Abstract
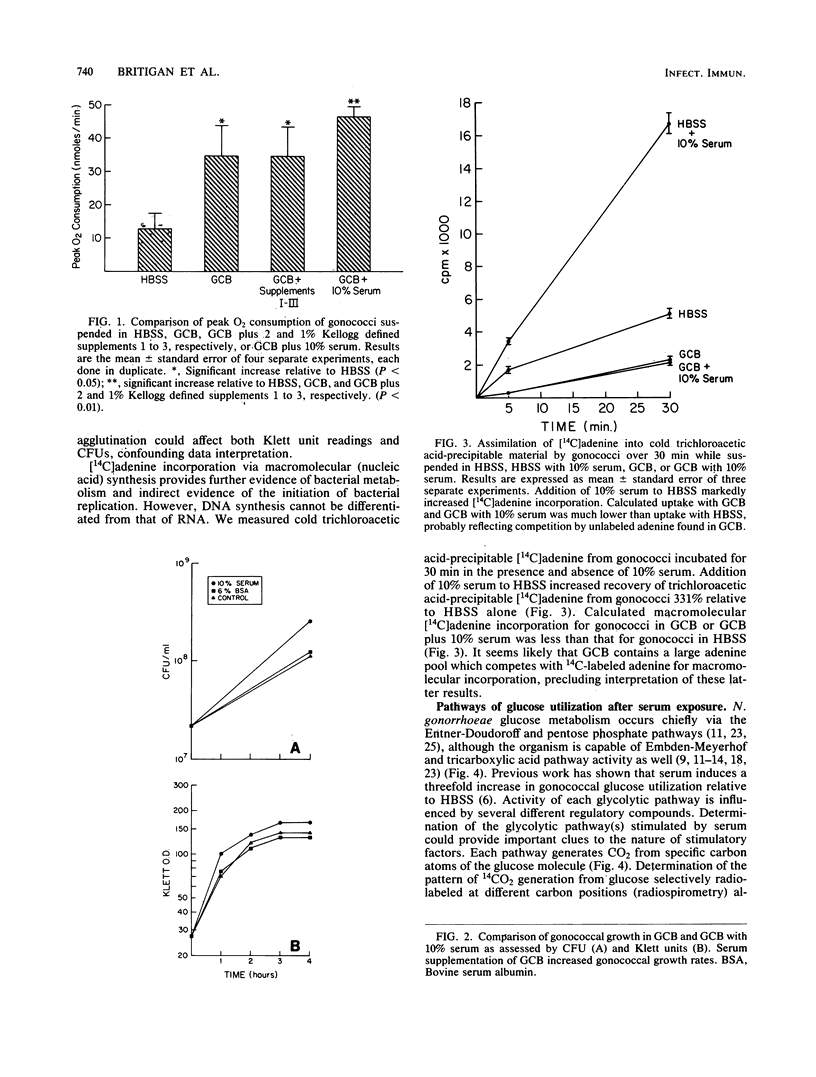
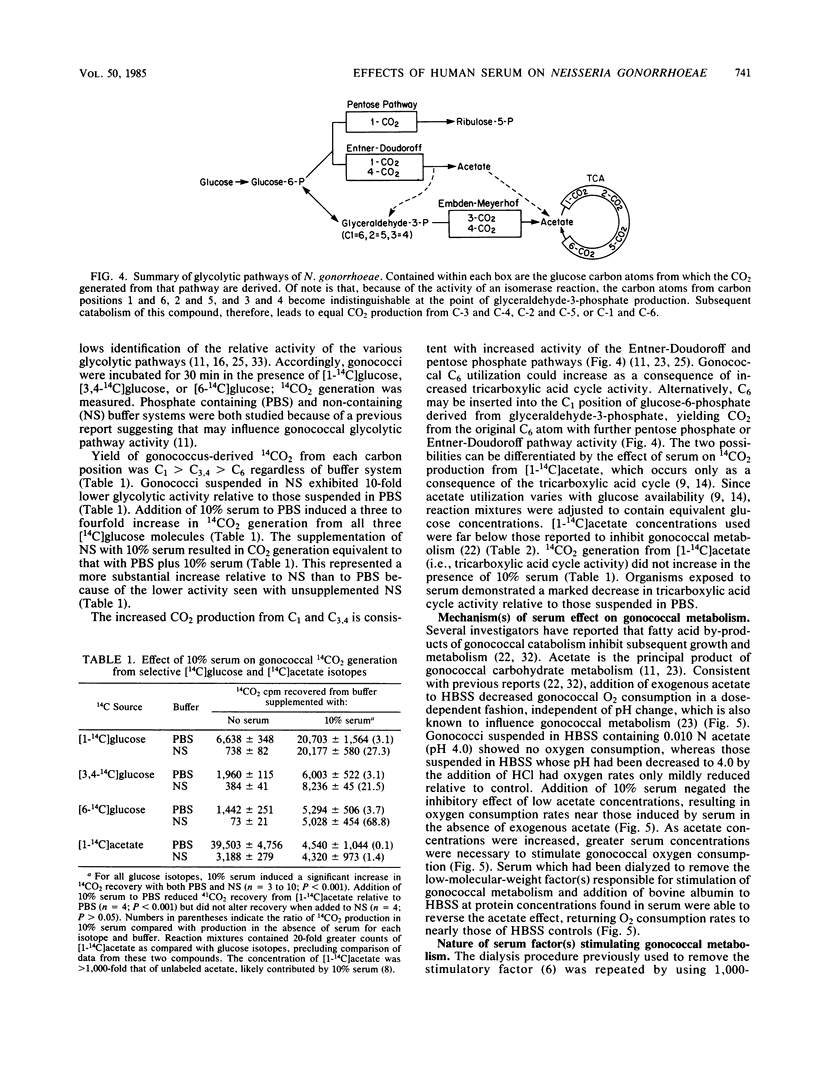
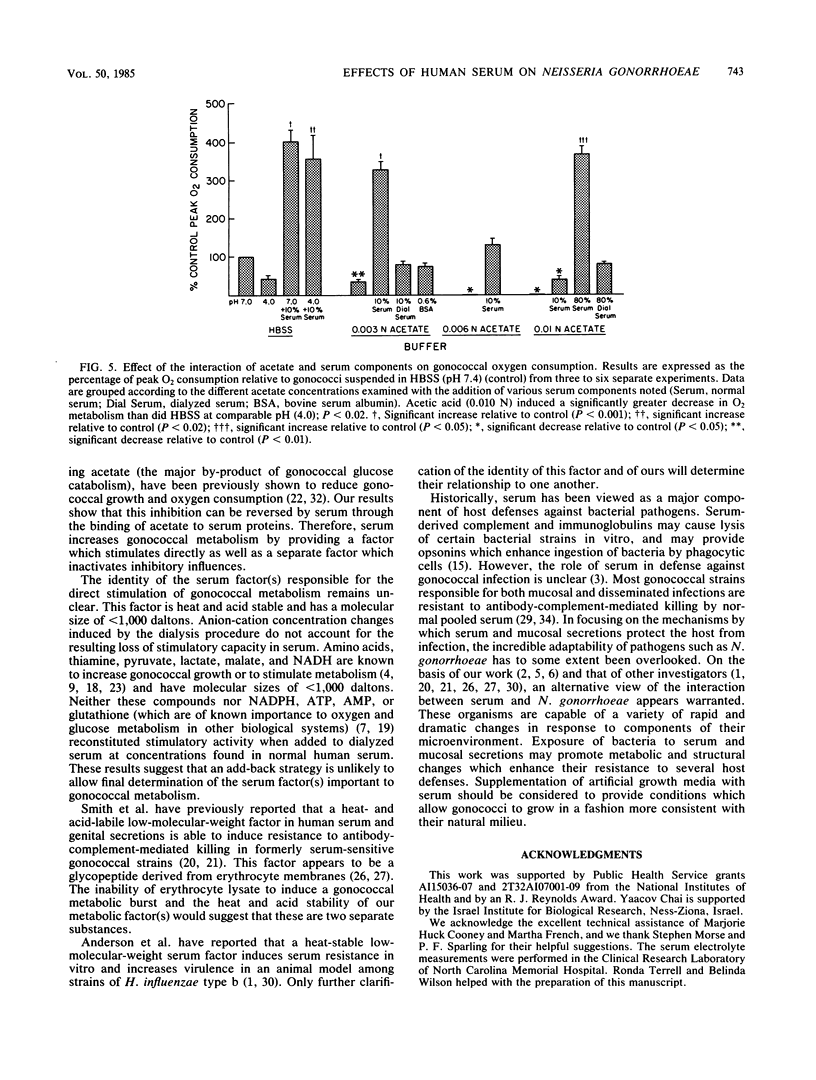
Humans are the sole reservoir of Neisseria gonorrhoeae, an organism which undergoes a marked increase in metabolic rate after exposure to a low-molecular-weight, heat-stable component(s) of human serum. Further studies on the effect of serum on gonococcal metabolism were undertaken. Gonococcal broth (GCB) is commonly used for in vitro cultivation of gonococci. Gonococci suspended in GCB plus 10% serum exhibited oxygen consumption rates of 139% (P less than 0.01) and 456% (P less than 0.01) of those suspended in GCB or Hanks balanced salt solution, respectively. A twofold increase in growth rate also resulted from the addition of 10% serum to GCB. Gonococcal 14C-labeled adenine incorporation increased threefold with 10% serum supplementation of Hanks balanced salt solution. Dialysis of serum in 1,000-molecular-weight exclusion tubing removed the stimulatory factor(s). Neither correction of anion-cation concentrations altered by dialysis nor addition of substances of known importance to the metabolism of gonococci (i.e., lactate, pyruvate, cysteine, ATP, AMP, NADPH, amino acids, malate, and glutathione) to dialyzed serum reconstituted stimulatory capacity. The effect of serum on gonococcal glucose-catabolic pathways was measured by modified radiospirometry. An apparent threefold increase in Entner-Doudoroff and pentose phosphate pathway activities was induced by 10% serum, as was the increased shunting of glucose-derived glyceraldehyde-3-phosphate into these pathways. These metabolic changes did not allow specific identification of the serum stimulatory factor(s). Acetate, the major by-product of gonococcal glucose catabolism, inhibited gonococcal oxygen consumption as previously reported. A high-molecular-weight serum component, probably albumin, reversed