Abstract
A few Yersinia pseudotuberculosis strains form biofilms on the head of the nematode Caenorhabditis elegans, but numerous others do not. We show that a widely used Y. pseudotuberculosis strain, YPIII, is biofilm positive because of a mutation in phoP, which encodes the response regulator of a two-component system. For two wild-type Y. pseudotuberculosis that do not make biofilms on C. elegans, deletion of phoP was sufficient to produce robust biofilms. In Yersinia pestis, a phoP mutant made more extensive biofilms in vitro than did the wild type. Expression of HmsT, a diguanylate cyclase that positively regulates biofilms, is diminished in Y. pseudotuberculosis strains with functional PhoP.
Introduction
Yersinia pseudotuberculosis and Yersinia pestis are closely related bacteria (Achtman, et al., 1999, Chain, et al., 2004). Y. pseudotuberculosis, a prototroph, is a relatively mild gastrointestinal pathogen (Smego, et al., 1999). Y. pestis, the agent of bubonic plague, is a frequently lethal bacterium that evolved recently (Achtman, et al., 1999); it is an auxotroph that that infects mammals (usually rodents) and their fleas and is believed to be an obligate parasite (Perry & Fetherston, 1997). An important part of the Y. pestis life cycle is formation of a biofilm to colonize the digestive tracts of fleas (Jarrett, et al., 2004). Y. pseudotuberculosis is apparently incapable of forming biofilms in fleas (Erickson, et al., 2006), and a role for biofilms in its natural life cycle has not been reported.
In a laboratory model, both species form biofilms on the head of the nematode Caenorhabditis elegans (Darby, et al., 2002). Multiple Y. pestis strains make these in vivo biofilms (Darby, et al., 2002, Joshua, et al., 2003), but under the same conditions relatively few Y. pseudotuberculosis strains do so. A study that examined 40 Y. pseudotuberculosis strain backgrounds found that only four made “severe” biofilms, while four others had an intermediate phenotype, two made barely detectable biofilms and 30 produced none (Joshua, et al., 2003).
Y. pseudotuberculosis strains that fail to make biofilms in fleas or on C. elegans are nevertheless capable of forming biofilms under some conditions. In one study, strains that failed to make biofilms in fleas formed biofilms in vitro on borosilicate glass (Erickson, et al., 2006). In another report, deletion of rcsA, encoding a component of a phosphorelay signaling system, resulted in robust biofilm formation on C. elegans (Sun, et al., 2008).
YPIII, a widely used laboratory strain, is among the few Y. pseudotuberculosis that make strong biofilms on C. elegans (Darby, et al., 2002, Joshua, et al., 2003). YPIII has an inactivating mutation in phoP, which encodes the response regulator of a two-component signal transduction system (Grabenstein, et al., 2004). In the present study we show that PhoP negatively regulates Y. pestis and Y. pseudotuberculosis biofilms.
Materials and methods
Bacterial strains and plasmids
The strains and plasmids used are shown in Table 1. Bacteria were grown in LB except as noted below, and plasmids were maintained with 100 µg/ml ampicillin. CDY664 and CDY665 were made by electroporating plasmid pCBD26 into the indicated parent strains. CDY618 was made using the λ Red recombination method (Datsenko & Wanner, 2000, Sun, et al., 2008) to delete phoP from Y. pestis KIM6+ and replace it with a kanamycin resistance gene; the PCR primers used were 5′-GATAATATCCTGTTATCCGGTTAACGTTTTATCAAGGATTGGTGTATTCCGGGGATCCGTCGACC and 5′-CTAGTTGACGTCAAAACGATATCCCTGACTACGAATAGTCGTAATGTGTAGGCTGGAGCTGCTTCG. All Y. pestis strains in this study are avirulent in mammals due to absence of the pCD1 virulence plasmid. To make pCBD26, the hmsT gene and its promoter were PCR amplified from Y. pestis KIM6+ using the primers 5′-CGCGGATCCACGTGGTACAACATGCTGAC and 5′-CTTCGGCCGTCCCGAGTGATAAGGTGTGG, then cloned into pCR2.1-TOPO (Invitrogen). The fidelity of the open reading frame was verified by DNA sequencing.
Table 1.
