Abstract
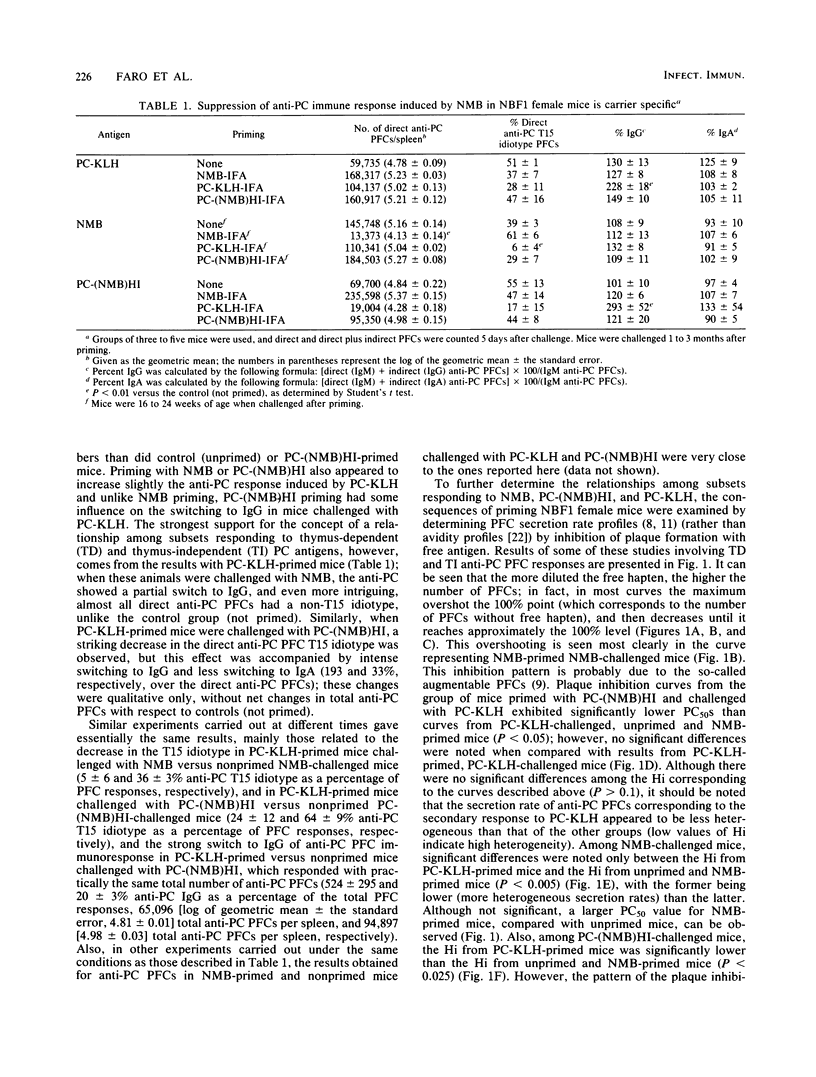
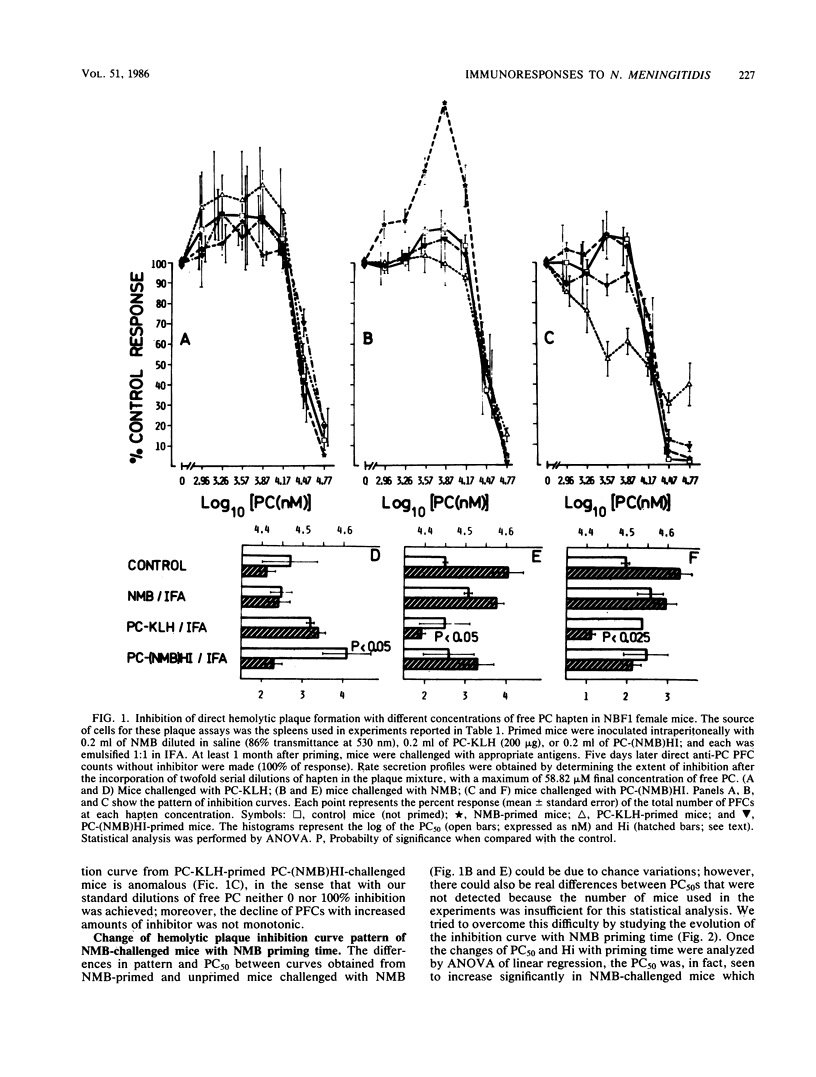
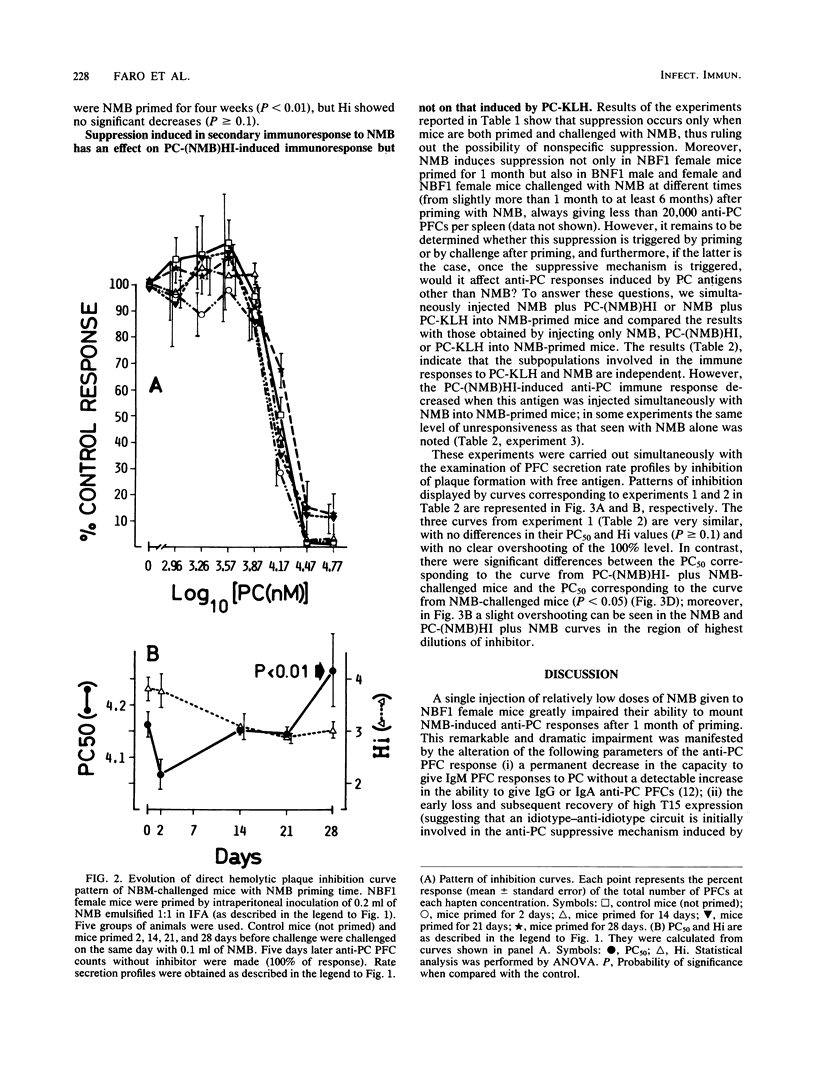
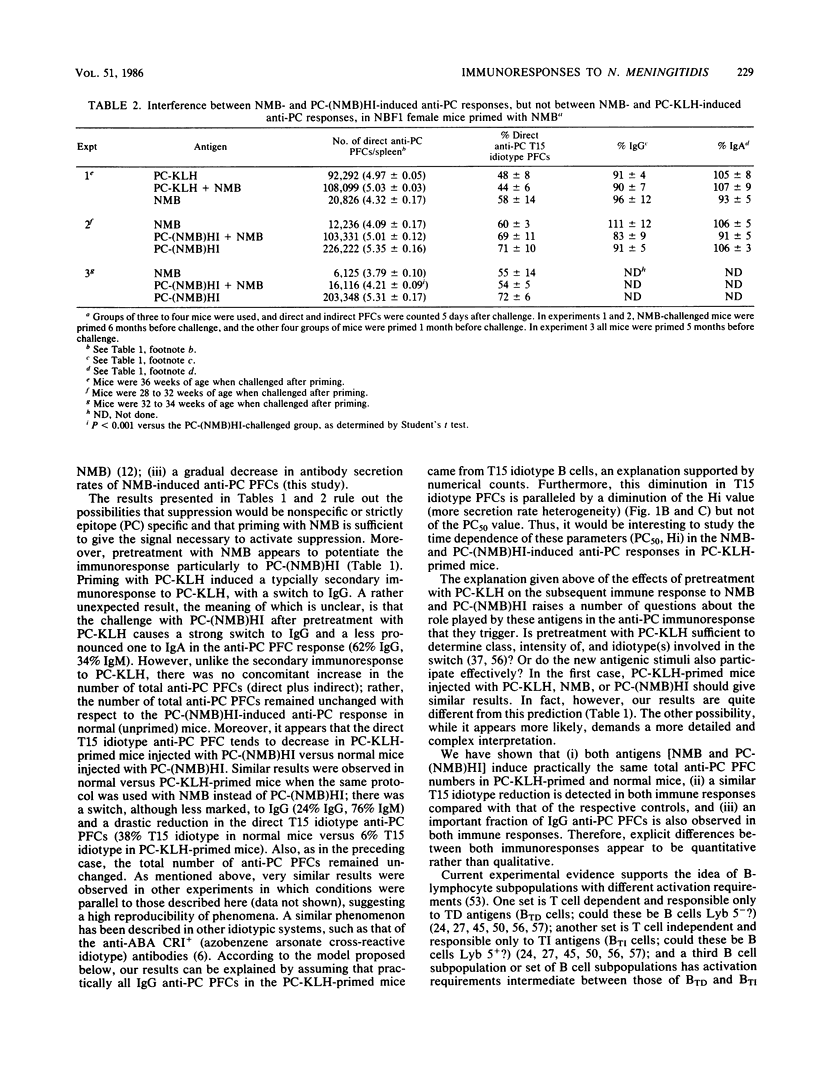
Results of our previous work have shown that Neisseria meningitidis serogroup B M986 can induce a phosphorylcholine (PC)-specific plaque-forming cell immunoresponse in mice. Also, a single injection of a relatively low dose of meningococci in NBF1 female mice induced a priming time-dependent suppression on subsequent meningococcus challenge. This suppression was not due to switching to another class of immunoglobulin nor to the presence of a capsule on N. meningitidis. In this study we show that suppression induced by meningococcus is carrier specific. Furthermore, we offer evidence suggesting that the structure(s) on meningococcus that trigger this suppression is heat labile and different from the antigenic structure(s) recognized by the suppressed B cells. In addition, we found that there is a gradual increase in antibody secretion rates of N. meningitidis-induced anti-PC plaque-forming cells that correlates with N. meningitidis priming time. Rather unexpected was the fact that pretreatment of mice with PC-keyhole limpet hemocyanin (thymus-dependent antigen) had a great influence on the subsequent PC-specific immunoresponses induced by N. meningitidis and PC-coupled heat-inactivated meningococcus [PC-(NMB)HI], as shown by (i) a striking decrease in T15 idiotype expression, (ii) concomitant direct anti-PC plaque-forming cells reduction, (iii) switching to immunoglobulin G (N. meningitidis-induced immunoresponse) or immunoglobulin G plus immunoglobulin A [PC-(NMB)HI-induced immunoresponse], and (iv) a significant increase in heterogeneity of plaque-forming cell secretion rates. The possibility that N. meningitidis, PC-(NMB)HI, and PC-KLH stimulate B lymphocytes pertaining to three different subpopulations embedded in distinct regulatory circuits is discussed, with emphasis on the interrelationships between T-dependent and T-independent lymphocyte compartments. We focus on the possibility of the existence of high-level regulatory circuits in which lymphocyte subpopulations or sets of lymphocyte subpopulations with different requirements of activation are connected.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Calkins C. E. Interactions between primed and unprimed cells in the regulation of in vitro antibody responses. I. Role of "plasma cells" as inducers of suppression. Eur J Immunol. 1982 Jan;12(1):70–75. doi: 10.1002/eji.1830120113. [DOI] [PubMed] [Google Scholar]
