Abstract
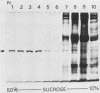
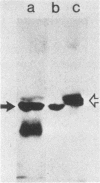
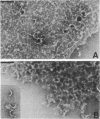
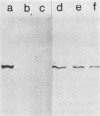
Listeriolysin was isolated from target rabbit erythrocyte membranes after lysis of the cells with partially purified toxin derived from a culture supernatant of Listeria ivanovii. The membrane form of the toxin exhibited properties similar to those previously found for streptolysin O. Detergent-solubilized, delipidated listeriolysin was found to comprise a heterogeneous population of partially and fully circularized, amphiphilic oligomers whose embedment within the lipid bilayer generated large transmembrane pores. The molecular weight of the toxin monomer was estimated to be 55,000 to 60,000 by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Immunological cross-reactions between the toxin and streptolysin O were demonstrable by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and immunoblotting. An immunoblot assay for detecting listeriolysin in agar-incorporated, lysed erythrocyte membranes was developed, and 28 defined, clinical isolates of Listeria monocytogenes were examined for toxin production. These isolates caused beta-hemolysis on the agar plates and had previously been regarded as listeriolysin producers. However, we found that only two isolates produced genuine listeriolysin, since the sensitive immunoblot assay entirely failed to detect the toxin in all other cases. We excluded that this finding derived from proteolytic degradation of membrane-bound toxin. Thus, the great majority of human pathogenic Listeria strains appear to produce one or several hemolysins that are immunologically and, by inference, molecularly distinct from the streptolysin O-related listeriolysin. We propose that the streptolysin O-related toxin be designated alpha-listeriolysin and that the other hemolysin(s) be termed beta-listeriolysin.
Full text
PDF


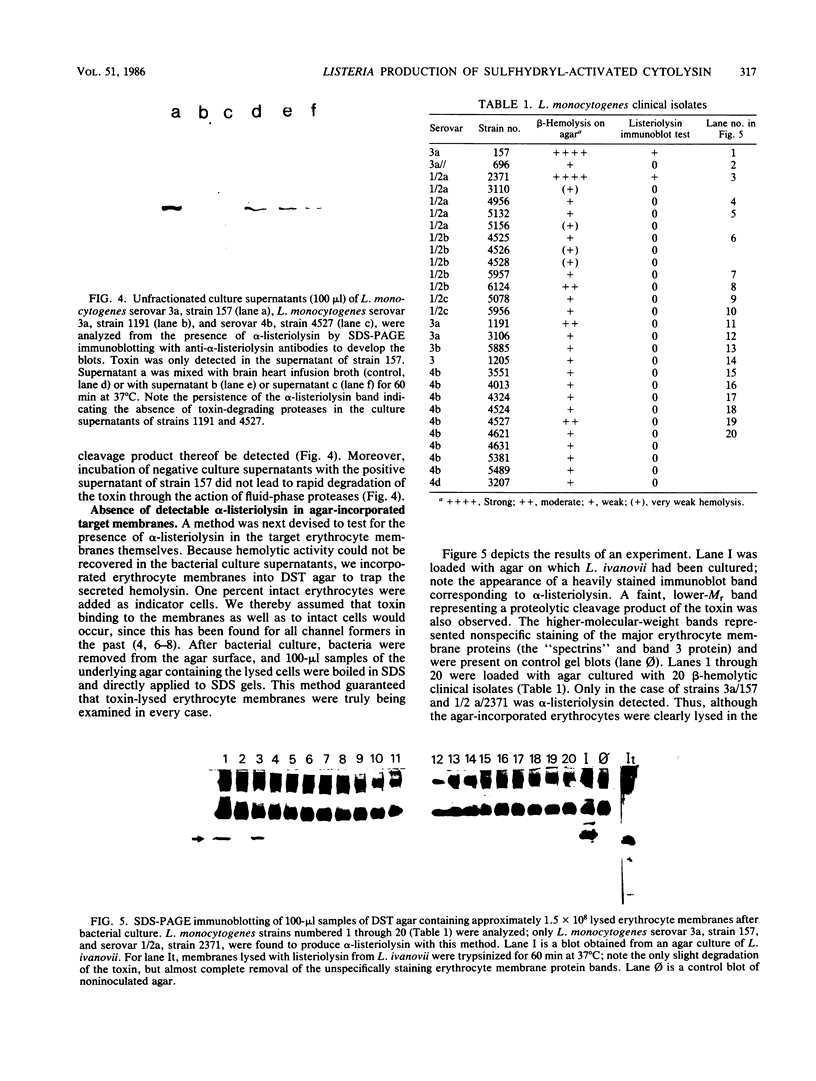


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bhakdi S., Muhly M., Füssle R. Correlation between toxin binding and hemolytic activity in membrane damage by staphylococcal alpha-toxin. Infect Immun. 1984 Nov;46(2):318–323. doi: 10.1128/iai.46.2.318-323.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhakdi S., Roth M., Sziegoleit A., Tranum-Jensen J. Isolation and identification of two hemolytic forms of streptolysin-O. Infect Immun. 1984 Nov;46(2):394–400. doi: 10.1128/iai.46.2.394-400.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhakdi S., Tranum-Jensen J. Mechanism of complement cytolysis and the concept of channel-forming proteins. Philos Trans R Soc Lond B Biol Sci. 1984 Sep 6;306(1129):311–324. doi: 10.1098/rstb.1984.0092. [DOI] [PubMed] [Google Scholar]
- Bhakdi S., Tranum-Jensen J., Sziegoleit A. Mechanism of membrane damage by streptolysin-O. Infect Immun. 1985 Jan;47(1):52–60. doi: 10.1128/iai.47.1.52-60.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckingham L., Duncan J. L. Approximate dimensions of membrane lesions produced by streptolysin S and streptolysin O. Biochim Biophys Acta. 1983 Mar 23;729(1):115–122. doi: 10.1016/0005-2736(83)90462-5. [DOI] [PubMed] [Google Scholar]