Bacterial strains and plasmids
| Strain or plasmid | Genotype and/or description | Reference or source |
|---|---|---|
| Y. pseudotuberculosis | ||
| YPIII | Serogroup O3, phoPT160P | (Gemski, et al., 1980) |
| YPIII/MMB67EH | phoPT160P/pMMB67EH | (Grabenstein, et al., 2004) |
| YPIII/pPhoPKIM | phoPT160P/pPhoPKIM | (Grabenstein, et al., 2004) |
| CDY103 | YPIIIphoPT160P/hmsT::TnphoA | (Darby, et al., 2002) |
| IP2666c | Serogroup O3, wild type | (Simonet & Falkow, 1992) |
| IP2666cphoPΔ | phoPΔ127–429 | (Grabenstein, et al., 2004) |
| IP2666cphoPΔ/pPhoPKIM | phoPΔ127–429/pPhoPKIM | (Grabenstein, et al., 2004) |
| CDY664 | IP2666c/pCBD26 | This study |
| IP32777 | Serogroup O1, wild type | (Collyn, et al., 2002) |
| IP32777phoP::kan | phoP::kan | (Grabenstein, et al., 2004) |
| IP32777phoP::kan/pPhoPKIM | phoP::kan/pPhoPKIM | (Grabenstein, et al., 2004) |
| CDY665 | IP32777/pCBD26 | This study |
| Y. pestis | ||
| KIM6+ | wild type (ΔpCD1) | (Deng, et al., 2002) |
| CDY618 | KIM6+ ΔphoP::kan | This study |
| CDY187 | ΔhmsS::cat | (Sun, et al., 2008) |
| Plasmids | ||
| pMMB67EH | expression vector | (Furste, et al., 1986) |
| pPhoPKIM | phoP in pMMB67EH | (Grabenstein, et al., 2004) |
| pCR2.1-TOPO | cloning vector | Invitrogen |
| pCBD26 (pHmsT) | hmsT in pCR2.1-TOPO | This study |
C. elegans biofilms
For Y. pseudotuberculosis YPIII and IP2666c and their derivatives, nematode growth was assayed as described (Darby, et al., 2005). Briefly, adult C. elegans were allowed to lay eggs on lawns of bacteria for 2–4 h; after the adults were removed, the plates were incubated for 2 d at 20°C, and the worm development to fourth larval (L4) stage was scored. To assay the biofilm capability of IP32777 and its derivatives, and for photographs of all Y. pseudotuberculosis strains, adult C. elegans were placed on bacterial lawns for approx. 16 h.
In vitro biofilms
Y. pestis biofilms grown on polystyrene culture dishes were assayed as described (Sun, et al., 2008). Briefly, bacteria were grown in the dishes in 40% brain-heart infusion broth with shaking for 16 h at 26°C. The wells were washed gently with water, adherent biofilms were stained with crystal violet, and the wells were washed again. Crystal violet was resolubilized in ethanol-acetone and quantified by spectrophotometry. For each strain, four replicates were grown in parallel; wild-type and isogenic mutants were grown in a single 24-well dish. Mean and standard deviations were plotted, and significance was determined using the two-tailed Student’s t-test.
Western blotting
Bacteria were grown in LB at 26°C to an OD600 of 4 (YPIII strains) or 2 (IP2666c strains), with 1mM IPTG added for strains with a phoP plasmid or empty vector. Approx. 20 µg of total protein per strain was electrophoresed on 12% Bis-Tris polyacrylamide gels (Invitrogen) and blotted onto nitrocellulose. Primary rabbit polyclonal antibody against HmsT (Perry, et al., 2004) was diluted 1:10,000 in either phosphate-buffered saline (PBS) plus 5% skim milk or TBST buffer (20mM Tris HCl pH 7.5, 150mM NaCl, 0.05% Tween-20) plus 5% skim milk and incubated overnight at 4°C. After three buffer washes, goat anti-rabbit secondary antibody conjugated to horseradish peroxidase (Invitrogen) was diluted 1:2,000 in buffer plus skim milk and the membrane incubated for 1 h, then washed three times in buffer. Bound antibody was detected with Immobilon Western HRP Substrate (Millipore).