- Cebra J. J., Komisar J. L., Schweitzer P. A. CH isotype 'switching' during normal B-lymphocyte development. Annu Rev Immunol. 1984;2:493–548. doi: 10.1146/annurev.iy.02.040184.002425. [DOI] [PubMed] [Google Scholar]
- Chesebro B., Metzger H. Affinity labeling of a phosphorylcholine binding mouse myeloma protein. Biochemistry. 1972 Feb 29;11(5):766–771. doi: 10.1021/bi00755a014. [DOI] [PubMed] [Google Scholar]
- Conger J. D., Lewis G. K., Goodman J. W. Idiotype profile of an immune response. I. Contrasts in idiotypic dominance between primary and secondary responses and between IgM and IgG plaque-forming cells. J Exp Med. 1981 May 1;153(5):1173–1186. doi: 10.1084/jem.153.5.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham A. J., Szenberg A. Further improvements in the plaque technique for detecting single antibody-forming cells. Immunology. 1968 Apr;14(4):599–600. [PMC free article] [PubMed] [Google Scholar]
- DeLisi C. Hemolytic plaque inhibition: the physical chemical limits on its use as an affinity assay. J Immunol. 1976 Dec;117(6):2249–2257. [PubMed] [Google Scholar]
- Doria G., Mancini C., DeLisi C. Secretion rate independent evaluation of IgM antibody avidity at the level of single immunocytes. J Immunol. 1978 Nov;121(5):2030–2034. [PubMed] [Google Scholar]
- Faro J., Seoane R., Puentes E., Martínez Ubeira F., Regueiro B. J. Immunoresponses to Neisseria meningitidis epitopes: primary versus secondary antiphosphorylcholine responses. Infect Immun. 1985 May;48(2):428–432. doi: 10.1128/iai.48.2.428-432.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson T. A., Krieger N. J., Pesce A., Michael J. G. Enhancement of antigen-specific suppression by muramyl dipeptide. Infect Immun. 1983 Feb;39(2):800–806. doi: 10.1128/iai.39.2.800-806.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frasch C. E., Peppler M. S. Protection against group B Neisseria meningitidis disease: preparation of soluble protein and protein-polysaccharide immunogens. Infect Immun. 