acetate-mediated inhibition of gonococcal oxygen consumption, identifying a second mechanism by which serum increases gonococcal metabolism. These results suggest that supplementation of growth media with serum should be considered to provide N. gonorrhoeae with conditions more consistent with its normal environment.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson P., Flesher A., Shaw S., Harding A. L., Smith D. H. Phenotypic and genetic variation in the susceptibility of Haemophilus influenzae type b to antibodies to somatic antigens. J Clin Invest. 1980 Apr;65(4):885–891. doi: 10.1172/JCI109741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britigan B. E., Cohen M. S., Sparling P. F. Gonococcal infection: a model of molecular pathogenesis. N Engl J Med. 1985 Jun 27;312(26):1683–1694. doi: 10.1056/NEJM198506273122606. [DOI] [PubMed] [Google Scholar]
- Britigan B., Cohen M. S. Effect of growth in serum on uptake of Neisseria gonorrhoeae by human neutrophils. J Infect Dis. 1985 Aug;152(2):330–338. doi: 10.1093/infdis/152.2.330. [DOI] [PubMed] [Google Scholar]
- Catlin B. W. Nutritional profiles of Neisseria gonorrhoeae, Neisseria meningitidis, and Neisseria lactamica in chemically defined media and the use of growth requirements for gonococcal typing. J Infect Dis. 1973 Aug;128(2):178–194. doi: 10.1093/infdis/128.2.178. [DOI] [PubMed] [Google Scholar]
- Cohen M. S., Black J. R., Proctor R. A., Sparling P. F. Host defences and the vaginal mucosa. A re-evaluation. Scand J Urol Nephrol Suppl. 1984;86:13–22. [PubMed] [Google Scholar]
- Hebeler B. H., Morse S. A. Physiology and metabolism of pathogenic neisseria: tricarboxylic acid cycle activity in Neisseria gonorrhoeae. J Bacteriol. 1976 Oct;128(1):192–201. doi: 10.1128/jb.128.1.192-201.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holten E. 6-Phosphogluconate dehydrogenase and enzymes of the Entner-Doudoroff pathway in Neisseria. Acta Pathol Microbiol Scand B Microbiol Immunol. 1974 Apr;82(2):207–213. doi: 10.1111/j.1699-0463.1974.tb02313.x. [DOI] [PubMed] [Google Scholar]