Results
Because Yersinia sp. biofilms cover the mouth of C. elegans and inhibit the nematode’s feeding, the ability of newly hatched C. elegans to grow on a bacterial lawn is a measure of the bacteria’s biofilm formation (Darby, et al., 2002, Darby, et al., 2005, Sun, et al., 2008). Beginning with eggs deposited on a lawn, almost all animals will develop to the fourth larval (L4) stage if feeding on biofilm-negative bacteria, while almost none will become L4 if the bacteria make robust biofilms.
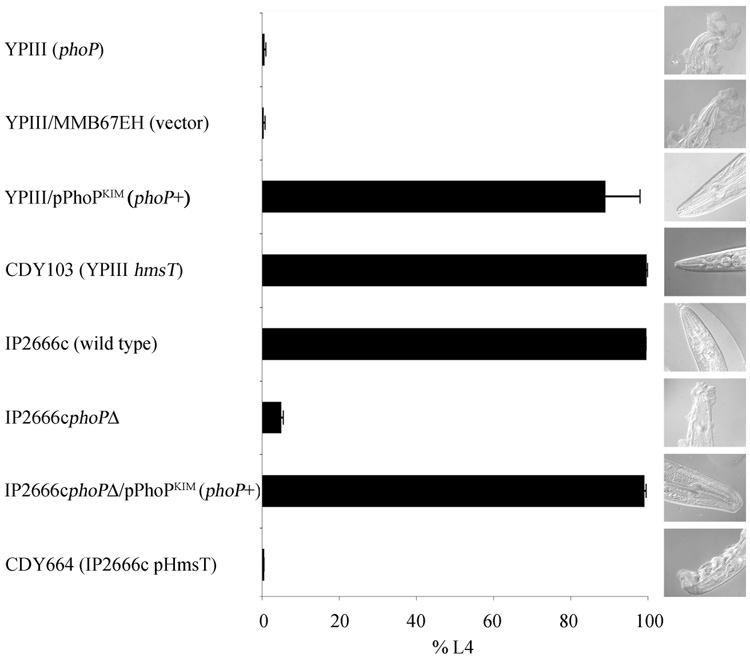
We used this C. elegans growth assay to determine whether the phoP mutation in Y. pseudotuberculosis YPIII is responsible for the biofilm phenotype. Consistent with previous reports (Darby, et al., 2002, Darby, et al., 2005), YPIII made large biofilms on the worms and strongly inhibited growth (Fig. 1). When YPIII was transformed with a plasmid expressing wild-type phoP, it failed to make biofilms detectable by microscopy, and worms grew normally (Fig. 1). The phenotype of the phoP+ strain was essentially the same as YPIII with a mutation in the positive regulator hmsT (Fig. 1). To further establish the role of phoP in biofilm regulation, we examined another Y. pseudotuberculosis strain background. IP2666c failed to make biofilms and failed to inhibit growth, but an isogenic strain with a phoP deletion made visible biofilms and inhibited growth almost as well as YPIII (Fig. 1). Plasmid complementation restored biofilm repression, confirming that biofilm production was due to the phoP mutation (Fig. 1).
Fig. 1.
PhoP represses Y. pseudotuberculosis biofilms on C. elegans. Growth of C. elegans to L4 stage in 2 d on lawns of the indicated strains is plotted; data are mean ± S.D. of assays performed on three separate occasions, with at least 1,200 worms per sample. Low growth indicates biofilm is made that inhibits feeding, while high growth indicates reduction or absence of biofilm. Photographs show typical adult worms after 16 h on bacterial lawns.
We also tested a third Y. pseudotuberculosis strain background, IP32777. The growth assay could not be used, because the strain has an uncharacterized activity that kills C. elegans eggs before they hatch. Instead, we placed adult C. elegans on IP32777 lawns for approx. 16 h and scored biofilm accumulation by microscopy. IP32777 failed to make a detectable biofilm. When phoP was deleted, substantial biofilms were made; complementation with plasmid-expressed phoP restored the biofilm-negative phenotype (data not shown). Judged by this visual assay, biofilm production by the IP32777 phoP mutant was somewhat weaker than that of its counterpart in the IP2666c background.
