Abstract
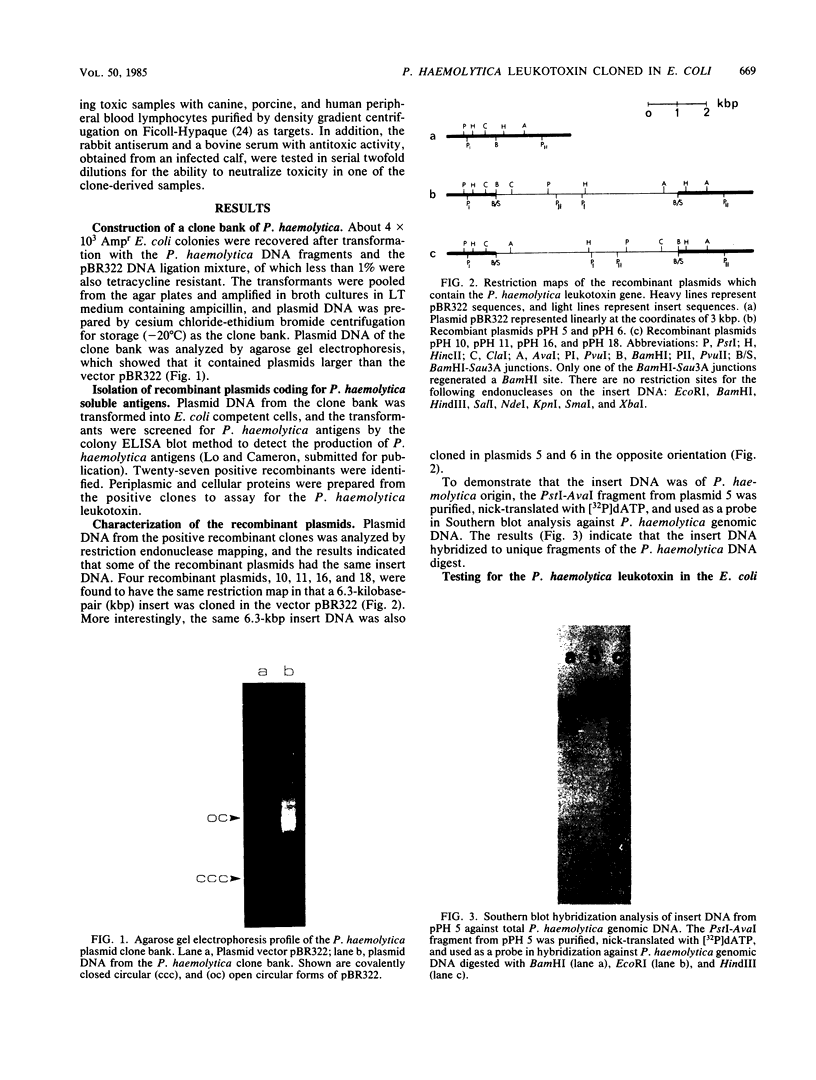
A clone bank of Pasteurella haemolytica A1 was constructed by partial digestion of the genomic DNA with Sau3A and ligation of 5- to 10-kilobase-pair fragments into the BamHI site of the plasmid vector pBR322. After transformation into Escherichia coli K-12, a total of 4 X 10(3) recombinant clones was obtained. These were screened for the production of P. haemolytica soluble antigens by a colony enzyme-linked immunosorbent assay blot method with a rabbit antiserum raised against the soluble antigens. The clones producing P. haemolytica soluble antigens were then analyzed for the production of the leukotoxin by a cytotoxicity assay with cells from a bovine leukemia-derived B-lymphocyte cell line as the target cells. Positive clones were identified, and subsequent restriction analysis of the recombinant plasmids showed that the same 6.3 kilobase pairs of insert DNA was cloned in either of the two orientations into the plasmid vector pBR322. One of the clones was selected for further characterization of the leukotoxin as produced in E. coli. Tests for heat lability and target cell species specificity with canine, porcine, and human peripheral blood lymphocytes indicated that the activity of the cloned leukotoxin was identical to that of the P. haemolytica leukotoxin. Furthermore, the E. coli-produced leukotoxin was also neutralized by bovine or rabbit antiserum known to have antitoxic activity. When cellular proteins from the E. coli clones were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western blot analysis, a 100,000-dalton protein was identified which corresponded to one of the soluble antigens found in the leukotoxic culture supernatant of P. haemolytica. These results demonstrated that the gene(s) for the P. haemolytica leukotoxin have been cloned and that the leukotoxin was expressed in E. coli.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baluyut C. S., Simonson R. R., Bemrick W. J., Maheswaran S. K. Interaction of Pasteurella haemolytica with bovine neutrophils: identification and partial characterization of a cytotoxin. Am J Vet Res. 1981 Nov;42(11):1920–1926. [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Clarke L., Carbon J. A colony bank containing synthetic Col El hybrid plasmids representative of the entire E. coli genome. Cell. 1976 Sep;9(1):91–99. doi: 10.1016/0092-8674(76)90055-6. [DOI] [PubMed] [Google Scholar]
- Coleman K., Dougan G., Arbuthnott J. P. Cloning, and expression in Escherichia coli K-12, of the chromosomal hemolysin (phospholipase C) determinant of Pseudomonas aeruginosa. J Bacteriol. 1983 Feb;153(2):909–915. doi: 10.1128/jb.153.2.909-915.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Confer A. W., Panciera R. J., Fulton R. W., Gentry M. J., Rummage J. A. Effect of vaccination with live or killed Pasteurella haemolytica on resistance to experimental bovine pneumonic pasteurellosis. Am J Vet Res. 1985 Feb;46(2):342–347. [PubMed] [Google Scholar]