- Holten E. Pyridine nucleotide independent oxidation of L-malate in genus Neisseria. Acta Pathol Microbiol Scand B. 1976 Feb;84(1):17–21. doi: 10.1111/j.1699-0463.1976.tb01895.x. [DOI] [PubMed] [Google Scholar]
- Holten E. Radiorespirometric studies in genus Neisserai. I. The catabolism of glucose. Acta Pathol Microbiol Scand B. 1975 Aug;83(4):353–366. [PubMed] [Google Scholar]
- Holten E. Radiorespirometric studies in genus Neisseria. 2. The catabolism of glutamate and fumarate. Acta Pathol Microbiol Scand B. 1976 Feb;84(1):1–8. [PubMed] [Google Scholar]
- Holten E. Radiorespirometric studies in genus Neisseria. 3. The catabolism of pyruvate and acetate. Acta Pathol Microbiol Scand B. 1976 Feb;84(1):9–16. [PubMed] [Google Scholar]
- Horwitz M. A. Phagocytosis of microorganisms. Rev Infect Dis. 1982 Jan-Feb;4(1):104–123. doi: 10.1093/clinids/4.1.104. [DOI] [PubMed] [Google Scholar]
- KATZ J., WOOD H. G. The use of C14O2 yields from glucose-1- and -6-C14 for the evaluation of the pathways of glucose metabolism. J Biol Chem. 1963 Feb;238:517–523. [PubMed] [Google Scholar]
- KELLOGG D. S., Jr, PEACOCK W. L., Jr, DEACON W. E., BROWN L., PIRKLE D. I. NEISSERIA GONORRHOEAE. I. VIRULENCE GENETICALLY LINKED TO CLONAL VARIATION. J Bacteriol. 1963 Jun;85:1274–1279. doi: 10.1128/jb.85.6.1274-1279.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenimer E. A., Lapp D. F. Effects of selected inhibitors on electron transport in Neisseria gonorrhoeae. J Bacteriol. 1978 May;134(2):537–545. doi: 10.1128/jb.134.2.537-545.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin P. M., Patel P. V., Parsons N. J., Smith H. Induction of phenotypically determined resistance of Neisseria gonorrhoeae to human serum by factors in human serum. J Gen Microbiol. 1981 Nov;127(1):213–217. doi: 10.1099/00221287-127-1-213. [DOI] [PubMed] [Google Scholar]
