Abstract
The ability of 21 nonencapsulated and 15 encapsulated coagulase-negative staphylococci (CNS) to adhere to xylene in xylene-water emulsions and to fluorinated poly(ethylenepropylene) (FEP) films revealed remarkable differences. Nonencapsulated CNS strains adhered well to FEP, whereas their adherence to xylene ranged widely. Encapsulated strains with low adherence to xylene showed slight adherence to FEP. Encapsulated strains which adhered well to xylene ranged widely in their adherence to FEP. It was concluded that results obtained from the xylene adherence test were not predictive of the adherence of CNS to the hydrophobic FEP surface. The number of nonwashed, slime-producing CNS strains adhering to FEP was similar to that of washed bacteria of the same strains. Bacterial adherence to FEP was decreased when FEP films were exposed to a solution containing extracellular products (EP) obtained from a slime-producing CNS strain. Bacterial adherence to xylene also decreased when the bacterial suspensions contained EP. Apparently, initial adherence of CNS to FEP and xylene is hampered by EP. Nonencapsulated and encapsulated CNS pretreated with proteolytic enzymes failed to adhere to xylene and FEP, indicating that intact surface proteins or constituents associated with surface proteins mediated their adherence to xylene and FEP. Freeze-etch replicas of a CNS strain adhering to FEP showed a smooth, flattened area on the bacterial surface at the contact site of the bacteria with the FEP, indicating that an external layer was present at the bacterial surface.
Full text
PDF

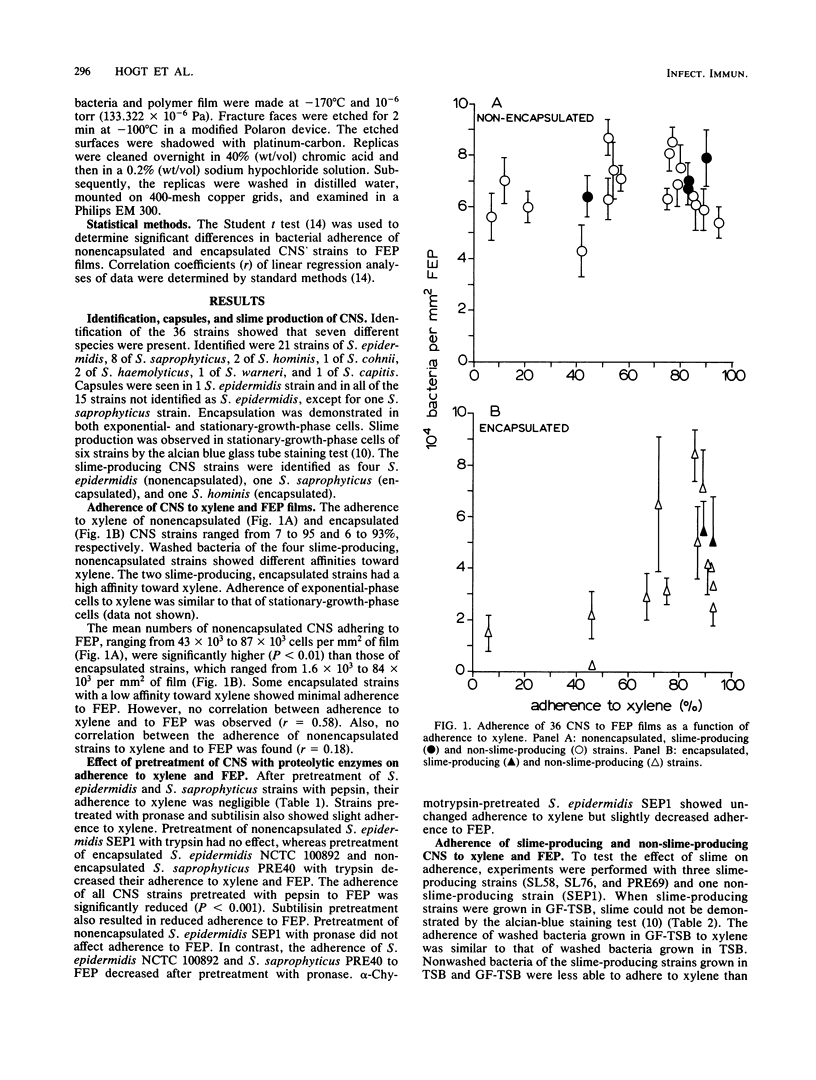


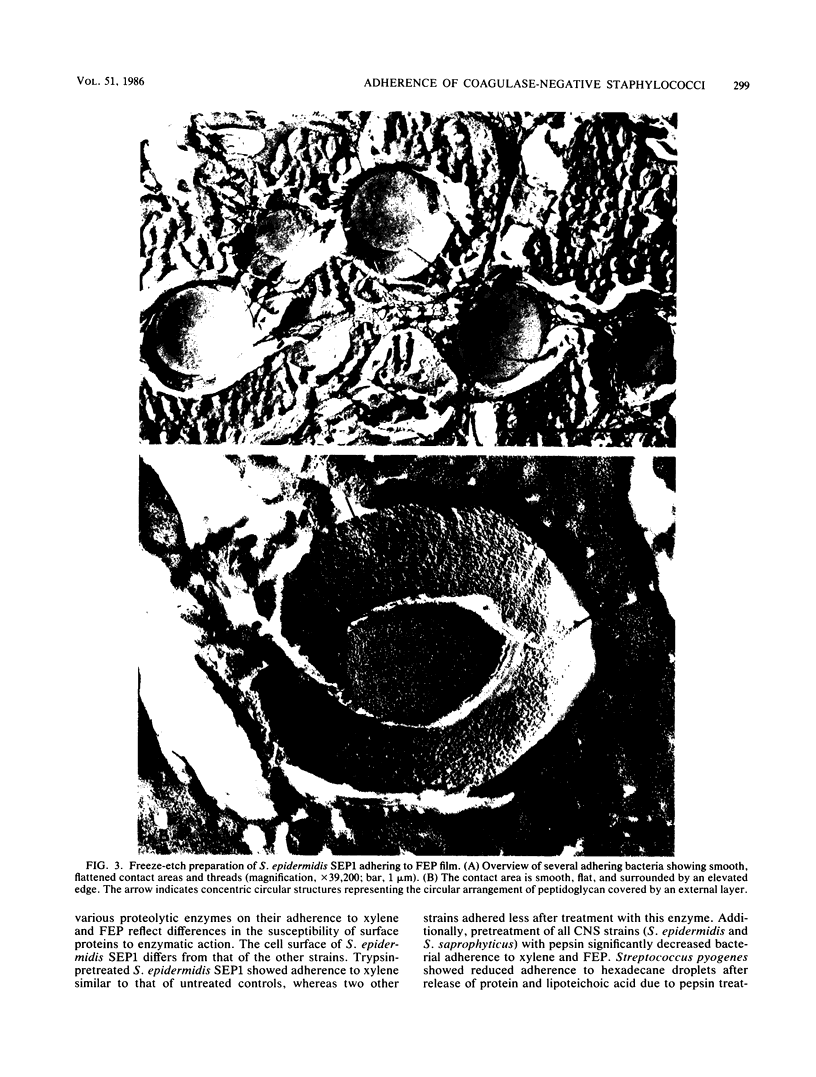


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amako K., Umeda A., Murata K. Arrangement of peptidoglycan in the cell wall of Staphylococcus spp. J Bacteriol. 1982 May;150(2):844–850. doi: 10.1128/jb.150.2.844-850.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson J. C., Wilson C. D. Encapsulated, coagulase-negative strain of Staphylococcus simulans. Infect Immun. 1981 Jul;33(1):304–308. doi: 10.1128/iai.33.1.304-308.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baddour L. M., Christensen G. D., Hester M. G., Bisno A. L. Production of experimental endocarditis by coagulase-negative staphylococci: variability in species virulence. J Infect Dis. 1984 Nov;150(5):721–727. doi: 10.1093/infdis/150.5.721. [DOI] [PubMed] [Google Scholar]