HmsT is a diguanylate cyclase required for both Y. pestis and Y. pseudotuberculosis biofilms (Darby, et al., 2002, Kirillina, et al., 2004); few other positive regulators have been identified. To examine whether PhoP negatively regulates biofilms by repressing hmsT, we transformed biofilm-negative IP2666c and IP32777 with a high-copy hmsT plasmid (pHmsT). Biofilms were made on C. elegans, similar to those observed when phoP is deleted (Fig. 1 and data not shown).
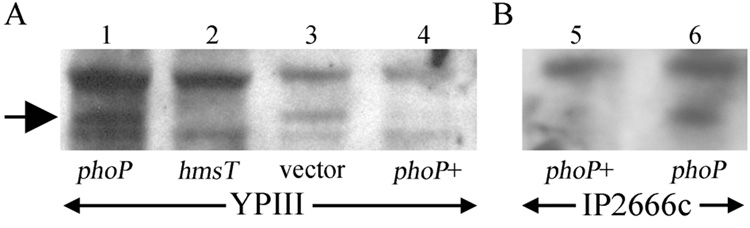
To confirm that PhoP represses HmsT, we used Western blotting. YPIII, a phoP mutant, produced substantial HmsT when transformed with an empty vector, but when wild-type phoP was on the plasmid, the protein level was reduced to a nearly undetectable level (Fig. 2A). With IP2666c, which has wild-type phoP, HmsT was not observed at all, but deletion of phoP produced detectable protein (Fig. 2B). We conclude that PhoP inhibits biofilms at least in part by negatively regulating HmsT. It has not been established whether the regulation is direct or through intermediates.
Fig. 2.
phoP mutations increase HmsT expression in Y. pseudotuberculosis. Total protein from strains in the YPIII (A) and IP2666c (B) backgrounds was immunoblotted with a rabbit polyclonal antibody against HmsT. The antibody is known to cross-react with multiple other proteins (Perry, et al., 2004); the HmsT band (arrow) is identified by its absence in the hmsT mutant. 1, YPIII; 2, CDY103; 3, YPIII/MMB67EH; 4, YPIII/pPhoPKIM; 5, IP2666c; 6, IP266cphoPΔ.
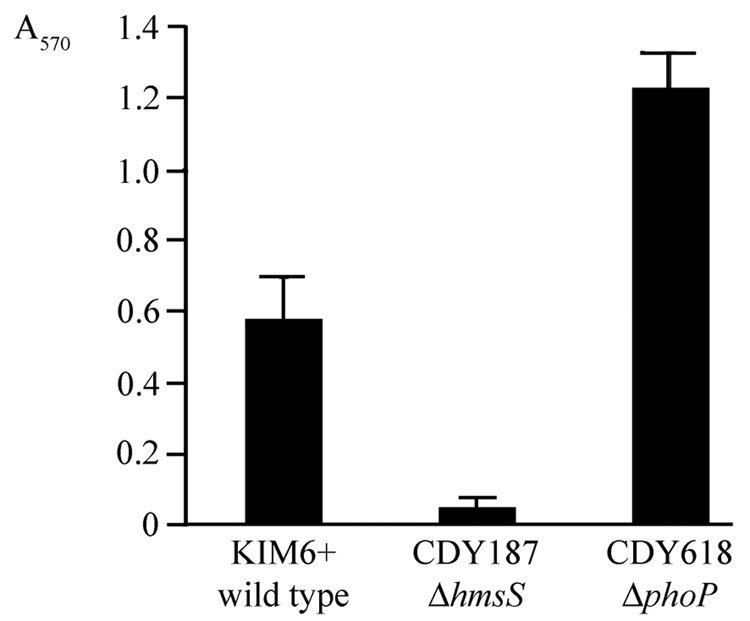
To determine whether PhoP negatively regulates Y. pestis biofilms, we deleted phoP from the biofilm-positive strain KIM6+. Because KIM6+ makes biofilms that completely inhibit C. elegans growth (Sun, et al., 2008), an increase in biofilms in the nematode assay would not be detectable. Instead, we examined Y. pestis biofilms in vitro by growing them on polystyrene dishes and staining with crystal violet. The values for an hmsS mutant were barely above background (Fig. 3), confirming that the in vito biofilm is hmsHFRS-dependent. The phoP mutant biofilms were consistently about twice as extensive as the wild-type (Fig. 3), confirming that PhoP negatively regulates Y. pestis biofilms. During initial trials it was observed that biofilms made by the phoP mutant adhered more loosely to the dish than did wild-type biofilms, and gentle washing was required to avoid dislodging them.
