Abstract
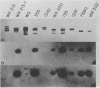
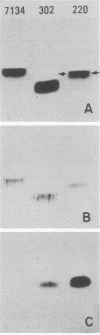
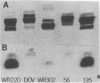
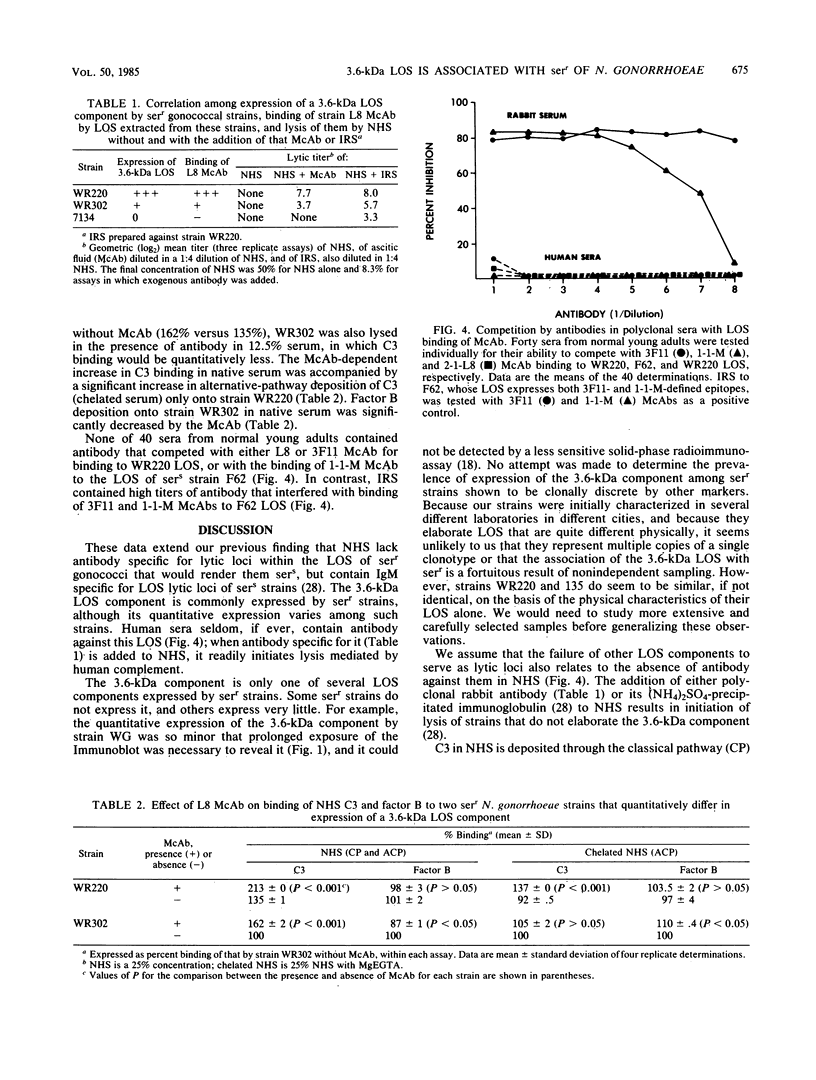
Neisseria gonorrhoeae strains that resist lysis by normal human sera (NHS) do so, in part, because NHS contain immunoglobulin M (IgM) specific for lipooligosaccharide (LOS) antigens of serum-sensitive strains, but lack antibodies for LOS antigens that can serve as loci for immune lysis of serum-resistant (serr) strains. We used a monoclonal antibody (McAb), specific for an epitope within a 3.6-kilodalton (kDa) component of Neisseria meningitidis L8 LOS, that binds a 3.6-kDa gonococcal LOS component so that we could explore further serr gonococcal strains. The McAb bound to the LOS of 6 of 7 serr of strains but not to the LOS of 0 of 14 serum-sensitive and serum-intermediate gonococcal strains of diverse origin. We studied three serr strains further. Strain 7134 does not elaborate the 3.6-kDa LOS component and does not bind the McAb; strains WR220 and WR302 do elaborate the 3.6-kDa LOS component. The titer (log2) at which the McAb, diluted in NHS, lysed strain WR220 was 7.7; for WR302 it was 3.7, and for 7134 it was 0. Addition of McAb to NHS caused increased classical and alternative-pathway C3 deposition onto strain WR220, but only classical-pathway-activated C3 deposition onto strain WR302. The difference in lytic effectiveness of the McAb for the two strains, therefore, may result from differences in alternative-pathway augmentation of McAb-dependent classical-pathway activation on their surfaces. None of 40 randomly selected normal young adults had serum antibody that could compete with the McAb for binding to WR220 LOS in a solid-phase RIA. We conclude that the 3.6-kDa LOS component is commonly expressed by serr strains of N. gonorrhoeae and that antibody to it would be lytic if present in human serum, but that it is infrequently, if ever, present. As a result, strains elaborating this LOS are resistant to lysis by NHS.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apicella M. A., Bennett K. M., Hermerath C. A., Roberts D. E. Monoclonal antibody analysis of lipopolysaccharide from Neisseria gonorrhoeae and Neisseria meningitidis. Infect Immun. 1981 Dec;34(3):751–756. doi: 10.1128/iai.34.3.751-756.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertram M. A., Griffiss J. M., Broud D. D. Response to antigenic determinants of Neisseria meningitidis lipopolysaccharide investigated with a new radioactive antigen-binding assay. J Immunol. 1976 Mar;116(3):842–846. [PubMed] [Google Scholar]
- Brade V., Lee G. D., Nicholson A., Shin H. S., Mayer M. M. The reaction of zymosan with the properdin system in normal and C4-deficienct guinea pig serum. Demonstration of C3- and C5-cleaving multi-unit enzymes, both containing factor B, and acceleration of their formation by the classical complement pathway. J Immunol. 1973 Nov;111(5):1389–1400. [PubMed] [Google Scholar]