- Martin P. M., Patel P. V., Parsons N. J., Smith H. Induction of serum resistance in recent isolates of Neisseria gonorrhoeae by a low-molecular-weight fraction of guinea pig serum. J Infect Dis. 1983 Aug;148(2):334–334. doi: 10.1093/infdis/148.2.334. [DOI] [PubMed] [Google Scholar]
- Miller R. D., Brown K. E., Morse S. A. Inhibitory action of fatty acids on the growth of Neisseria gonorrhoeae. Infect Immun. 1977 Aug;17(2):303–312. doi: 10.1128/iai.17.2.303-312.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse S. A., Hebeler B. H. Effect of pH on the growth and glucose metabolism of Neisseria gonorrhoeae. Infect Immun. 1978 Jul;21(1):87–95. doi: 10.1128/iai.21.1.87-95.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse S. A., Stein S., Hines J. Glucose metabolism in Neisseria gonorrhoeae. J Bacteriol. 1974 Nov;120(2):702–714. doi: 10.1128/jb.120.2.702-714.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel P. V., Martin P. M., Goldner M., Parsons N. J., Smith H. Red blood cells, a source of factors which induce Neisseria gonorrhoeae to resistance to complement-mediated killing by human serum. J Gen Microbiol. 1984 Nov;130(11):2767–2770. doi: 10.1099/00221287-130-11-2767. [DOI] [PubMed] [Google Scholar]
- Patel P. V., Veale D. R., Fox J. E., Martin P. M., Parsons N. J., Smith H. Fractionation of guinea pig serum for an inducer of gonococcal resistance to killing by human serum: active fractions containing glucopeptides similar to those from human red blood cells. J Gen Microbiol. 1984 Nov;130(11):2757–2766. doi: 10.1099/00221287-130-11-2757. [DOI] [PubMed] [Google Scholar]
- Root R. K., Cohen M. S. The microbicidal mechanisms of human neutrophils and eosinophils. Rev Infect Dis. 1981 May-Jun;3(3):565–598. doi: 10.1093/clinids/3.3.565. [DOI] [PubMed] [Google Scholar]