Fig. 3.
phoP negatively regulates Y. pestis biofilms in vitro. Broth cultures were grown in a multi-well polystyrene culture dish for 16 h. After washing, the adherent material was stained with crystal violet, which was resolubilized and quantified by absorption at 570 nm. The hmsS mutant is a negative control; hmsS is known to be required for Y. pestis biofilms in vitro and in vivo (Forman, et al., 2006, Sun, et al., 2008). The hmsS (p = 0.002) and phoP (p = 0.0016) mutants both differed significantly from wild type. The experiment was repeated with similar results.
Discussion
The role of biofilms in the Y. pestis life cycle is known. Y. pestis forms biofilms in the digestive tracts of fleas that block the insect’s feeding, stimulating it to bite repeatedly in search of food and thereby transmit the bacteria to new hosts (Jarrett, et al., 2004, Darby, 2008). Biofilms play no apparent role in Y. pestis mammalian pathogenesis. A mutation in hmsR, a gene required for biofilms, did not reduce virulence in a mouse infection model (Lillard, et al., 1999), and biofilm formation in vitro is repressed at 37°C (Kirillina, et al., 2004).
Despite the close relationship between the two Yersinia species, multiple Y. pseudotuberculosis strains failed to form biofilms in fleas, including those that were able to make biofilms in vitro (Erickson, et al., 2006). No published reports examine biofilms in Y. pseudotuberculosis mammalian infections. However, an hmsT mutant that was completely defective for biofilm formation in the C. elegans assay (Darby, et al., 2002) was fully virulent in a mouse oral infection model (J.W. Hsu, C. Darby and S. Falkow, unpublished data), suggesting that biofilms are not required in a mammalian host.
Y. pestis and Y. pseudotuberculosis have virtually identical exopolysaccharide biosynthetic operons, hmsHFRS (Darby, 2008). This operon is required for Y. pestis biofilms in fleas (Hinnebusch, et al., 1996, Jarrett, et al., 2004) and for either species to make biofilms on C. elegans (Darby, et al., 2002). Multiple C. elegans mutants isolated on the basis of resistance to Y. pseudotuberculosis biofilm attachment were all cross-resistant to Y. pestis (Darby, et al., 2007). Together, these findings suggest that differences between the two bacteria in the C. elegans biofilm model are not at the level of exopolysaccharide composition and structure. Consistent with this interpretation, we recently reported a regulatory difference affecting biofilms. During Y. pestis evolution, a mutation occurred that inactivated rcsA, encoding a phosphorelay accessory protein; the mutation relieved biofilm repression and was apparently crucial for the bacterium’s ability to form biofilms in fleas (Sun, et al., 2008).
In the current work we examined another regulatory signaling pathway, the PhoP-PhoQ two-component system. Our investigation was prompted by the finding that the widely used Y. pseudotuberculosis laboratory strain YPIII has an inactivating mutation in phoP (Grabenstein, et al., 2004). Because YPIII makes robust biofilms in the C. elegans assay while many other Y. pseudotuberculosis do not (Darby, et al., 2002, Joshua, et al., 2003, Sun, et al., 2008), we asked whether the mutation in phoP is responsible. The answer was affirmative. Restoration of functional phoP in YPIII resulted in almost complete absence of biofilms in the C. elegans model (Fig. 1). In two other Y. pseudotuberculosis wild-type backgrounds that do not make biofilms on C. elegans, deletion of phoP was sufficient to produce extensive biofilms that blocked nematode feeding (Fig. 1 and data not shown).
We conclude that PhoP negatively regulates biofilms in Y. pseudotuberculosis. This appears to occur at least in part by downregulation, directly or indirectly, of the diguanylate cyclase HmsT (Fig. 2). In two different backgrounds, HmsT was absent or barely detectable with wild-type phoP present but highly expressed when phoP was mutated or deleted.