- Bryan C. S. Sensitization of E. coli to the serum bactericidal system and to lysozyme by ethyleneglycoltetraacetic acid. Proc Soc Exp Biol Med. 1974 Apr;145(4):1431–1433. doi: 10.3181/00379727-145-38028. [DOI] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Clas F., Loos M. Antibody-independent binding of the first component of complement (C1) and its subcomponent C1q to the S and R forms of Salmonella minnesota. Infect Immun. 1981 Mar;31(3):1138–1144. doi: 10.1128/iai.31.3.1138-1144.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Counts G. W., Gregory D. F., Spearman J. G., Lee B. A., Filice G. A., Holmes K. K., Griffiss J. M. Group A meningococcal disease in the U.S. Pacific Northwest: epidemiology, clinical features, and effect of a vaccination control program. Rev Infect Dis. 1984 Sep-Oct;6(5):640–648. doi: 10.1093/clinids/6.5.640. [DOI] [PubMed] [Google Scholar]
- Eads M. E., Levy N. J., Kasper D. L., Baker C. J., Nicholson-Weller A. Antibody-independent activation of C1 by type Ia group B streptococci. J Infect Dis. 1982 Nov;146(5):665–672. doi: 10.1093/infdis/146.5.665. [DOI] [PubMed] [Google Scholar]
- Edwards M. S., Kasper D. L., Jennings H. J., Baker C. J., Nicholson-Weller A. Capsular sialic acid prevents activation of the alternative complement pathway by type III, group B streptococci. J Immunol. 1982 Mar;128(3):1278–1283. [PubMed] [Google Scholar]
- Griffiss J. M., Brandt B. L., Broud D. D. Human immune response to various doses of group Y and W135 meningococcal polysaccharide vaccines. Infect Immun. 1982 Jul;37(1):205–208. doi: 10.1128/iai.37.1.205-208.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiss J. M. Epidemiological value of lipopolysaccharide and heat-modifiable outer-membrane protein serotyping of group-A strains of Neisseria meningitidis. J Med Microbiol. 1982 Aug;15(3):327–330. doi: 10.1099/00222615-15-3-327. [DOI] [PubMed] [Google Scholar]
- Joiner K. A., Schmetz M. A., Goldman R. C., Leive L., Frank M. M. Mechanism of bacterial resistance to complement-mediated killing: inserted C5b-9 correlates with killing for Escherichia coli O111B4 varying in O-antigen capsule and O-polysaccharide coverage of lipid A core oligosaccharide. Infect Immun. 1984 Jul;45(1):113–117. doi: 10.1128/iai.45.1.113-117.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Platts-Mills T. A., Ishizaka K. Activation of the alternate pathway of human complements by rabbit cells. J Immunol. 1974 Jul;113(1):348–358. [PubMed] [Google Scholar]
- Reynolds B. L., Rother U. A., Rother K. O. Interaction of complement components with a serum-resistant strain of Salmonella typhimurium. Infect Immun. 1975 May;11(5):944–948. doi: 10.1128/iai.11.5.944-948.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice P. A., Kasper D. L. Characterization of serum resistance of Neisseria gonorrhoeae that disseminate. Roles of blocking antibody and gonococcal outer membrane proteins. J Clin Invest. 1982 Jul;70(1):157–167. doi: 10.1172/JCI110589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowley D. Endotoxins and bacterial virulence. J Infect Dis. 1971 Mar;123(3):317–327. doi: 10.1093/infdis/123.3.317. [DOI] [PubMed] [Google Scholar]