- Füssle R., Bhakdi S., Sziegoleit A., Tranum-Jensen J., Kranz T., Wellensiek H. J. On the mechanism of membrane damage by Staphylococcus aureus alpha-toxin. J Cell Biol. 1981 Oct;91(1):83–94. doi: 10.1083/jcb.91.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIRARD K. F., SBARRA A. J., BARDAWIL W. A. Serology of Listeria monocytogenes. I. Characteristics of the soluble hemolysin. J Bacteriol. 1963 Feb;85:349–355. doi: 10.1128/jb.85.2.349-355.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giavedoni E. B., Chow Y. M., Dalmasso A. P. The functional size of the primary complement lesion in resealed erythrocyte membrane ghosts. J Immunol. 1979 Jan;122(1):240–245. [PubMed] [Google Scholar]
- Groves R. D., Welshimer H. J. Separation of pathogenic from apathogenic Listeria monocytogenes by three in vitro reactions. J Clin Microbiol. 1977 Jun;5(6):559–563. doi: 10.1128/jcm.5.6.559-563.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JENKINS E. M., NJOKU-OBI A. N., ADAMS E. W. PURIFICATION OF THE SOLUBLE HEMOLYSINS OF LISTERIA MONOCYTOGENES. J Bacteriol. 1964 Aug;88:418–424. doi: 10.1128/jb.88.2.418-424.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins E. M., Watson B. B. Extracellular Antigens from Listeria monocytogenes I. Purification and Resolution of Hemolytic and Lipolytic Antigens from Culture Filtrates of Listeria monocytogenes. Infect Immun. 1971 Apr;3(4):589–594. doi: 10.1128/iai.3.4.589-594.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan M. A., Seaman T. A., Woodbine M. Listeria monocytogenes-haemolysin: lecithinase. Zentralbl Bakteriol Orig A. 1973 Oct;225(1):66–79. [PubMed] [Google Scholar]
- Kingdon G. C., Sword C. P. Biochemical and Immunological Effects of Listeria monocytogenes Hemolysin. Infect Immun. 1970 Apr;1(4):363–372. doi: 10.1128/iai.1.4.363-372.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingdon G. C., Sword C. P. Cardiotoxic and Lethal Effects of Listeria monocytogenes Hemolysin. Infect Immun. 1970 Apr;1(4):373–379. doi: 10.1128/iai.1.4.373-379.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingdon G. C., Sword C. P. Effects of Listeria monocytogenes Hemolysin on Phagocytic Cells and Lysosomes. Infect Immun. 1970 Apr;1(4):356–362. doi: 10.1128/iai.1.4.356-362.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NJOKU-OBI A. N., JENKINS E. M., NJOKU-OBI J. C., ADAMS J., COVINGTON V. PRODUCTION AND NATURE OF LISTERIA MONOCYTOGENES HEMOLYSINS. J Bacteriol. 1963 Jul;86:1–8. doi: 10.1128/jb.86.1.1-8.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeliger H. P., Schrettenbrunner A., Pongratz G., Hof H. Zur Sonderstellung stark hämolysierender Stämme der Gattung Listeria. Zentralbl Bakteriol Mikrobiol Hyg A. 1982 Jun;252(2):176–190. [PubMed] [Google Scholar]
- Skalka B., Smola J., Elischerová K. Routine test for in vitro differentiation of pathogenic and apathogenic Listeria monocytogenes strains. J Clin Microbiol. 1982 Mar;15(3):503–507. doi: 10.1128/jcm.15.3.503-507.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson B. B., Lavizzo J. C. Extracellular antigens from Listeria monocytogenes. II. Cytotoxicity of hemolytic and lipolytic antigens of Listeria for cultured mouse macrophages. Infect Immun. 1973 May;7(5):753–758. doi: 10.1128/iai.7.5.753-758.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]