1982 Jul;37(1):271–280. doi: 10.1128/iai.37.1.271-280.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frasch C. E. Role of protein serotype antigens in protection against disease due to Neisseria meningitidis. J Infect Dis. 1977 Aug;136 (Suppl):S84–S90. doi: 10.1093/infdis/136.supplement.s84. [DOI] [PubMed] [Google Scholar]
- Fung J., Köhler H. Immune response to phosphorylcholine. VII. Functional evidence for three separate B cell subpopulations responding to TI and TD PC-antigens. J Immunol. 1980 Aug;125(2):640–646. [PubMed] [Google Scholar]
- Griffiss J. M. Bactericidal activity of meningococcal antisera. Blocking by IgA of lytic antibody in human convalescent sera. J Immunol. 1975 Jun;114(6):1779–1784. [PubMed] [Google Scholar]
- Griffiss J. M., Goroff D. K. IgA blocks IgM and IgG-initiated immune lysis by separate molecular mechanisms. J Immunol. 1983 Jun;130(6):2882–2885. [PubMed] [Google Scholar]
- Haregewoin A., Godal T., Mustafa A. S., Belehu A., Yemaneberhan T. T-cell conditioned media reverse T-cell unresponsiveness in lepromatous leprosy. Nature. 1983 May 26;303(5915):342–344. doi: 10.1038/303342a0. [DOI] [PubMed] [Google Scholar]
- Jerne N. K., Henry C., Nordin A. A., Fuji H., Koros A. M., Lefkovits I. Plaque forming cells: methodology and theory. Transplant Rev. 1974;18:130–191. doi: 10.1111/j.1600-065x.1974.tb01588.x. [DOI] [PubMed] [Google Scholar]
- Jerne N. K., Nordin A. A. Plaque Formation in Agar by Single Antibody-Producing Cells. Science. 1963 Apr 26;140(3565):405–405. doi: 10.1126/science.140.3565.405. [DOI] [PubMed] [Google Scholar]
- Julius M. H., von Boehmer H., Sidman C. L. Dissociation of two signals required for activation of resting B cells. Proc Natl Acad Sci U S A. 1982 Mar;79(6):1989–1993. doi: 10.1073/pnas.79.6.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanellopoulos-Langevin C., Mathieson B. J., Perkins A., Maynard A., Asofsky R. Regulation of anti-hapten antibody responses in vivo: memory, carrier-specific Lyt-2+ T cells can terminate antibody production without altering the peak of response. J Immunol. 1984 Apr;132(4):1639–1646. [PubMed] [Google Scholar]