In Y. pestis, a phoP deletion resulted in a doubling of biofilm production, as assayed by crystal violet staining of biofilms adhering to polystyrene (Fig. 3). This indicates that in Y. pestis as well as Y. pseudotuberculosis, PhoP negatively regulates biofilms. We also observed that the Y. pestis phoP biofilms are less adherent than normal. Interestingly, Erickson et al. observed that Y. pseudotuberculosis biofilms in glass flow cells tend to slough off the surface more readily than those made by Y. pestis. Differences in adhesion are not easily explained by mere changes in the amount of exopolysaccharide; rather, they suggest variation in composition or structure of the extracellular matrix. Our findings suggest that in Y. pestis, PhoP regulates both the volume and the adhesive properties of the biofilm.
PhoP, a response regulator, functions with the sensor kinase PhoQ in a two-component system. PhoP-PhoQ has been studied most extensively in Salmonella enterica serovar Typhimurium, where it responds to Mg2+ and Ca2+ concentrations and is essential for virulence (Groisman, 2001). Of particular relevance to the present study, a S. enterica phoP null mutant had enhanced biofilm production on gallstones and on glass, while a phoP constitutive mutant was biofilm defective, indicating that PhoP negatively regulates S. enterica biofilms (Prouty & Gunn, 2003).
In Y. pseudotuberculosis as in S. enterica, PhoP mediates a response to Mg2+ (Grabenstein, et al., 2004). A Y. pseudotuberculosis phoP mutant was attenuated in a mouse infection model, with a 100-fold higher LD50 (Grabenstein, et al., 2004), and phoP is a virulence factor in a Y. pseudotuberculosis lung infection model (Fisher, et al., 2007). In both Y. pseudotuberculosis and Y. pestis, PhoP increases survival inside macrophages (Oyston,et al., 2000, Grabenstein, et al., 2004). Although PhoP has not been examined as extensively in Yersinia sp. as it has in S. enterica, the available evidence indicates that it functions as a positive virulence regulator in mammalian infections.
Y. pestis is an obligate parasite with two disparate hosts, and as it shuttles between fleas at lower temperatures and mammals at higher ones, it regulates its functions accordingly (Prentice & Rahalison, 2007). In fleas, Y. pestis makes biofilms required to persist in the insect, while repressing the Yop proteins that disrupt and evade mammalian immunity. When the bacteria are transmitted to a mammalian host, the profile is reversed: biofilms are repressed while Yops are expressed at high levels. Our finding that Y. pestis PhoP negatively regulates biofilms is consistent with this bimodal life cycle. During mammalian infections, PhoP positively regulates multiple genes that promote survival and virulence (Grabenstein, et al., 2006), and our results suggest that at the same time PhoP negatively regulates biofilm functions that are unneeded in mammalian hosts.
The place of biofilms in the Y. pseudotuberculosis life cycle is not known. However, the two Yersinia have a close evolutionary relationship, and they use many of the same genetic elements (hmsHFRS, hmsT, rcs, phoP) in making biofilms. In addition, the genomes of both species encode multiple insecticidal proteins (Parkhill, et al., 2001, Gendlina, et al., 2007, Pinheiro & Ellar, 2007, Waterfield, et al., 2007). Thus, even though Y. pseudotuberculosis appears unable to make biofilms in fleas, it likely encounters insects in its life cycle. The specialized ability of Y. pestis to make biofilms in fleas may have evolved from a more general capability in the ancestral Y. pseudotuberculosis to make biofilms in an invertebrate host.
Acknowledgements
We thank James Bliska for strains and plasmids and Robert Perry for anti-HmsT antibody. This work was funded by U.S. Public Health Service grant AI057512 to C.D.