- Bandyk D. F., Berni G. A., Thiele B. L., Towne J. B. Aortofemoral graft infection due to Staphylococcus epidermidis. Arch Surg. 1984 Jan;119(1):102–108. doi: 10.1001/archsurg.1984.01390130084015. [DOI] [PubMed] [Google Scholar]
- Bayston R. A model of catheter colonisation in vitro and its relationship to clinical catheter infections. J Infect. 1984 Nov;9(3):271–276. doi: 10.1016/s0163-4453(84)90596-6. [DOI] [PubMed] [Google Scholar]
- Blumenkrantz N., Asboe-Hansen G. New method for quantitative determination of uronic acids. Anal Biochem. 1973 Aug;54(2):484–489. doi: 10.1016/0003-2697(73)90377-1. [DOI] [PubMed] [Google Scholar]
- Caputy G. G., Costerton J. W. Morphological examination of the glycocalyces of Staphylococcus aureus strains Wiley and Smith. Infect Immun. 1982 May;36(2):759–767. doi: 10.1128/iai.36.2.759-767.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choo M. H., Holmes D. R., Jr, Gersh B. J., Maloney J. D., Merideth J., Pluth J. R., Trusty J. Permanent pacemaker infections: characterization and management. Am J Cardiol. 1981 Sep;48(3):559–564. doi: 10.1016/0002-9149(81)90088-6. [DOI] [PubMed] [Google Scholar]
- Christensen G. D., Bisno A. L., Parisi J. T., McLaughlin B., Hester M. G., Luther R. W. Nosocomial septicemia due to multiply antibiotic-resistant Staphylococcus epidermidis. Ann Intern Med. 1982 Jan;96(1):1–10. doi: 10.7326/0003-4819-96-1-1. [DOI] [PubMed] [Google Scholar]
- Christensen G. D., Simpson W. A., Bisno A. L., Beachey E. H. Adherence of slime-producing strains of Staphylococcus epidermidis to smooth surfaces. Infect Immun. 1982 Jul;37(1):318–326. doi: 10.1128/iai.37.1.318-326.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen G. D., Simpson W. A., Bisno A. L., Beachey E. H. Experimental foreign body infections in mice challenged with slime-producing Staphylococcus epidermidis. Infect Immun. 1983 Apr;40(1):407–410. doi: 10.1128/iai.40.1.407-410.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUGUID J. P. The demonstration of bacterial capsules and slime. J Pathol Bacteriol. 1951 Oct;63(4):673–685. doi: 10.1002/path.1700630413. [DOI] [PubMed] [Google Scholar]
- Ekstedt R. D., Bernhard J. M. Preparation and characterization of a slime layer material produced by Staphylococcus aureus. Proc Soc Exp Biol Med. 1973 Jan;142(1):86–91. doi: 10.3181/00379727-142-36964. [DOI] [PubMed] [Google Scholar]