- Kennedy M. W., Thomas D. B. A regulatory role for the memory B cell as suppressor-inducer of feedback control. J Exp Med. 1983 Feb 1;157(2):547–558. doi: 10.1084/jem.157.2.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny J. J., Yaffe L. J., Ahmed A., Metcalf E. S. Contribution of Lyb 5+ and Lyb 5- B cells to the primary and secondary phosphocholine-specific antibody response. J Immunol. 1983 Jun;130(6):2574–2579. [PubMed] [Google Scholar]
- Koenig S., Hoffmann M. K. Bacterial lipopolysaccharide activates suppressor B lymphocytes. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4608–4612. doi: 10.1073/pnas.76.9.4608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leinonen M., Frasch C. E. Class-specific antibody response to group B Neisseria meningitidis capsular polysaccharide: use of polylysine precoating in an enzyme-linked immunosorbent assay. Infect Immun. 1982 Dec;38(3):1203–1207. doi: 10.1128/iai.38.3.1203-1207.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowell G. H., Smith L. F., Artenstein M. S., Nash G. S., MacDermott R. P., Jr Antibody-dependent cell-mediated antibacterial activity of human mononuclear cells. I. K lymphocytes and monocytes are effective against meningococi in cooperation with human imune sera. J Exp Med. 1979 Jul 1;150(1):127–137. doi: 10.1084/jem.150.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowell G. H., Smith L. F., Griffiss J. M., Brandt B. L., MacDermott R. P. Antibody-dependent mononuclear cell-mediated antimeningococcal activity. Comparison of the effects of convalescent and postimmunization immunoglobulins G, M, and A. J Clin Invest. 1980 Aug;66(2):260–267. doi: 10.1172/JCI109852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackie E. B., Longenecker B. M., Rabin H. R., Di Ninno V. L., Bryan L. E. Immune response of the mouse to gram-negative bacterial outer membrane extracts as assessed with monoclonal antibodies. J Immunol. 1982 Aug;129(2):829–832. [PubMed] [Google Scholar]
- McDaniel L. S., Benjamin W. H., Jr, Forman C., Briles D. E. Blood clearance by anti-phosphocholine antibodies as a mechanism of protection in experimental pneumococcal bacteremia. J Immunol. 1984 Dec;133(6):3308–3312. [PubMed] [Google Scholar]