References
- Achtman v, Zurth K, Morelli G, Torrea G, Guiyoule A, Carniel E. Yersinia pestis, the cause of plague, is a recently emerged clone of Yersinia pseudotuberculosis. Proc Natl Acad Sci U S A. 1999;96:14043–14048. doi: 10.1073/pnas.96.24.14043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chain PS, Carniel E, Larimer FW, et al. Insights into the evolution of Yersinia pestis through whole-genome comparison with Yersinia pseudotuberculosis. Proc Natl Acad Sci U S A. 2004;101:13826–13831. doi: 10.1073/pnas.0404012101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collyn F, Lety MA, Nair S, Escuyer V, Ben Younes A, Simonet M, Marceau M. Yersinia pseudotuberculosis harbors a type IV pilus gene cluster that contributes to pathogenicity. Infect Immun. 2002;70:6196–6205. doi: 10.1128/IAI.70.11.6196-6205.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darby C. Uniquely insidious: Yersinia pestis biofilms. Trends Microbiol. 2008;16:158–164. doi: 10.1016/j.tim.2008.01.005. [DOI] [PubMed] [Google Scholar]
- Darby C, Hsu JW, Ghori N, Falkow S. Caenorhabditis elegans: plague bacteria biofilm blocks food intake. Nature. 2002;417:243–244. doi: 10.1038/417243a. [DOI] [PubMed] [Google Scholar]
- Darby C, Ananth SL, Tan L, Hinnebusch BJ. Identification of gmhA, a Yersinia pestis gene required for flea blockage, by using a Caenorhabditis elegans biofilm system. Infect Immun. 2005;73:7236–7242. doi: 10.1128/IAI.73.11.7236-7242.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darby C, Chakraborti A, Politz SM, Daniel CC, Tan L, Drace K. Caenorhabditis elegans Mutants Resistant to Attachment of Yersinia Biofilms. Genetics. 2007;176:221–230. doi: 10.1534/genetics.106.067496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datsenko KA, Wanner BL. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci U S A. 2000;97:6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng W, Burland V, Plunkett G, 3rd, et al. Genome sequence of Yersinia pestis KIM. J Bacteriol. 2002;184:4601–4611. doi: 10.1128/JB.184.16.4601-4611.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson DL, Jarrett CO, Wren BW, Hinnebusch BJ. Serotype differences and lack of biofilm formation characterize Yersinia pseudotuberculosis infection of the Xenopsylla cheopis flea vector of Yersinia pestis. J Bacteriol. 2006;188:1113–1119. doi: 10.1128/JB.188.3.1113-1119.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher ML, Castillo C, Mecsas J. Intranasal inoculation of mice with Yersinia pseudotuberculosis causes a lethal lung infection that is dependent on Yersinia outer proteins and PhoP. Infect Immun. 2007;75:429–442. doi: 10.1128/IAI.01287-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman S, Bobrov AG, Kirillina O, Craig SK, Abney J, Fetherston JD, Perry RD. Identification of critical amino acid residues in the plague biofilm Hms proteins. Microbiology. 2006;152:3399–3410. doi: 10.1099/mic.0.29224-0. [DOI] [PubMed] [Google Scholar]
- Furste JP, Pansegrau W, Frank R, Blocker H, Scholz P, Bagdasarian M, Lanka E. Molecular cloning of the plasmid RP4 primase region in a multi-host-range tacP expression vector. Gene. 1986;48:119–131. doi: 10.1016/0378-1119(86)90358-6. [DOI] [PubMed] [Google Scholar]
- Gemski P, Lazere JR, Casey T, Wohlhieter JA. Presence of a virulence-associated plasmid in Yersinia pseudotuberculosis. Infect Immun. 1980;28:1044–1047. doi: 10.1128/iai.28.3.1044-1047.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendlina I, Held KG, Bartra SS, et al. Identification and type III-dependent secretion of the Yersinia pestis insecticidal-like proteins. Mol Microbiol. 2007;64:1214–1227. doi: 10.1111/j.1365-2958.2007.05729.x. [DOI] [PubMed] [Google Scholar]
