Abstract
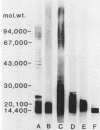
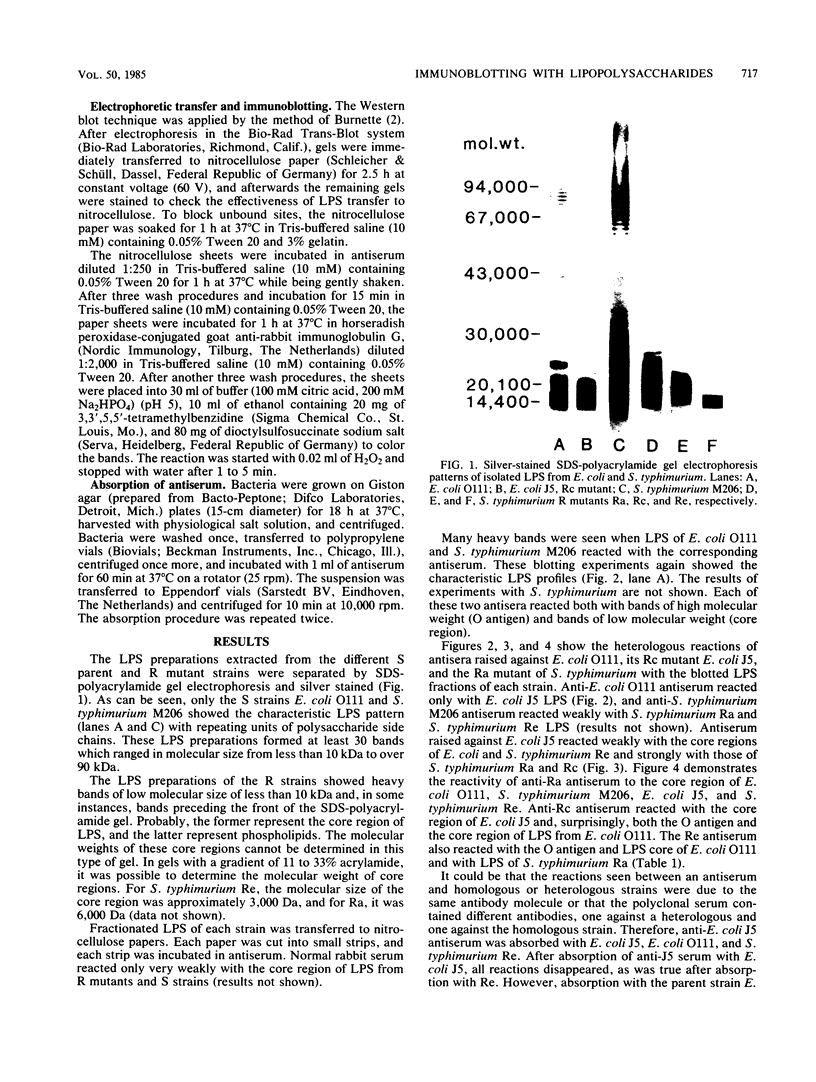
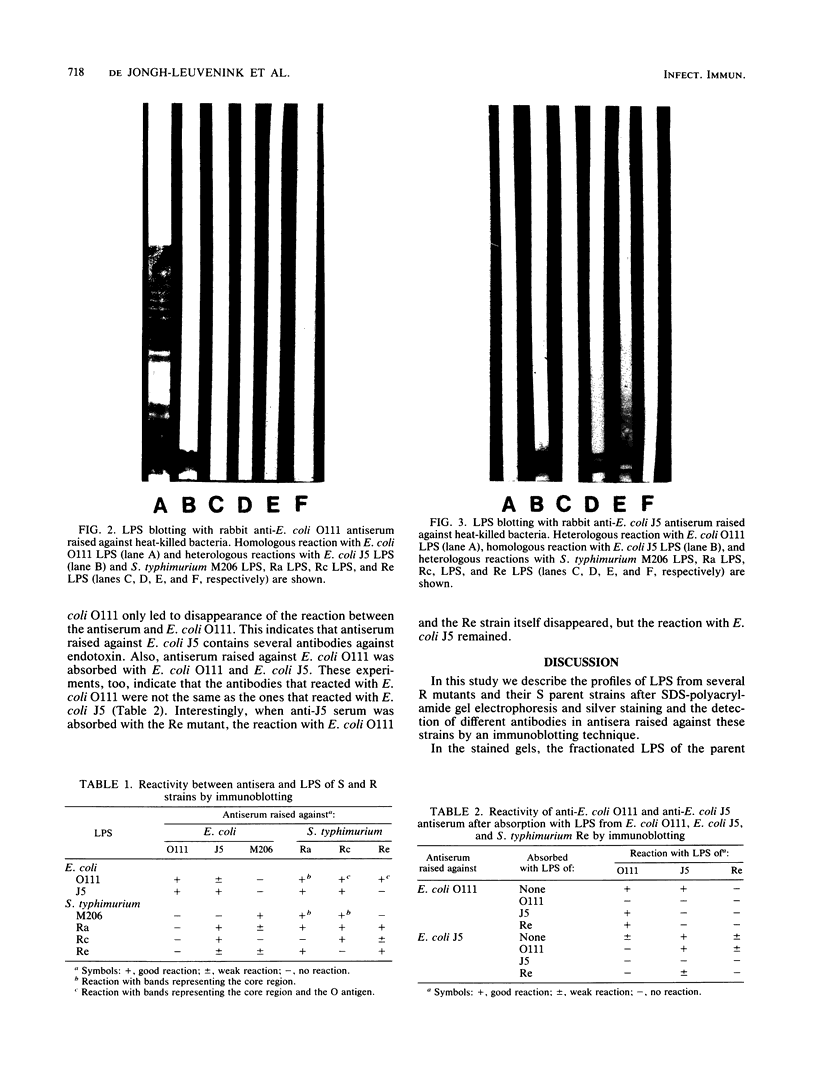
Antisera raised against several smooth and rough strains of Escherichia coli and Salmonella typhimurium were tested against lipopolysaccharides (LPS) of homologous and heterologous strains. The LPS were separated by sodium dodecyl sulfate-gel electrophoresis, transferred to nitrocellulose paper, and overlaid with antisera. The results showed that antisera raised against smooth strains reacted with high- as well as low-molecular-weight bands of their corresponding LPS and showed very few cross-reactions. Anti-E. coli J5 antiserum cross-reacted with few strains in the core region. But, anti-S. typhimurium Ra antiserum cross-reacted with many more strains. When these sera were absorbed with either the homologous- or a heterologous-positive strain, reactions were abolished. It appears that reactions of anti-E. coli J5 antiserum and anti-S. typhimurium Ra antiserum with homologous and heterologous strains were not due to the same antibody. This immunoblotting technique proved to be a useful method to distinguish different antibodies in antiserum raised against LPS of gram-negative bacteria.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Ivanoff B., André C., Fontanges R., Jourdan G. Secondary immune response to oral and nasal rough mutant strains of Salmonella typhimurium. Ann Immunol (Paris) 1982 Jul-Aug;133D(1):61–70. [PubMed] [Google Scholar]
- Izui S., Morrison D. C., Curry B., Dixon F. J. Effect of lipid A-associated protein and lipid A on the expression of lipopolysaccharide activity. I. Immunological activity. Immunology. 1980 Jul;40(3):473–482. [PMC free article] [PubMed] [Google Scholar]
- Johns M., Skehill A., McCabe W. R. Immunization with rough mutants of Salmonella minnesota. IV. Protection by antisera to O and rough antigens against endotoxin. J Infect Dis. 1983 Jan;147(1):57–67. doi: 10.1093/infdis/147.1.57. [DOI] [PubMed] [Google Scholar]
- Kirkland T. N., Ziegler E. J. An immunoprotective monoclonal antibody to lipopolysaccharide. J Immunol. 1984 May;132(5):2590–2592. [PubMed] [Google Scholar]
