Abstract
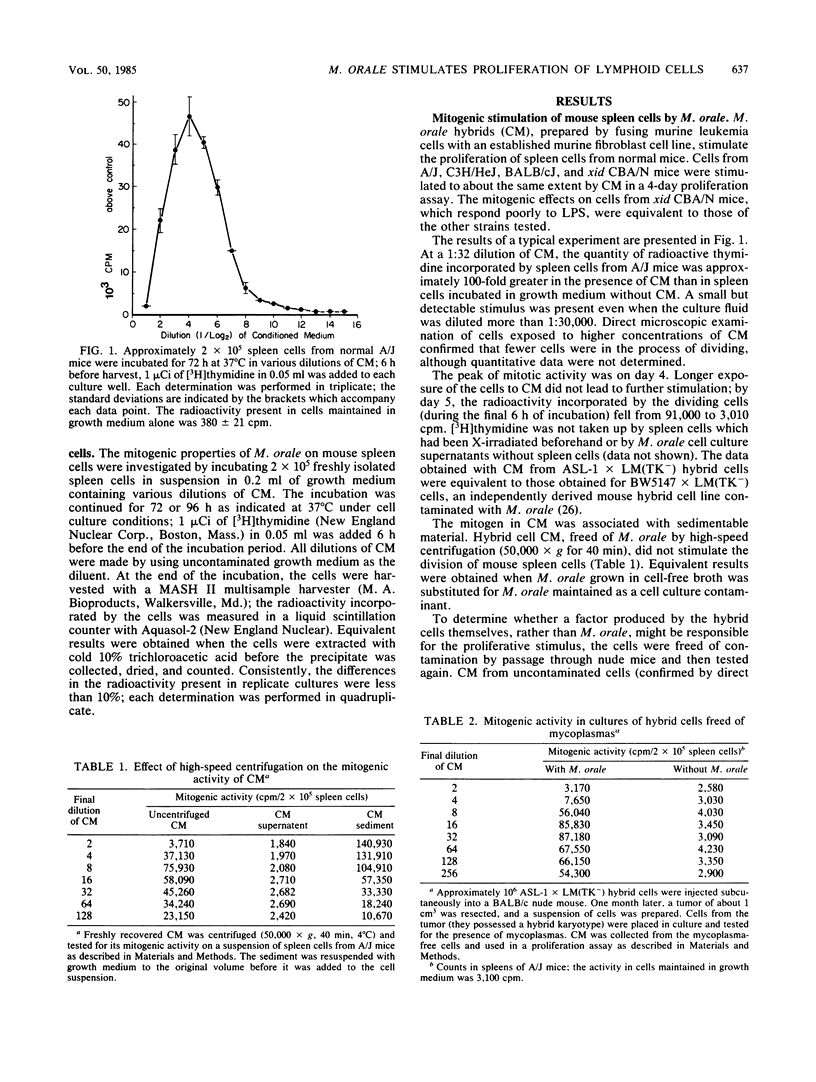
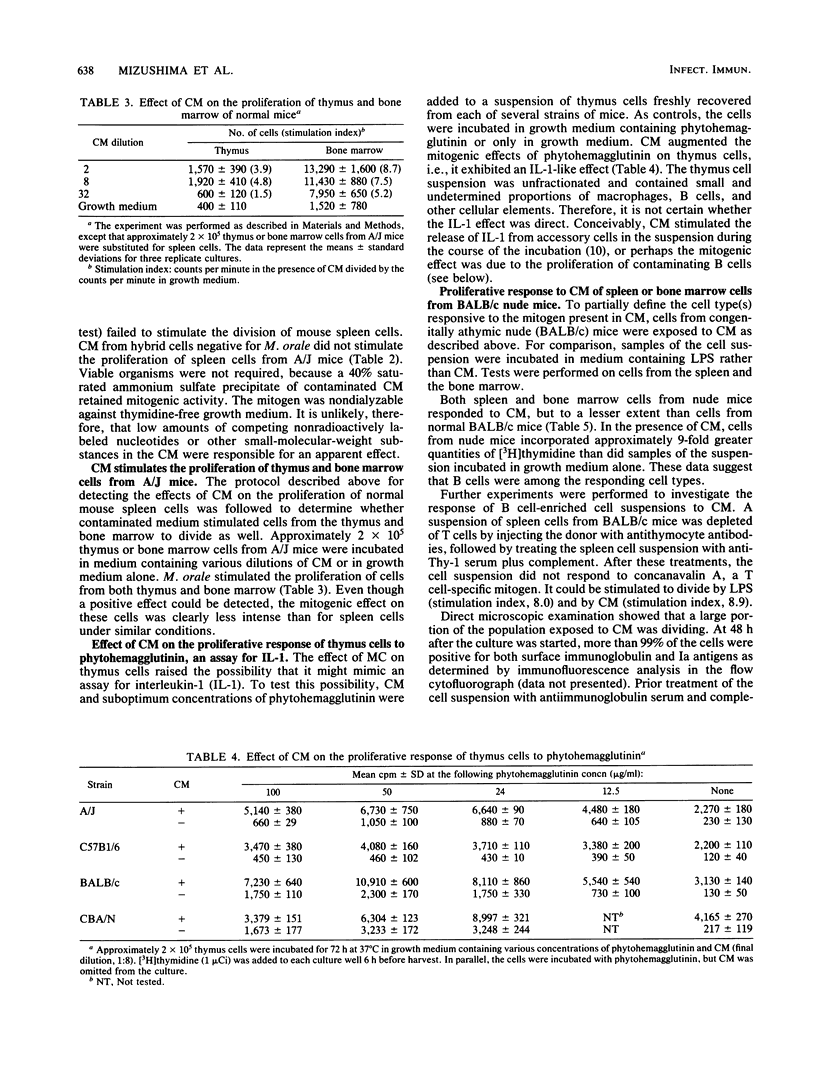
Mycoplasma orale, maintained as a contaminant of a mouse hybrid cell line, induces an intense proliferation in short-term culture of lymphoid cells of inbred mice. Cell division induced by the contaminated cell culture fluid reaches a maximum on day four and declines rapidly thereafter. Culture fluids from hybrid cells freed of contamination do not cause proliferation. Cells from the spleen, bone marrow, and thymus of each of several strains of inbred mice, including xid CBA/N, poorly responsive to lipopolysaccharide, are stimulated by the mitogen, as are cells from BALB/c nude mice. The characteristics of the stimulatory effect are analogous in several important aspects to those of naturally occurring T cell-derived growth factors. In the absence of detectable numbers of T cells, both small and large B lymphocytes undergo mitosis in the presence of contaminated cell culture fluid, and B cells stimulated to divide by lipopolysaccharide are sustained for further rounds of replication by M. orale-containing cell culture fluid. The fluid also augments the stimulatory effect on thymocytes of suboptimum concentrations of phytohemagglutinin mimicking the effect of interleukin-1. Unlike with most naturally occurring lymphoid cell mitogens, however, the dividing cells do not go on to immunoglobulin secretion.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Butler G. H., Göbel U., Stanbridge E. J. Blast transformation of mouse splenic B-lymphocytes with extracts of Mycoplasma hyorhinis. Isr J Med Sci. 1984 Sep;20(9):891–894. [PubMed] [Google Scholar]
- Cole B. C., Daynes R. A., Ward J. R. Stimulation of mouse lymphocytes by a mitogen derived from Mycoplasma arthritidis. III. Ir gene control of lymphocyte transformation correlates with binding of the mitogen to specific Ia-bearing cells. J Immunol. 1982 Oct;129(4):1352–1359. [PubMed] [Google Scholar]
- Cole B. C., Thorpe R. N. Induction of human gamma interferons by a mitogen derived form Mycoplasma arthritidis and by Phytohemagglutinin: differential inhibition with monoclonal anti-HLA.DR antibodies. J Immunol. 1983 Nov;131(5):2392–2396. [PubMed] [Google Scholar]
- Cole B. C., Washburn L. R., Sullivan G. J., Ward J. R. Specificity of a mycoplasma mitogen for lymphocytes from human and various animal hosts. Infect Immun. 1982 May;36(2):662–666. doi: 10.1128/iai.36.2.662-666.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrar J. J., Howard M., Fuller-Farrar J., Paul W. E. Biochemical and physicochemical characterization of mouse B cell growth factor: a lymphokine distinct from interleukin 2. J Immunol. 1983 Oct;131(4):1838–1842. [PubMed] [Google Scholar]