- Sarafian S. K., Tam M. R., Morse S. A. Gonococcal protein I-specific opsonic IgG in normal human serum. J Infect Dis. 1983 Dec;148(6):1025–1032. doi: 10.1093/infdis/148.6.1025. [DOI] [PubMed] [Google Scholar]
- Schneider H., Griffiss J. M. A bactericidal microassay for testing serum sensitivity of Neisseria gonorrhoeae. J Immunol Methods. 1982 Oct 15;54(1):101–105. doi: 10.1016/0022-1759(82)90118-1. [DOI] [PubMed] [Google Scholar]
- Schneider H., Griffiss J. M., Williams G. D., Pier G. B. Immunological basis of serum resistance of Neisseria gonorrhoeae. J Gen Microbiol. 1982 Jan;128(1):13–22. doi: 10.1099/00221287-128-1-13. [DOI] [PubMed] [Google Scholar]
- Schneider H., Hale T. L., Zollinger W. D., Seid R. C., Jr, Hammack C. A., Griffiss J. M. Heterogeneity of molecular size and antigenic expression within lipooligosaccharides of individual strains of Neisseria gonorrhoeae and Neisseria meningitidis. Infect Immun. 1984 Sep;45(3):544–549. doi: 10.1128/iai.45.3.544-549.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tramont E. C., Sadoff J. C., Wilson C. Variability of the lytic susceptibility of Neisseria gonorrhoeae to human sera. J Immunol. 1977 May;118(5):1843–1851. [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 1;119(1):115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- Verbrugh H. A., van Dijk W. C., van Erne M. E., Peters R., Peterson P. K., Verhoef J. Quantitation of the third component of human complement attached to the surface of opsonized bacteria: opsonin-deficient sera and phagocytosis-resistant strains. Infect Immun. 1979 Dec;26(3):808–814. doi: 10.1128/iai.26.3.808-814.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhoef J., Peterson P., Kim Y., Sabath L. D., Quie P. G. Opsonic requirements for staphylococcal phagocytosis. Heterogeneity among strains. Immunology. 1977 Aug;33(2):191–197. [PMC free article] [PubMed] [Google Scholar]
- Winkelstein J. A., Shin H. S., Wood W. B., Jr Heat labile opsonins to Pneumococcus. 3. The participation of immunoglobulin and of the alternate pathway of C3 activation. J Immunol. 1972 Jun;108(6):1681–1689. [PubMed] [Google Scholar]
- Zollinger W. D., Dalrymple J. M., Artenstein M. S. Analysis of parameters affecting the solid phase radioimmunoassay quantitation of antibody to meningococcal antigens. J Immunol. 1976 Nov;117(5 PT2):1788–1798. [PubMed] [Google Scholar]
- Zollinger W. D., Mandrell R. E. Importance of complement source in bactericidal activity of human antibody and murine monoclonal antibody to meningococcal group B polysaccharide. Infect Immun. 1983 Apr;40(1):257–264. doi: 10.1128/iai.40.1.257-264.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]