- Franson T. R., Sheth N. K., Rose H. D., Sohnle P. G. Scanning electron microscopy of bacteria adherent to intravascular catheters. J Clin Microbiol. 1984 Sep;20(3):500–505. doi: 10.1128/jcm.20.3.500-505.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grov A., Helgeland S. M. Immunochemical characterization of Stapylococcus epidermidis and Micrococcus cell walls. Acta Pathol Microbiol Scand B Microbiol Immunol. 1971;79(6):812–818. doi: 10.1111/j.1699-0463.1971.tb00116.x. [DOI] [PubMed] [Google Scholar]
- Hogt A. H., Dankert J., Feijen J. Adhesion of Staphylococcus epidermidis and Staphylococcus saprophyticus to a hydrophobic biomaterial. J Gen Microbiol. 1985 Sep;131(9):2485–2491. doi: 10.1099/00221287-131-9-2485. [DOI] [PubMed] [Google Scholar]
- Hogt A. H., Dankert J., de Vries J. A., Feijen J. Adhesion of coagulase-negative staphylococci to biomaterials. J Gen Microbiol. 1983 Sep;129(9):2959–2968. doi: 10.1099/00221287-129-9-2959. [DOI] [PubMed] [Google Scholar]
- ITZHAKI R. F., GILL D. M. A MICRO-BIURET METHOD FOR ESTIMATING PROTEINS. Anal Biochem. 1964 Dec;9:401–410. doi: 10.1016/0003-2697(64)90200-3. [DOI] [PubMed] [Google Scholar]
- Ichiman Y., Yoshida K. The relationship of capsular-type of Staphylococcus epidermidis to virulence and induction of resistance in the mouse. J Appl Bacteriol. 1981 Oct;51(2):229–241. doi: 10.1111/j.1365-2672.1981.tb01237.x. [DOI] [PubMed] [Google Scholar]
- Inman R. D., Gallegos K. V., Brause B. D., Redecha P. B., Christian C. L. Clinical and microbial features of prosthetic joint infection. Am J Med. 1984 Jul;77(1):47–53. doi: 10.1016/0002-9343(84)90434-0. [DOI] [PubMed] [Google Scholar]
- Karchmer A. W., Archer G. L., Dismukes W. E. Staphylococcus epidermidis causing prosthetic valve endocarditis: microbiologic and clinical observations as guides to therapy. Ann Intern Med. 1983 Apr;98(4):447–455. doi: 10.7326/0003-4819-98-4-447. [DOI] [PubMed] [Google Scholar]
- Kloos W. E., Schleifer K. H. Simplified scheme for routine identification of human Staphylococcus species. J Clin Microbiol. 1975 Jan;1(1):82–88. doi: 10.1128/jcm.1.1.82-88.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locci R., Peters G., Pulverer G. Microbial colonization of prosthetic devices. III. Adhesion of staphylococci to lumina of intravenous catheters perfused with bacterial suspensions. Zentralbl Bakteriol Mikrobiol Hyg B. 1981;173(5):300–307. [PubMed] [Google Scholar]
- Lowy F. D., Hammer S. M. Staphylococcus epidermidis infections. Ann Intern Med. 1983 Dec;99(6):834–839. doi: 10.7326/0003-4819-99-6-834. [DOI] [PubMed] [Google Scholar]
