Abstract
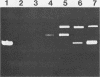
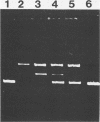
Mycoplasma pulmonis has substantial DNase activity exposed on the cell surface. At least part of this activity is attributable to an endonuclease. The activity is destroyed at 56 degrees C and inhibited by either 5 mM EDTA or 10 mM zinc chloride. It can also be eliminated by treatment of intact organisms with trypsin and is regenerated by incubation of the treated organisms in a medium that supports protein synthesis. DNase exposed at the cell surface constitutes 20% of the total DNase activity present in M. pulmonis extracts.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birnboim H. C. A rapid alkaline extraction method for the isolation of plasmid DNA. Methods Enzymol. 1983;100:243–255. doi: 10.1016/0076-6879(83)00059-2. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Brubaker R. R. The genus Yersinia: biochemistry and genetics of virulence. Curr Top Microbiol Immunol. 1972;57:111–158. doi: 10.1007/978-3-642-65297-4_4. [DOI] [PubMed] [Google Scholar]
- Chang A. C., Cohen S. N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol. 1978 Jun;134(3):1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayflick L. Tissue cultures and mycoplasmas. Tex Rep Biol Med. 1965 Jun;23(Suppl):285+–285+. [PubMed] [Google Scholar]
- McIvor R. S., Kenny G. E. Differences in incorporation of nucleic acid bases and nucleosides by various Mycoplasma and Acholeplasma species. J Bacteriol. 1978 Aug;135(2):483–489. doi: 10.1128/jb.135.2.483-489.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minion F. C., Cassell G. H., Pnini S., Kahane I. Multiphasic interactions of Mycoplasma pulmonis with erythrocytes defined by adherence and hemagglutination. Infect Immun. 1984 May;44(2):394–400. doi: 10.1128/iai.44.2.394-400.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ne'eman Z., Razin S. Characterization of the mycoplasma membrane proteins. V. Release and localization of membrane-bound enzymes in Acholeplasma laidlawii. Biochim Biophys Acta. 1975 Jan 14;375(1):54–68. doi: 10.1016/0005-2736(75)90072-3. [DOI] [PubMed] [Google Scholar]
- Pollack J. D., Hoffmann P. J. Properties of the nucleases of mollicutes. J Bacteriol. 1982 Oct;152(1):538–541. doi: 10.1128/jb.152.1.538-541.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollack J. D., Razin S., Cleverdon R. C. Localization of Enzymes in Mycoplasma. J Bacteriol. 1965 Sep;90(3):617–622. doi: 10.1128/jb.90.3.617-622.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razin S. The mycoplasmas. Microbiol Rev. 1978 Jun;42(2):414–470. doi: 10.1128/mr.42.2.414-470.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens M. A. Partial purification and cleavage specificity of a site-specific endonuclease, SciNI, isolated from Spiroplasma citri. J Bacteriol. 1982 Feb;149(2):508–514. doi: 10.1128/jb.149.2.508-514.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]