- Gourlay R. N. Mycoplasma-induced arthritis in farm animals. Isr J Med Sci. 1981 Jul;17(7):626–627. [PubMed] [Google Scholar]
- Hefeneider S. H., Conlon P. J., Henney C. S., Gillis S. In vivo interleukin 2 administration augments the generation of alloreactive cytolytic T lymphocytes and resident natural killer cells. J Immunol. 1983 Jan;130(1):222–227. [PubMed] [Google Scholar]
- Howard M., Farrar J., Hilfiker M., Johnson B., Takatsu K., Hamaoka T., Paul W. E. Identification of a T cell-derived b cell growth factor distinct from interleukin 2. J Exp Med. 1982 Mar 1;155(3):914–923. doi: 10.1084/jem.155.3.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawase I., Brooks C. G., Kuribayashi K., Olabuenaga S., Newman W., Gillis S., Henney C. S. Interleukin 2 induces gamma-interferon production: participation of macrophages and NK-like cells. J Immunol. 1983 Jul;131(1):288–292. [PubMed] [Google Scholar]
- Lynch D. H., Gurish M. F., Cole B. C., Daynes R. A. T cell proliferative responses to a mitogen derived from Mycoplasma arthritidis are controlled by the accessory cell. J Immunol. 1983 Oct;131(4):1702–1706. [PubMed] [Google Scholar]
- Maizel A. L., Morgan J. W., Mehta S. R., Kouttab N. M., Bator J. M., Sahasrabuddhe C. G. Long-term growth of human B cells and their use in a microassay for B-cell growth factor. Proc Natl Acad Sci U S A. 1983 Aug;80(16):5047–5051. doi: 10.1073/pnas.80.16.5047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maizel A., Sahasrabuddhe C., Mehta S., Morgan J., Lachman L., Ford R. Biochemical separation of a human B cell mitogenic factor. Proc Natl Acad Sci U S A. 1982 Oct;79(19):5998–6002. doi: 10.1073/pnas.79.19.5998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochizuki D., Watson J., Gillis S. Biochemical and biologic characterization of lymphocyte regulatory molecules. IV. Purification of Interleukin 2 from a murine T cell lymphoma. J Immunol. 1980 Dec;125(6):2579–2583. [PubMed] [Google Scholar]
- Muraguchi A., Butler J. L., Kehrl J. H., Fauci A. S. Differential sensitivity of human B cell subsets to activation signals delivered by anti-mu antibody and proliferative signals delivered by a monoclonal B cell growth factor. J Exp Med. 1983 Feb 1;157(2):530–546. doi: 10.1084/jem.157.2.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi K., Howard M., Muraguchi A., Farrar J., Takatsu K., Hamaoka T., Paul W. E. Soluble factors involved in B cell differentiation: identification of two distinct T cell-replacing factors (TRF). J Immunol. 1983 May;130(5):2219–2224. [PubMed] [Google Scholar]