- Gray G. L., Smith D. H., Baldridge J. S., Harkins R. N., Vasil M. L., Chen E. Y., Heyneker H. L. Cloning, nucleotide sequence, and expression in Escherichia coli of the exotoxin A structural gene of Pseudomonas aeruginosa. Proc Natl Acad Sci U S A. 1984 May;81(9):2645–2649. doi: 10.1073/pnas.81.9.2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock R. E., Carey A. M. Outer membrane of Pseudomonas aeruginosa: heat- 2-mercaptoethanol-modifiable proteins. J Bacteriol. 1979 Dec;140(3):902–910. doi: 10.1128/jb.140.3.902-910.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himmel M. E., Yates M. D., Lauerman L. H., Squire P. G. Purification and partial characterization of a macrophage cytotoxin from Pasteurella haemolytica. Am J Vet Res. 1982 May;43(5):764–767. [PubMed] [Google Scholar]
- Kaehler K. L., Markam R. J., Muscoplat C. C., Johnson D. W. Evidence of cytocidal effects of Pasteurella haemolytica on bovine peripheral blood mononuclear leukocytes. Am J Vet Res. 1980 Oct;41(10):1690–1693. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Maniatis T., Hardison R. C., Lacy E., Lauer J., O'Connell C., Quon D., Sim G. K., Efstratiadis A. The isolation of structural genes from libraries of eucaryotic DNA. Cell. 1978 Oct;15(2):687–701. doi: 10.1016/0092-8674(78)90036-3. [DOI] [PubMed] [Google Scholar]
- Martin S. W., Meek A. H., Davis D. G., Thomson R. G., Johnson J. A., Lopez A., Stephens L., Curtis R. A., Prescott J. F., Rosendal S. Factors associated with mortality in feedlot cattle: the Bruce County Beef Cattle Project. Can J Comp Med. 1980 Jan;44(1):1–10. [PMC free article] [PubMed] [Google Scholar]
- Martin S. W. Vaccination: Is it Effective in Preventing Respiratory Disease or Influencing Weight Gains in Feedlot Calves? Can Vet J. 1983 Jan;24(1):10–19. [PMC free article] [PubMed] [Google Scholar]
- Nasmyth K. A., Reed S. I. Isolation of genes by complementation in yeast: molecular cloning of a cell-cycle gene. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2119–2123. doi: 10.1073/pnas.77.4.2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neu H. C., Heppel L. A. The release of enzymes from Escherichia coli by osmotic shock and during the formation of spheroplasts. J Biol Chem. 1965 Sep;240(9):3685–3692. [PubMed] [Google Scholar]
- Otulakowski G. L., Shewen P. E., Udoh A. E., Mellors A., Wilkie B. N. Proteolysis of sialoglycoprotein by Pasteurella haemolytica cytotoxic culture supernatant. Infect Immun. 1983 Oct;42(1):64–70. doi: 10.1128/iai.42.1.64-70.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Shewen P. E., Wilkie B. N. Antibody titers to Pasteurella haemolytica A1 in Ontario beef cattle. Can J Comp Med. 1982 Oct;46(4):354–356. [PMC free article] [PubMed] [Google Scholar]
- Shewen P. E., Wilkie B. N. Cytotoxin of Pasteurella haemolytica acting on bovine leukocytes. Infect Immun. 1982 Jan;35(1):91–94. doi: 10.1128/iai.35.1.91-94.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu M., Pan I. C., Hess W. R. T and B lymphocytes in porcine blood. Am J Vet Res. 1976 Mar;37(3):309–317. [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Thompson R., Hughes S. G., Broda P. Plasmid identification using specific endonucleases. Mol Gen Genet. 1974;133(2):141–149. doi: 10.1007/BF00264835. [DOI] [PubMed] [Google Scholar]
- Wieslander L. A simple method to recover intact high molecular weight RNA and DNA after electrophoretic separation in low gelling temperature agarose gels. Anal Biochem. 1979 Oct 1;98(2):305–309. doi: 10.1016/0003-2697(79)90145-3. [DOI] [PubMed] [Google Scholar]