- Kusecek B., Wloch H., Mercer A., Vaisänen V., Pluschke G., Korhonen T., Achtman M. Lipopolysaccharide, capsule, and fimbriae as virulence factors among O1, O7, O16, O18, or O75 and K1, K5, or K100 Escherichia coli. Infect Immun. 1984 Jan;43(1):368–379. doi: 10.1128/iai.43.1.368-379.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugtenberg B., Meijers J., Peters R., van der Hoek P., van Alphen L. Electrophoretic resolution of the "major outer membrane protein" of Escherichia coli K12 into four bands. FEBS Lett. 1975 Oct 15;58(1):254–258. doi: 10.1016/0014-5793(75)80272-9. [DOI] [PubMed] [Google Scholar]
- Lüderitz O., Tanamoto K., Galanos C., McKenzie G. R., Brade H., Zähringer U., Rietschel E. T., Kusumoto S., Shiba T. Lipopolysaccharides: structural principles and biologic activities. Rev Infect Dis. 1984 Jul-Aug;6(4):428–431. doi: 10.1093/clinids/6.4.428. [DOI] [PubMed] [Google Scholar]
- Mattsby-Baltzer I., Gemski P., Alving C. R. Heterogeneity of lipid A: comparison of lipid A types from different gram-negative bacteria. J Bacteriol. 1984 Sep;159(3):900–904. doi: 10.1128/jb.159.3.900-904.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison D. C. Bacterial endotoxins and pathogenesis. Rev Infect Dis. 1983 Sep-Oct;5 (Suppl 4):S733–S747. doi: 10.1093/clinids/5.supplement_4.s733. [DOI] [PubMed] [Google Scholar]
- Mutharia L. M., Crockford G., Bogard W. C., Jr, Hancock R. E. Monoclonal antibodies specific for Escherichia coli J5 lipopolysaccharide: cross-reaction with other gram-negative bacterial species. Infect Immun. 1984 Sep;45(3):631–636. doi: 10.1128/iai.45.3.631-636.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rietschel E. T., Wollenweber H. W., Russa R., Brade H., Zähringer U. Concepts of the chemical structure of lipid A. Rev Infect Dis. 1984 Jul-Aug;6(4):432–438. doi: 10.1093/clinids/6.4.432. [DOI] [PubMed] [Google Scholar]
- Rowley D. Sensitivity of rough gram-negative bacteria to the bactericidal action of serum. J Bacteriol. 1968 May;95(5):1647–1650. doi: 10.1128/jb.95.5.1647-1650.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 1;119(1):115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- Young L. S., Stevens P., Ingram J. Functional role of antibody against "core" glycolipid of Enterobacteriaceae. J Clin Invest. 1975 Oct;56(4):850–861. doi: 10.1172/JCI108164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler E. J., McCutchan J. A., Fierer J., Glauser M. P., Sadoff J. C., Douglas H., Braude A. I. Treatment of gram-negative bacteremia and shock with human antiserum to a mutant Escherichia coli. N Engl J Med. 1982 Nov 11;307(20):1225–1230. doi: 10.1056/NEJM198211113072001. [DOI] [PubMed] [Google Scholar]