- Naot Y., Ginsburg H. Activation of B lymphocytes by mycoplasma mitogen(s). Immunology. 1978 Apr;34(4):715–720. [PMC free article] [PubMed] [Google Scholar]
- Naot Y., Merchav S., Ben-David E., Ginsburg H. Mitogenic activity of Mycoplasma pulmonis. I. Stimulation of rat B and T lymphocytes. Immunology. 1979 Mar;36(3):399–406. [PMC free article] [PubMed] [Google Scholar]
- Naot Y., Merchav S., Ginsburg H. Mycoplasma neurolyticum: a potent mitogen for rat B lymphocytes. Eur J Immunol. 1979 Mar;9(3):185–189. doi: 10.1002/eji.1830090303. [DOI] [PubMed] [Google Scholar]
- Parker D. C., Fothergill J. J., Wadsworth D. C. B lymphocyte activation by insoluble anti-immunoglobulin: induction of immunoglobulin secretion by a T cell-dependent soluble factor. J Immunol. 1979 Aug;123(2):931–941. [PubMed] [Google Scholar]
- Parker D. C., Wadsworth D. C., Schneider G. B. Activation of murine B lymphocytes by anti-immunoglobulin is an inductive signal leading to immunoglobulin secretion. J Exp Med. 1980 Jul 1;152(1):138–150. doi: 10.1084/jem.152.1.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proust J. J., Buchholz M. A., Nordin A. A. A "lymphokine-like" soluble product that induces proliferation and maturation of B cells appears in the serum-free supernatant of a T cell hybridoma as a consequence of mycoplasmal contamination. J Immunol. 1985 Jan;134(1):390–396. [PubMed] [Google Scholar]
- Ruuth E., Wieslander A., Persson H., Friedrich B., Lundgren E. Lymphokine-like activity of a strain of Mycoplasma arginini. Isr J Med Sci. 1984 Sep;20(9):886–890. [PubMed] [Google Scholar]
- Sidman C. L., Paige C. J., Schreier M. H. B cell maturation factor (BMF): a lymphokine or family of lymphokines promoting the maturation of B lymphocytes. J Immunol. 1984 Jan;132(1):209–222. [PubMed] [Google Scholar]
- Slomski R., Wang D. R., Cohen E. P. Surface antigens of immunoprotective leukaemia x fibroblast hybrid cells which have lost malignant properties in histocompatible mice differ from the malignant parental cells. Immunology. 1984 Jun;52(2):281–290. [PMC free article] [PubMed] [Google Scholar]
- Stanbridge E. J., Weiss R. L. Mycoplasma capping on lymphocytes. Nature. 1978 Dec 7;276(5688):583–587. doi: 10.1038/276583a0. [DOI] [PubMed] [Google Scholar]
- Welte K., Wang C. Y., Mertelsmann R., Venuta S., Feldman S. P., Moore M. A. Purification of human interleukin 2 to apparent homogeneity and its molecular heterogeneity. J Exp Med. 1982 Aug 1;156(2):454–464. doi: 10.1084/jem.156.2.454. [DOI] [PMC free article] [PubMed] [Google Scholar]