- Grabenstein JP, Fukuto HS, Palmer LE, Bliska JB. Characterization of phagosome trafficking and identification of PhoP-regulated genes important for survival of Yersinia pestis in macrophages. Infect Immun. 2006;74:3727–3741. doi: 10.1128/IAI.00255-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabenstein JP, Marceau M, Pujol C, Simonet M, Bliska JB. The response regulator PhoP of Yersinia pseudotuberculosis is important for replication in macrophages and for virulence. Infect Immun. 2004;72:4973–4984. doi: 10.1128/IAI.72.9.4973-4984.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groisman EA. The pleiotropic two-component regulatory system PhoP-PhoQ. J Bacteriol. 2001;183:1835–1842. doi: 10.1128/JB.183.6.1835-1842.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinnebusch BJ, Perry RD, Schwan TG. Role of the Yersinia pestis hemin storage (hms) locus in the transmission of plague by fleas. Science. 1996;273:367–370. doi: 10.1126/science.273.5273.367. [DOI] [PubMed] [Google Scholar]
- Jarrett CO, Deak E, Isherwood KE, et al. Transmission of Yersinia pestis from an infectious biofilm in the flea vector. J Infect Dis. 2004;190:783–792. doi: 10.1086/422695. [DOI] [PubMed] [Google Scholar]
- Joshua GW, Karlyshev AV, Smith MP, Isherwood KE, Titball RW, Wren BW. A Caenorhabditis elegans model of Yersinia infection: biofilm formation on a biotic surface. Microbiology. 2003;149:3221–3229. doi: 10.1099/mic.0.26475-0. [DOI] [PubMed] [Google Scholar]
- Kirillina O, Fetherston JD, Bobrov AG, Abney J, Perry RD. HmsP, a putative phosphodiesterase, and HmsT, a putative diguanylate cyclase, control Hms-dependent biofilm formation in Yersinia pestis. Mol Microbiol. 2004;54:75–88. doi: 10.1111/j.1365-2958.2004.04253.x. [DOI] [PubMed] [Google Scholar]
- Lillard JW, Jr, Bearden SW, Fetherston JD, Perry RD. The haemin storage (Hms+) phenotype of Yersinia pestis is not essential for the pathogenesis of bubonic plague in mammals. Microbiology. 1999;145(Pt 1):197–209. doi: 10.1099/13500872-145-1-197. [DOI] [PubMed] [Google Scholar]
- Oyston PC, Dorrell N, Williams K, Li SR, Green M, Titball RW, Wren BW. The response regulator PhoP is important for survival under conditions of macrophage-induced stress and virulence in Yersinia pestis. Infect Immun. 2000;68:3419–3425. doi: 10.1128/iai.68.6.3419-3425.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkhill J, Wren BW, Thomson NR, et al. Genome sequence of Yersinia pestis, the causative agent of plague. Nature. 2001;413:523–527. doi: 10.1038/35097083. [DOI] [PubMed] [Google Scholar]
- Perry RD, Fetherston JD. Yersinia pestis--etiologic agent of plague. Clin Microbiol Rev. 1997;10:35–66. doi: 10.1128/cmr.10.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry RD, Bobrov AG, Kirillina O, Jones HA, Pedersen L, Abney J, Fetherston JD. Temperature regulation of the hemin storage (Hms+) phenotype of Yersinia pestis is posttranscriptional. J Bacteriol. 2004;186:1638–1647. doi: 10.1128/JB.186.6.1638-1647.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinheiro VB, Ellar DJ. Expression and insecticidal activity of Yersinia pseudotuberculosis and Photorhabdus luminescens toxin complex proteins. Cell Microbiol. 2007;9:2372–2380. doi: 10.1111/j.1462-5822.2007.00966.x. [DOI] [PubMed] [Google Scholar]
- Prentice MB, Rahalison L. Plague. Lancet. 2007;369:1196–1207. doi: 10.1016/S0140-6736(07)60566-2. [DOI] [PubMed] [Google Scholar]
- Prouty AM, Gunn JS. Comparative analysis of Salmonella enterica serovar Typhimurium biofilm formation on gallstones and on glass. Infect Immun. 2003;71:7154–7158. doi: 10.1128/IAI.71.12.7154-7158.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonet M, Falkow S. Invasin expression in Yersinia pseudotuberculosis. Infect Immun. 1992;60:4414–4417. doi: 10.1128/iai.60.10.4414-4417.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smego RA, Frean J, Koornhof HJ. Yersiniosis I: microbiological and clinicoepidemiological aspects of plague and non-plague Yersinia infections. Eur J Clin Microbiol Infect Dis. 1999;18:1–15. doi: 10.1007/s100960050219. [DOI] [PubMed] [Google Scholar]
- Sun YC, Hinnebusch BJ, Darby C. Experimental evidence for negative selection in the evolution of a Yersinia pestis pseudogene. Proc Natl Acad Sci U S A. 2008;105:8097–8101. doi: 10.1073/pnas.0803525105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterfield N, Hares M, Hinchliffe S, Wren B, ffrench-Constant R. The insect toxin complex of Yersinia. Adv Exp Med Biol. 2007;603:247–257. doi: 10.1007/978-0-387-72124-8_22. [DOI] [PubMed] [Google Scholar]