- Mehra V., Brennan P. J., Rada E., Convit J., Bloom B. R. Lymphocyte suppression in leprosy induced by unique M. leprae glycolipid. Nature. 1984 Mar 8;308(5955):194–196. doi: 10.1038/308194a0. [DOI] [PubMed] [Google Scholar]
- Melchers F., Andersson J. B cell activation: three steps and their variations. Cell. 1984 Jul;37(3):713–720. doi: 10.1016/0092-8674(84)90407-0. [DOI] [PubMed] [Google Scholar]
- Moreno C., Esdaile J. Immunoglobulin isotype in the murine response to polysaccharide antigens. Eur J Immunol. 1983 Mar;13(3):262–264. doi: 10.1002/eji.1830130317. [DOI] [PubMed] [Google Scholar]
- Nakayama S., Du Clos T. W., Gewurz H., Mold C. Inhibition of antibody responses to phosphocholine by C-reactive protein. J Immunol. 1984 Mar;132(3):1336–1340. [PubMed] [Google Scholar]
- Phipps R. P., Scott D. W. A novel role for macrophages: the ability of macrophages to tolerize B cells. J Immunol. 1983 Nov;131(5):2122–2127. [PubMed] [Google Scholar]
- Plaut A. G., Gilbert J. V., Artenstein M. S., Capra J. D. Neisseria gonorrhoeae and neisseria meningitidis: extracellular enzyme cleaves human immunoglobulin A. Science. 1975 Dec 12;190(4219):1103–1105. doi: 10.1126/science.810892. [DOI] [PubMed] [Google Scholar]
- Plaut A. G. The IgA1 proteases of pathogenic bacteria. Annu Rev Microbiol. 1983;37:603–622. doi: 10.1146/annurev.mi.37.100183.003131. [DOI] [PubMed] [Google Scholar]
- Poolman J. T., Hopman C. T., Zanen H. C. Immunogenicity of meningococcal antigens as detected in patient sera. Infect Immun. 1983 Apr;40(1):398–406. doi: 10.1128/iai.40.1.398-406.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reth M., Kelsoe G., Rajewsky K. Idiotypic regulation by isologous monoclonal anti-idiotope antibodies. Nature. 1981 Mar 19;290(5803):257–259. doi: 10.1038/290257a0. [DOI] [PubMed] [Google Scholar]
- Roberts R. B. The interaction in vitro between group B meningococci and rabbit polymorphonuclear leukocytes. Demonstration of type specific opsonins and bactericidins. J Exp Med. 1967 Nov 1;126(5):795–818. doi: 10.1084/jem.126.5.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider H., Hale T. L., Zollinger W. D., Seid R. C., Jr, Hammack C. A., Griffiss J. M. Heterogeneity of molecular size and antigenic expression within lipooligosaccharides of individual strains of Neisseria gonorrhoeae and Neisseria meningitidis. Infect Immun. 