- Minagi S., Miyake Y., Inagaki K., Tsuru H., Suginaka H. Hydrophobic interaction in Candida albicans and Candida tropicalis adherence to various denture base resin materials. Infect Immun. 1985 Jan;47(1):11–14. doi: 10.1128/iai.47.1.11-14.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofek I., Whitnack E., Beachey E. H. Hydrophobic interactions of group A streptococci with hexadecane droplets. J Bacteriol. 1983 Apr;154(1):139–145. doi: 10.1128/jb.154.1.139-145.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters G., Locci R., Pulverer G. Adherence and growth of coagulase-negative staphylococci on surfaces of intravenous catheters. J Infect Dis. 1982 Oct;146(4):479–482. doi: 10.1093/infdis/146.4.479. [DOI] [PubMed] [Google Scholar]
- Peters G., Locci R., Pulverer G. Microbial colonization of prosthetic devices. II. Scanning electron microscopy of naturally infected intravenous catheters. Zentralbl Bakteriol Mikrobiol Hyg B. 1981;173(5):293–299. [PubMed] [Google Scholar]
- Ponce de Leon S., Wenzel R. P. Hospital-acquired bloodstream infections with Staphylococcus epidermidis. Review of 100 cases. Am J Med. 1984 Oct;77(4):639–644. doi: 10.1016/0002-9343(84)90354-1. [DOI] [PubMed] [Google Scholar]
- Press O. W., Ramsey P. G., Larson E. B., Fefer A., Hickman R. O. Hickman catheter infections in patients with malignancies. Medicine (Baltimore) 1984 Jul;63(4):189–200. doi: 10.1097/00005792-198407000-00001. [DOI] [PubMed] [Google Scholar]
- Price E. H. Staphylococcus epidermidis infections of cerebrospinal fluid shunts. J Hosp Infect. 1984 Mar;5(1):7–17. doi: 10.1016/0195-6701(84)90096-3. [DOI] [PubMed] [Google Scholar]
- Wade J. C., Schimpff S. C., Newman K. A., Wiernik P. H. Staphylococcus epidermidis: an increasing cause of infection in patients with granulocytopenia. Ann Intern Med. 1982 Oct;97(4):503–508. doi: 10.7326/0003-4819-97-4-503. [DOI] [PubMed] [Google Scholar]
- Yoshida K., Ekstedt R. D. Relation of mucoid growth of Staphylococcus aureus to clumping factor reaction, morphology in serum-soft agar, and virulence. J Bacteriol. 1968 Oct;96(4):902–908. doi: 10.1128/jb.96.4.902-908.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida K., Minegishi Y. Isolation of an encapsulated strain of Staphylococcus epidermidis. J Appl Bacteriol. 1979 Oct;47(2):299–301. doi: 10.1111/j.1365-2672.1979.tb01758.x. [DOI] [PubMed] [Google Scholar]



