl. Acad. Sci. U. S. A. 1992;89:6333–6337. doi: 10.1073/pnas.89.14.6333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ryter SW, Alam J, Choi AM. Heme oxygenase-1/carbon monoxide: from basic science to therapeutic applications. Physiol Rev. 2006;86:583–650. doi: 10.1152/physrev.00011.2005. [DOI] [PubMed] [Google Scholar]
- 32.Chitambar CR, Purpi DP, Woodliff J, Yang M, Wereley JP. Development of gallium compounds for treatment of lymphoma: Gallium maltolate, a novel hydroxypyrone gallium compound induces apoptosis and circumvents lymphoma cell resistance to gallium nitrate. J. Pharmacol. Exp. Ther. 2007;322:1228–1236. doi: 10.1124/jpet.107.126342. [DOI] [PubMed] [Google Scholar]
- 33.Bittel D, Dalton T, Samson SL, Gedamu L, Andrews GK. The DNA binding activity of metal response element-binding transcription factor-1 is activated in vivo and in vitro by zinc, but not by other transition metals. J. Biol. Chem. 1998;273:7127–7133. doi: 10.1074/jbc.273.12.7127. [DOI] [PubMed] [Google Scholar]
- 34.Lichtlen P, Schaffner W. The "metal transcription factor" MTF-1: biological facts and medical implications. Swiss. Med. Wkly. 2001;131:647–652. doi: 10.4414/smw.2001.09672. [DOI] [PubMed] [Google Scholar]
- 35.Johnson GL, Lapadat R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science. 2002;298:1911–1912. doi: 10.1126/science.1072682. [DOI] [PubMed] [Google Scholar]
- 36.Lee JM, Johnson JA. An important role of Nrf2-ARE pathway in the cellular defense mechanism. J. Biochem. Mol. Biol. 2004;37:139–143. doi: 10.5483/bmbrep.2004.37.2.139. [DOI] [PubMed] [Google Scholar]
- 37.Fliss H, Menard M. Oxidant-induced mobilization of zinc from metallothionein. Arch. Biochem. Biophys. 1992;293:195–199. doi: 10.1016/0003-9861(92)90384-9. [DOI] [PubMed] [Google Scholar]
- 38.Maret W. Oxidative metal release from metallothionein via zinc-thiol/disulfide interchange. Proc. Natl. Acad. Sci. U. S. A. 1994;91:237–241. doi: 10.1073/pnas.91.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Suttner DM, Sridhar K, Lee CS, Tomura T, Hansen TN, Dennery PA. Protective effects of transient HO-1 overexpression on susceptibility to oxygen toxicity in lung cells. Am. J. Physiol. 1999;276:L443–L451. doi: 10.1152/ajplung.1999.276.3.L443. [DOI] [PubMed] [Google Scholar]
- 40.Dennery PA. Regulation and role of heme oxygenase in oxidative injury. Curr. Top. Cell Regul. 2000;36:181–199. doi: 10.1016/s0070-2137(01)80008-x. [DOI] [PubMed] [Google Scholar]
- 41.Kovacic P, Osuna JA., Jr Mechanisms of anti-cancer agents: emphasis on oxidative stress and electron transfer. Curr. Pharm. Des. 2000;6:277–309. doi: 10.2174/1381612003401046. [DOI] [PubMed] [Google Scholar]
- 42.Poole PJ, Black PN. Oral mucolytic drugs for exacerbations of chronic obstructive pulmonary disease: systematic review. BMJ. 2001;322:1271–1274. doi: 10.1136/bmj.322.7297.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Marzullo Lq. An update of N-acetylcysteine treatment for acute acetaminophen toxicity in children. Curr. Opin. Pediatr. 2005;17:239–245. doi: 10.1097/01.mop.0000152622.05168.9e. [DOI] [PubMed] [Google Scholar]