1984 Sep;45(3):544–549. doi: 10.1128/iai.45.3.544-549.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer A., Morrissey P. J., Hathcock K. S., Ahmed A., Scher I., Hodes R. J. Role of the major histocompatibility complex in T cell activation of B cell subpopulations Lyb-5+ and Lyb-5- B cell subpopulations differ in their requirement for major histocompatibility complex-restricted T cell recognition. J Exp Med. 1981 Aug 1;154(2):501–516. doi: 10.1084/jem.154.2.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparkes B. G. Dual effect of meningococcal antigens on a T cell dependent immune response. Can J Microbiol. 1983 Dec;29(12):1619–1625. doi: 10.1139/m83-248. [DOI] [PubMed] [Google Scholar]
- Sparkes B. G. Immunomodulating activity of meningococcal antigens. Can J Microbiol. 1983 Dec;29(12):1611–1618. doi: 10.1139/m83-247. [DOI] [PubMed] [Google Scholar]
- Stein L. D., Sigal N. H. Heterogeneity of the human phosphocholine-specific B cell repertoire. J Immunol. 1984 Mar;132(3):1329–1335. [PubMed] [Google Scholar]
- Sugasawara R. J., Prato C., Sippel J. E. Monoclonal antibodies against Neisseria meningitidis lipopolysaccharide. Infect Immun. 1983 Dec;42(3):863–868. doi: 10.1128/iai.42.3.863-868.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaitukaitis J. L. Production of antisera with small doses of immunogen: multiple intradermal injections. Methods Enzymol. 1981;73(Pt B):46–52. doi: 10.1016/0076-6879(81)73055-6. [DOI] [PubMed] [Google Scholar]
- Wicker L. S., Guelde G., Scher I., Kenny J. J. Regulation of T15 idiotype dominance. II. Genes unlinked to the Igh locus regulate T15 dominance of the secondary adoptive transfer response to phosphocholine. J Immunol. 1985 Jan;134(1):16–22. [PubMed] [Google Scholar]
- Wicker L. S., Guelde G., Scher I., Kenny J. J. The asymmetry in idiotype-isotype expression in the response to phosphocholine is due to divergence in the expressed repertoires of Lyb-5+ and Lyb-5- B cells. J Immunol. 1983 Nov;131(5):2468–2476. [PubMed] [Google Scholar]
- Zollinger W. D., Dalrymple J. M., Artenstein M. S. Analysis of parameters affecting the solid phase radioimmunoassay quantitation of antibody to meningococcal antigens. J Immunol. 1976 Nov;117(5 PT2):1788–1798. [PubMed] [Google Scholar]