- Rosenthal R. S., Fulbright R. S., Eads M. E., Sawyer W. D. Ethylenediaminetetraacetic acid-sensitive antiphagocytic activity of Neisseria gonorrhoeae. Infect Immun. 1977 Mar;15(3):817–827. doi: 10.1128/iai.15.3.817-827.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoolnik G. K., Buchanan T. M., Holmes K. K. Gonococci causing disseminated gonococcal infection are resistant to the bactericidal action of normal human sera. J Clin Invest. 1976 Nov;58(5):1163–1173. doi: 10.1172/JCI108569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw S., Smith A. L., Anderson P., Smith D. H. The paradox of Hemophilus infuenzae type B bacteremia in the presence of serum bactericidal activity. J Clin Invest. 1976 Oct;58(4):1019–1029. doi: 10.1172/JCI108525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J., Kraus S. J., Gotschlich E. C. Studies on gonococcus infection. I. Pili and zones of adhesion: their relation to gonococcal growth patterns. J Exp Med. 1971 Oct 1;134(4):886–906. doi: 10.1084/jem.134.4.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WANG C. H., STERN I., GILMOUR C. M., KLUNGSOYR S., REED D. J., BIALY J. J., CHRISTENSEN B. E., CHELDELIN V. H. Comparative study of glucose catabolism by the radiorespirometric method. J Bacteriol. 1958 Aug;76(2):207–216. doi: 10.1128/jb.76.2.207-216.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walstad D. L., Reitz R. C., Sparling P. F. Growth inhibition among strains of Neisseria gonorrhoeae due to production of inhibitory free fatty acids and lysophosphatidylethanolamine: absence of bacteriocins. Infect Immun. 1974 Sep;10(3):481–488. doi: 10.1128/iai.10.3.481-488.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward M. E., Watt P. J., Glynn A. A. Gonococci in urethral exudates possess a virulence factor lost on subculture. Nature. 1970 Jul 25;227(5256):382–384. doi: 10.1038/227382a0. [DOI] [PubMed] [Google Scholar]


